Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review
Abstract
:1. Introduction
2. Cytokines in Physical Activity
2.1. Interleukins (ILs)
2.2. Chemokines
2.3. Interferons (IFNs)
2.4. Tumor Necrosis Factor (TNF)
2.5. Colony-Stimulating Factors (CSFs)
2.6. Growth Factors
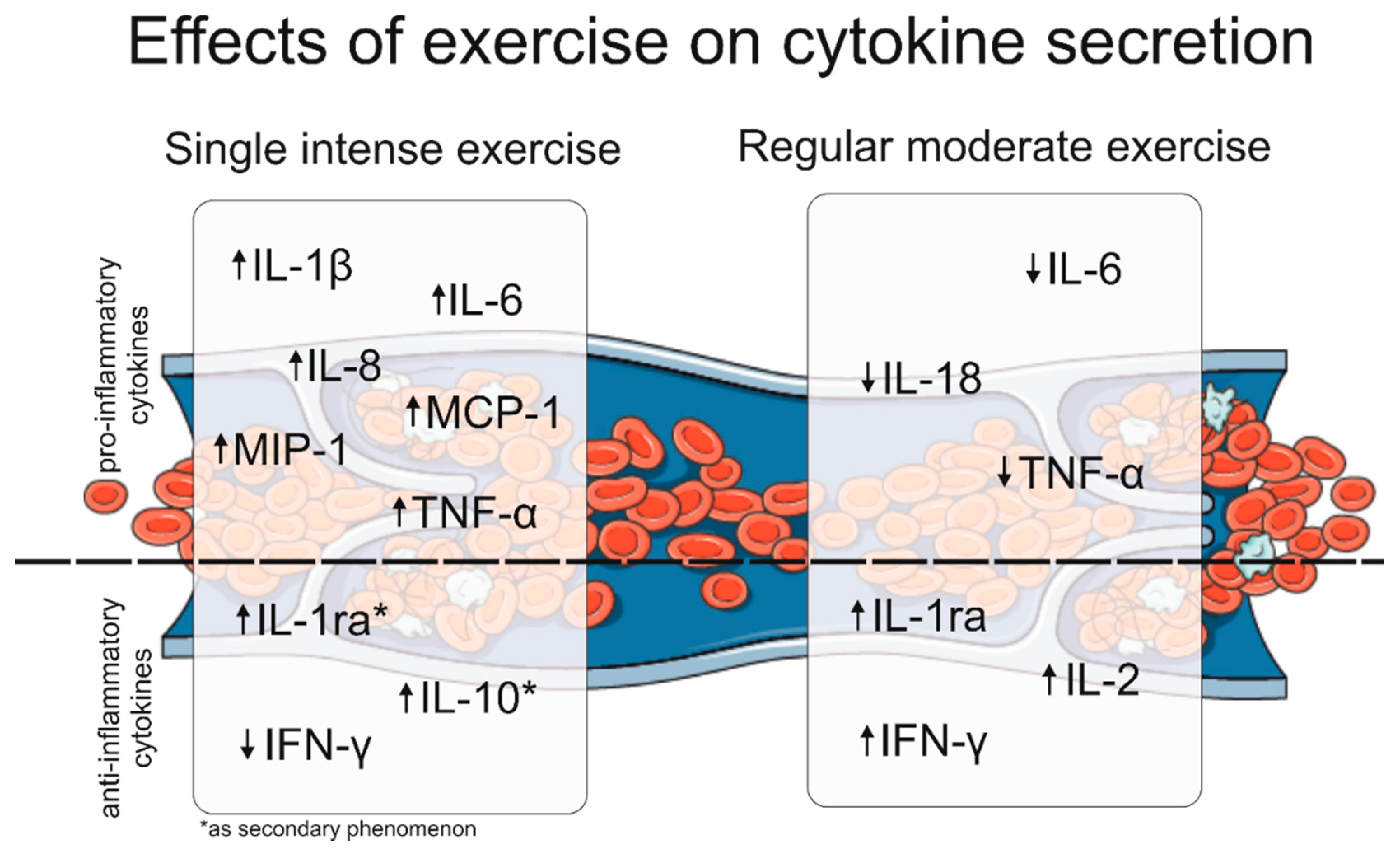
2.7. Summary of the Effects of Exercise on Cytokine Secretion
3. Effect of Training Level on the Amount of Secreted Cytokines
4. Cytokines as Biomarkers in Sport and Exercise
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Schuch, F.B.; Vancampfort, D. Physical Activity, Exercise, and Mental Disorders: It Is Time to Move On. Trends Psychiatry Psychother. 2021, 43, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. Biomed. Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Conn, V.S.; Koopman, R.J.; Ruppar, T.M.; Phillips, L.J.; Mehr, D.R.; Hafdahl, A.R. Insulin Sensitivity Following Exercise Interventions: Systematic Review and Meta-Analysis of Outcomes Among Healthy Adults. J. Prim. Care Community Health 2014, 5, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Schuch, F.B.; Stubbs, B.; Meyer, J.; Heissel, A.; Zech, P.; Vancampfort, D.; Rosenbaum, S.; Deenik, J.; Firth, J.; Ward, P.B.; et al. Physical Activity Protects from Incident Anxiety: A Meta-Analysis of Prospective Cohort Studies. Depress. Anxiety 2019, 36, 846–858. [Google Scholar] [CrossRef]
- Callaghan, P. Exercise: A Neglected Intervention in Mental Health Care? J. Psychiatr. Ment. Health Nurs. 2004, 11, 476–483. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.-Y.; Cheng, Y.-C.; Su, C.-H. Exercise Dosage in Reducing the Risk of Dementia Development: Mode, Duration, and Intensity—A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 13331. [Google Scholar] [CrossRef]
- Kline, C.E. The Bidirectional Relationship between Exercise and Sleep: Implications for Exercise Adherence and Sleep Improvement. Am. J. Lifestyle Med. 2014, 8, 375–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, M.; Rejeski, J.; Blair, S.; Duncan, P.; Judge, J.; King, A.; Macera, C.; Castaneda-Sceppa, C. Physical Activity and Public Health in Older Adults. Circulation 2007, 116, 1094–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mersy, D. Health Benefits of Aerobic Exercise. Postgrad. Med. 1991, 90, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Seguin, R.; Nelson, M.E. The Benefits of Strength Training for Older Adults. Am. J. Prev. Med. 2003, 25, 141–149. [Google Scholar] [CrossRef]
- Lim, E.-J.; Hyun, E.-J. The Impacts of Pilates and Yoga on Health-Promoting Behaviors and Subjective Health Status. Int. J. Environ. Res. Public Health 2021, 18, 3802. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Craighead, D.H.; Heinbockel, T.C.; Freeberg, K.A.; Rossman, M.J.; Jackman, R.A.; Jankowski, L.R.; Hamilton, M.N.; Ziemba, B.P.; Reisz, J.A.; D’Alessandro, A.; et al. Time-Efficient Inspiratory Muscle Strength Training Lowers Blood Pressure and Improves Endothelial Function, NO Bioavailability, and Oxidative Stress in Midlife/Older Adults with Above-Normal Blood Pressure. J. Am. Heart Assoc. 2021, 10, e020980. [Google Scholar] [CrossRef]
- Tesema, G.; George, M.; Mondal, S.; Mathivana, D. Serum Cardiac Markers Are Inversely Associated with VO2max of Amateur Athletes in Response to Endurance Training Adaptations. BMJ Open Sport Exerc. Med. 2019, 5, e000537. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-M.; An, J. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.L.; Borba, H.H.L.; Bonetti, A.d.F.; Leonart, L.; Pontarolo, R. Cytokines and Interferons: Types and Functions; IntechOpen: Vienna, Austria, 2018; ISBN 978-1-78984-853-3. [Google Scholar]
- Sims, J.E.; Smith, D.E. The IL-1 Family: Regulators of Immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The Cytokine Response to Physical Activity and Training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef] [PubMed]
- König, A.; Mühlbauer, R.C.; Fleisch, H. Tumor Necrosis Factor Alpha and Interleukin-1 Stimulate Bone Resorption in Vivo as Measured by Urinary [3H]Tetracycline Excretion from Prelabeled Mice. J. Bone Miner. Res. 1988, 3, 621–627. [Google Scholar] [CrossRef]
- Zamir, O.; Hasselgren, P.O.; O’Brien, W.; Thompson, R.C.; Fischer, J.E. Muscle Protein Breakdown during Endotoxemia in Rats and after Treatment with Interleukin-1 Receptor Antagonist (IL-1ra). Ann. Surg. 1992, 216, 381–385; discussion 386–387. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, H.; Jacobs, C.; Nain, M.; Gressner, A.M.; Prinz, H.; Wesemann, W.; Gemsa, D. Enhanced Release of Cytokines, Interleukin-2 Receptors, and Neopterin after Long-Distance Running. Clin. Immunol. Immunopathol. 1992, 63, 188–195. [Google Scholar] [CrossRef]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Callister, R.; Gleeson, M. Cytokine Responses to Treadmill Running in Healthy and Illness-Prone Athletes. Med. Sci. Sports Exerc. 2007, 39, 1918–1926. [Google Scholar] [CrossRef]
- Nieman, D.C.; Dumke, C.I.; Henson, D.A.; McAnulty, S.R.; McAnulty, L.S.; Lind, R.H.; Morrow, J.D. Immune and Oxidative Changes during and Following the Western States Endurance Run. Int. J. Sports Med. 2003, 24, 541–547. [Google Scholar] [CrossRef]
- Ronsen, O.; Lea, T.; Bahr, R.; Pedersen, B.K. Enhanced Plasma IL-6 and IL-1ra Responses to Repeated vs. Single Bouts of Prolonged Cycling in Elite Athletes. J Appl. Physiol. 2002, 92, 2547–2553. [Google Scholar] [CrossRef] [Green Version]
- Neumayr, G.; Ludwiczek, O.; Hoertnagl, H.; Pfister, R.; Mitterbauer, G.; Moschen, A.; Novick, D.; Rubinstein, M.; Tilg, H. The Impact of Prolonged Strenuous Endurance Exercise on Interleukin 18 and Interleukin 18 Binding Protein in Recreational Cyclists. Int. J. Sports Med. 2005, 26, 836–840. [Google Scholar] [CrossRef]
- Stensvold, D.; Slørdahl, S.A.; Wisløff, U. Effect of Exercise Training on Inflammation Status among People with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2012, 10, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Leick, L.; Lindegaard, B.; Stensvold, D.; Plomgaard, P.; Saltin, B.; Pilegaard, H. Adipose Tissue Interleukin-18 MRNA and Plasma Interleukin-18: Effect of Obesity and Exercise. Obesity 2007, 15, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.Y.; Selmi, C.; Gershwin, M.E. The Regulatory, Inflammatory, and T Cell Programming Roles of Interleukin-2 (IL-2). J. Autoimmun. 2008, 31, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Espersen, G.T.; ElbæK, A.; Ernst, E.; Toft, E.; Kaalund, S.; Jersild, C.; Grunnet, N. Effect of Physical Exercise on Cytokines and Lymphocyte Subpopulations in Human Peripheral Blood. APMIS 1990, 98, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating Cytokines and Hormones with Immunosuppressive but Neutrophil-Priming Potentials Rise after Endurance Exercise in Humans. Eur. J. Appl. Physiol. 2000, 81, 281–287. [Google Scholar] [CrossRef]
- Santos, V.C.; Levada-Pires, A.C.; Alves, S.R.; Pithon-Curi, T.C.; Curi, R.; Cury-Boaventura, M.F. Effects of DHA-Rich Fish Oil Supplementation on Lymphocyte Function before and after a Marathon Race. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 161–169. [Google Scholar] [CrossRef]
- Leelarungrayub, D.; Saidee, K.; Pothongsunun, P.; Pratanaphon, S.; YanKai, A.; Bloomer, R.J. Six Weeks of Aerobic Dance Exercise Improves Blood Oxidative Stress Status and Increases Interleukin-2 in Previously Sedentary Women. J. Bodyw. Mov. Ther. 2011, 15, 355–362. [Google Scholar] [CrossRef]
- Juszkiewicz, A.; Basta, P.; Trzeciak, J.; Petriczko, E.; Cieślicka, M.; Skarpańska-Stejnborn, A. Effect of Spirulina Supplementation on Selected Components of Th1/Th2 Balance in Rowers. Food Agric. Immunol. 2019, 30, 178–189. [Google Scholar] [CrossRef] [Green Version]
- Junior, L.A.L.; Santo, J.d.M.B.d.; Bachi, A.L.L.; Foster, R.; Amaro, A.S.; Oliveira, A.P.L.d.; Sierra, A.P.R.; Kiss, M.A.P.D.M.; Vaisberg, M.W. Relationship between Cytokines and Running Economy in Marathon Runners. Open Life Sci. 2016, 11, 308–312. [Google Scholar] [CrossRef]
- Clifford, T.; Allerton, D.M.; Brown, M.A.; Harper, L.; Horsburgh, S.; Keane, K.M.; Stevenson, E.J.; Howatson, G. Minimal Muscle Damage after a Marathon and No Influence of Beetroot Juice on Inflammation and Recovery. Appl. Physiol. Nutr. Metab. 2017, 42, 263–270. [Google Scholar] [CrossRef]
- Batatinha, H.; Tavares-Silva, E.; Leite, G.S.F.; Resende, A.S.; Albuquerque, J.A.T.; Arslanian, C.; Fock, R.A.; Lancha, A.H.; Lira, F.S.; Krüger, K.; et al. Probiotic Supplementation in Marathonists and Its Impact on Lymphocyte Population and Function after a Marathon: A Randomized Placebo-Controlled Double-Blind Study. Sci. Rep. 2020, 10, 18777. [Google Scholar] [CrossRef]
- Jürimäe, J.; Vaiksaar, S.; Purge, P. Circulating Inflammatory Cytokine Responses to Endurance Exercise in Female Rowers. Int. J. Sports Med. 2018, 39, 1041–1048. [Google Scholar] [CrossRef]
- Skinner, S.; Nader, E.; Stauffer, E.; Robert, M.; Boisson, C.; Cibiel, A.; Foschia, C.; Feasson, L.; Robach, P.; Millet, G.Y.; et al. Differential Impacts of Trail and Ultra-Trail Running on Cytokine Profiles: An Observational Study. Clin. Hemorheol. Microcirc. 2021, 78, 301–310. [Google Scholar] [CrossRef]
- Crane, L.J.; Miller, D.L. Plasma Protein Induction by Isolated Hepatocytes. Mol. Cell. Biochem. 1983, 53–54, 89–109. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, C.A.; Jones, S.A. IL-6 as a Keystone Cytokine in Health and Disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence That Interleukin-6 Is Produced in Human Skeletal Muscle during Prolonged Running. J. Physiol. 1998, 508 Pt 3, 949–953. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and Anti-Inflammatory Cytokine Balance in Strenuous Exercise in Humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- Castell, L.M.; Poortmans, J.R.; Leclercq, R.; Brasseur, M.; Duchateau, J.; Newsholme, E.A. Some Aspects of the Acute Phase Response after a Marathon Race, and the Effects of Glutamine Supplementation. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fensham, N.C.; McKay, A.K.A.; Tee, N.; Lundy, B.; Anderson, B.; Morabito, A.; Ross, M.L.R.; Burke, L.M. Sequential Submaximal Training in Elite Male Rowers Does Not Result in Amplified Increases in Interleukin-6 or Hepcidin. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.; Passos, M.E.P.; Silva, M.B.B.; Santos, V.C.; Momesso, C.M.; Pithon-Curi, T.C.; Gorjão, R.; Gray, S.R.; Lima, K.C.A.; de Freitas, P.B.; et al. Dance Training Improves Cytokine Secretion and Viability of Neutrophils in Diabetic Patients. Mediat. Inflamm. 2019, 2019, e2924818. [Google Scholar] [CrossRef] [Green Version]
- Halson, S.L.; Lancaster, G.I.; Jeukendrup, A.E.; Gleeson, M. Immunological Responses to Overreaching in Cyclists. Med. Sci. Sports Exerc. 2003, 35, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, P.; Dong, J. Effects of Overtraining on Skeletal Muscle Growth and Gene Expression. Int. J. Sports Med. 2012, 33, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Pauli, J.R.; de Souza, C.T.; Ropelle, E.R.; Cintra, D.E.; Rocha, E.M.; Freitas, E.C.; Papoti, M.; da Silva, L.; Lira, F.S.; et al. Nonfunctional Overreaching Leads to Inflammation and Myostatin Upregulation in Swiss Mice. Int. J. Sports Med. 2014, 35, 139–146. [Google Scholar] [CrossRef] [Green Version]
- da Rocha, A.L.; Pereira, B.C.; Teixeira, G.R.; Pinto, A.P.; Frantz, F.G.; Elias, L.L.K.; Lira, F.S.; Pauli, J.R.; Cintra, D.E.; Ropelle, E.R.; et al. Treadmill Slope Modulates Inflammation, Fiber Type Composition, Androgen, and Glucocorticoid Receptors in the Skeletal Muscle of Overtrained Mice. Front. Immunol. 2017, 8, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-Derived Interleukin-6: Possible Biological Effects. J. Physiol. 2001, 536, 329–337. [Google Scholar] [CrossRef]
- Vollmer-Conna, U.; Fazou, C.; Cameron, B.; Li, H.; Brennan, C.; Luck, L.; Davenport, T.; Wakefield, D.; Hickie, I.; Lloyd, A. Production of Pro-Inflammatory Cytokines Correlates with the Symptoms of Acute Sickness Behaviour in Humans. Psychol. Med. 2004, 34, 1289–1297. [Google Scholar] [CrossRef]
- Robson-Ansley, P.J.; de Milander, L.; Collins, M.; Noakes, T.D. Acute Interleukin-6 Administration Impairs Athletic Performance in Healthy, Trained Male Runners. Can. J. Appl. Physiol. 2004, 29, 411–418. [Google Scholar] [CrossRef]
- Verma, R.; Balakrishnan, L.; Sharma, K.; Khan, A.A.; Advani, J.; Gowda, H.; Tripathy, S.P.; Suar, M.; Pandey, A.; Gandotra, S.; et al. A Network Map of Interleukin-10 Signaling Pathway. J. Cell Commun. Signal. 2016, 10, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Koppelman, B.; Neefjes, J.J.; de Vries, J.E.; de Waal Malefyt, R. Interleukin-10 down-Regulates MHC Class II Alphabeta Peptide Complexes at the Plasma Membrane of Monocytes by Affecting Arrival and Recycling. Immunity 1997, 7, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Dulson, D. T-Cells and Their Cytokine Production: The Anti-Inflammatory and Immunosuppressive Effects of Strenuous Exercise. Cytokine 2018, 104, 136–142. [Google Scholar] [CrossRef]
- Cabral-Santos, C.; de Lima Junior, E.A.; Fernandes, I.M.d.C.; Pinto, R.Z.; Rosa-Neto, J.C.; Bishop, N.C.; Lira, F.S. Interleukin-10 Responses from Acute Exercise in Healthy Subjects: A Systematic Review. J. Cell. Physiol. 2019, 234, 9956–9965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. IL-6 Enhances Plasma IL-1ra, IL-10, and Cortisol in Humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef] [PubMed]
- Pretolani, M. Interleukin-10: An Anti-Inflammatory Cytokine with Therapeutic Potential. Clin. Exp. Allergy 1999, 29, 1164–1171. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J.S. Plasma Cytokine Changes in Relation to Exercise Intensity and Muscle Damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.R.; Pedersen, B.K. The Biological Roles of Exercise-Induced Cytokines: IL-6, IL-8, and IL-15. Appl. Physiol. Nutr. Metab. 2007, 32, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.S.; Anderson, B.G.; Drivdahl, R.H.; Alvarez, B.; Argilés, J.M. Overexpression of Interleukin-15 Induces Skeletal Muscle Hypertrophy in Vitro: Implications for Treatment of Muscle Wasting Disorders. Exp. Cell Res. 2002, 280, 55–63. [Google Scholar] [CrossRef]
- Carbó, N.; López-Soriano, J.; Costelli, P.; Alvarez, B.; Busquets, S.; Baccino, F.M.; Quinn, L.S.; López-Soriano, F.J.; Argilés, J.M. Interleukin-15 Mediates Reciprocal Regulation of Adipose and Muscle Mass: A Potential Role in Body Weight Control. Biochim. Biophys. Acta Gen. Subj. 2001, 1526, 17–24. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Ann. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [Green Version]
- Gilman-Sachs, A.; DuChateau, B. Clinical Relevance of Chemokines. Clin. Immunol. Newsl. 1997, 17, 93–98. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Chemokines Are Elevated in Plasma after Strenuous Exercise in Humans. Eur. J. Appl. Physiol. 2001, 84, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Gross, S.J.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; Mcanulty, S.R.; et al. Muscle Cytokine mRNA Changes after 2.5 h of Cycling: Influence of Carbohydrate. Med. Sci. Sports Exerc. 2005, 37, 1283. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a Competitive Marathon Race on Systemic Cytokine and Neutrophil Responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Maciejewska-Skrendo, A.; Tarnowski, M.; Kopytko, P.; Kochanowicz, A.; Mieszkowski, J.; Stankiewicz, B.; Sawczuk, M. CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls. Int. J. Environ. Res. Public Health 2022, 19, 9947. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; von Stebut, E. Macrophage Inflammatory Protein-1. Int. J. Biochem. Cell Biol. 2004, 36, 1882–1886. [Google Scholar] [CrossRef]
- Kline, J.N.; Kitagaki, K. Interferons. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 346–350. ISBN 978-0-12-370879-3. [Google Scholar]
- Parkin, J.; Cohen, B. An Overview of the Immune System. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Weerd, N.A.d.; Samarajiwa, S.A.; Hertzog, P.J. Type I Interferon Receptors: Biochemistry and Biological Functions. J. Biol. Chem. 2007, 282, 20053–20057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-J. IPC: Professional Type 1 Interferon-Producing Cells and Plasmacytoid Dendritic Cell Precursors. Ann. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef]
- Hermant, P.; Michiels, T. Interferon-λ in the Context of Viral Infections: Production, Response and Therapeutic Implications. JIN 2014, 6, 563–574. [Google Scholar] [CrossRef]
- Vijayaraghava, A.; Radhika, K. Alteration of Interferon Gamma (IFN-γ) in Human Plasma with Graded Physical Activity. J. Clin. Diagn. Res. 2014, 8, BC05–BC07. [Google Scholar] [CrossRef]
- Zamani, A.; Salehi, I.; Alahgholi-Hajibehzad, M. Moderate Exercise Enhances the Production of Interferon-γ and Interleukin-12 in Peripheral Blood Mononuclear Cells. Immune Netw. 2017, 17, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour Necrosis Factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Maldonado, A.; Montero, S.; Lemus, M.; Cerna-Cortés, J.; Rodríguez-Hernández, A.; Mendoza, M.A.; Melnikov, V.; Gamboa-Domínguez, A.; Muñiz, J.; Virgen-Ortiz, A.; et al. Moderate and High Intensity Chronic Exercise Reduces Plasma Tumor Necrosis Factor Alpha and Increases the Langerhans Islet Area in Healthy Rats. J. Musculoskelet. Neuronal Interact. 2019, 19, 354–361. [Google Scholar] [PubMed]
- Macêdo Santiago, L.Â.; Neto, L.G.L.; Borges Pereira, G.; Leite, R.D.; Mostarda, C.T.; de Oliveira Brito Monzani, J.; Sousa, W.R.; Rodrigues Pinheiro, A.J.M.; Navarro, F. Effects of Resistance Training on Immunoinflammatory Response, TNF-Alpha Gene Expression, and Body Composition in Elderly Women. J. Aging Res. 2018, 2018, 1467025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heir, R.; Stellwagen, D. TNF-Mediated Homeostatic Synaptic Plasticity: From in Vitro to in Vivo Models. Front. Cell. Neurosci. 2020, 14, 565841. [Google Scholar] [CrossRef] [PubMed]
- Gough, P.; Myles, I.A. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front. Immunol. 2020, 11, 585880. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Toft, A.D.; Jensen, L.B.; Bruunsgaard, H.; Ibfelt, T.; Halkjær-Kristensen, J.; Febbraio, M.; Pedersen, B.K. Cytokine Response to Eccentric Exercise in Young and Elderly Humans. Am. J. Physiol. Cell Physiol. 2002, 283, C289–C295. [Google Scholar] [CrossRef] [Green Version]
- Starkie, R.L.; Rolland, J.; Angus, D.J.; Anderson, M.J.; Febbraio, M.A. Circulating Monocytes Are Not the Source of Elevations in Plasma IL-6 and TNF-Alpha Levels after Prolonged Running. Am. J. Physiol. Cell Physiol. 2001, 280, C769–C774. [Google Scholar] [CrossRef]
- Hamada, K.; Vannier, E.; Sacheck, J.M.; Witsell, A.L.; Roubenoff, R. Senescence of Human Skeletal Muscle Impairs the Local Inflammatory Cytokine Response to Acute Eccentric Exercise. FASEB J. 2005, 19, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, D. The Colony-Stimulating Factors and Cancer. Nat. Rev. Cancer 2010, 10, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arellano, M.; Lonial, S. Clinical Uses of GM-CSF, a Critical Appraisal and Update. Biologics 2008, 2, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tojo, N.; Asakura, E.; Koyama, M.; Tanabe, T.; Nakamura, N. Effects of Macrophage Colony-Stimulating Factor (M-CSF) on Protease Production from Monocyte, Macrophage and Foam Cell in Vitro: A Possible Mechanism for Anti-Atherosclerotic Effect of M-CSF. Biochim. Biophys. Acta Mol. Cell Res. 1999, 1452, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Suzuki, K.; Kudo, S.; Totsuka, M.; Nakaji, S.; Sugawara, K. Raised Plasma G-CSF and IL-6 after Exercise May Play a Role in Neutrophil Mobilization into the Circulation. J. Appl. Physiol. 2002, 92, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.R.; Ward, A.C.; Russell, A.P. Granulocyte Colony-Stimulating Factor and Its Potential Application for Skeletal Muscle Repair and Regeneration. Mediat. Inflamm. 2017, 2017, 7517350. [Google Scholar] [CrossRef] [Green Version]
- Stone, W.L.; Leavitt, L.; Varacallo, M. Physiology, Growth Factor. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Letterio, J.J.; Roberts, A.B. Regulation of Immune Responses by TGF-β. Ann. Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef] [Green Version]
- Johann, K.; Kleinert, M.; Klaus, S. The Role of GDF15 as a Myomitokine. Cells 2021, 10, 2990. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic Obesity: Myokines as Potential Diagnostic Biomarkers and Therapeutic Targets? Exp. Gerontol. 2020, 139, 111022. [Google Scholar] [CrossRef]
- Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast Growth Factor 21 Controls Mitophagy and Muscle Mass. J. Cachexia Sarcopenia Muscle 2019, 10, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Eka Widiastuti, I.A.; Arsyad, A.; Idris, I.; Patellongi, I.; Kadriyan, H.; Buanayuda, G.W.; Sari, D.P.; Rosyidi, R.M. Exercise Adaptations and TGF-Β1 Levels in Recreational Cyclists. Ann. Med. Surg. 2021, 70, 102872. [Google Scholar] [CrossRef]
- Alexander, L.C.; Han, A.J.; Huebner, J.L.; Kraus, V.B. Transforming Growth Factor Beta Variation with Physical Activity in Knee Osteoarthritis. Osteoarthr. Cartil. 2020, 28, S332. [Google Scholar] [CrossRef]
- Czarkowska-Paczek, B.; Zendzian-Piotrowska, M.; Bartlomiejczyk, I.; Przybylski, J.; Gorski, J. The Influence of Physical Exercise on the Generation of TGF-Β1, PDGF-AA, and VEGF-A in Adipose Tissue. Eur. J. Appl. Physiol. 2011, 111, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campderrós, L.; Sánchez-Infantes, D.; Villarroya, J.; Nescolarde, L.; Bayès-Genis, A.; Cereijo, R.; Roca, E.; Villarroya, F. Altered GDF15 and FGF21 Levels in Response to Strenuous Exercise: A Study in Marathon Runners. Front. Physiol. 2020, 11, 550102. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Higuchi, M. Endurance Exercise Reduces Hepatic Fat Content and Serum Fibroblast Growth Factor 21 Levels in Elderly Men. J. Clin. Endocrinol. Metab. 2016, 101, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Abe, K.; Fujita, M.; Hayashi, M.; Okai, K.; Ohira, H. Simple Resistance Exercise Decreases Cytokeratin 18 and Fibroblast Growth Factor 21 Levels in Patients with Nonalcoholic Fatty Liver Disease: A Retrospective Clinical Study. Medicine 2020, 99, e20399. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.P.; Kiens, B.; Richter, E.A. Exercise Increases Circulating GDF15 in Humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Klein, A.B.; Nicolaisen, T.S.; Ørtenblad, N.; Gejl, K.D.; Jensen, R.; Fritzen, A.M.; Larsen, E.L.; Karstoft, K.; Poulsen, H.E.; Morville, T.; et al. Pharmacological but Not Physiological GDF15 Suppresses Feeding and the Motivation to Exercise. Nat. Commun. 2021, 12, 1041. [Google Scholar] [CrossRef]
- Kurowski, M.; Seys, S.; Bonini, M.; Del Giacco, S.; Delgado, L.; Diamant, Z.; Kowalski, M.L.; Moreira, A.; Rukhadze, M.; Couto, M. Physical Exercise, Immune Response, and Susceptibility to Infections—Current Knowledge and Growing Research Areas. Allergy 2022, 77, 2653–2664. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, X. Effects of Regular Exercise on Inflammasome Activation-Related Inflammatory Cytokine Levels in Older Adults: A Systematic Review and Meta-Analysis. J. Sports Sci. 2021, 39, 2338–2352. [Google Scholar] [CrossRef]
- Gokhale, R.; Chandrashekara, S.; Vasanthakumar, K.C. Cytokine Response to Strenuous Exercise in Athletes and Non-Athletes—An Adaptive Response. Cytokine 2007, 40, 123–127. [Google Scholar] [CrossRef]
- Kapilevich, L.V.; Zakharova, A.N.; Kabachkova, A.V.; Kironenko, T.A.; Orlov, S.N. Dynamic and Static Exercises Differentially Affect Plasma Cytokine Content in Elite Endurance- and Strength-Trained Athletes and Untrained Volunteers. Front. Physiol. 2017, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Henson, D.A.; Shannon, M.; Hjertman, J.M.E.; Schmitt, R.L.; Bolton, M.R.; Austin, M.D.; Schilling, B.K.; et al. Immune Function in Female Elite Rowers and Non-Athletes. Br. J. Sports Med. 2000, 34, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monastero, R.N.; Pentyala, S. Cytokines as Biomarkers and Their Respective Clinical Cutoff Levels. Int. J. Inflamm. 2017, 2017, 4309485. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.L. Cytokine hypothesis of overtraining: A physiological adaptation to excessive stress? Med. Sci. Sports Exerc. 2000, 32, 317. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J. Plasma MMP-9, TIMP-1, and TGF-Β1 Responses to Exercise-Induced Muscle Injury. Int. J. Environ. Rese. Public Health 2020, 17, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukarromah, S.B.; Ali, M.A.; Anggita, G.M.; Lesmana, R.; Rosdianto, A.M.; Komarudin; Hanief, Y.N.; Giang, N.T.; Park, S.H. The Effect of Aquarobics High Intensity Interval Training in on Interleukin-6 (IL-6) Serum Changes for over 8 Weeks. JPES 2022, 22, 3114–3121. [Google Scholar]
- Rall, L.C.; Roubenoff, R.; Cannon, J.G.; Abad, L.W.; Dinarello, C.A.; Meydani, S.N. Effects of Progressive Resistance Training on Immune Response in Aging and Chronic Inflammation. Med. Sci. Sports Exerc. 1996, 28, 1356–1365. [Google Scholar] [CrossRef]
- Ligthart, G.J.; Corberand, J.X.; Fournier, C.; Galanaud, P.; Hijmans, W.; Kennes, B.; Müller-Hermelink, H.K.; Steinmann, G.G. Admission Criteria for Immunogerontological Studies in Man: The Senieur Protocol. Mech. Ageing Dev. 1984, 28, 47–55. [Google Scholar] [CrossRef]
- Wegner, A.; Benson, S.; Rebernik, L.; Spreitzer, I.; Jäger, M.; Schedlowski, M.; Elsenbruch, S.; Engler, H. Sex Differences in the Pro-Inflammatory Cytokine Response to Endotoxin Unfold In Vivo but Not Ex Vivo in Healthy Humans. Innate Immun. 2017, 23, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef]
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Lone, K.P. Serum Profile of Cytokines and Their Genetic Variants in Metabolic Syndrome and Healthy Subjects: A Comparative Study. Biosci. Rep. 2019, 39, BSR20181202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Esposito, V.; Di Tolla, M.F.; Lecce, M.; Cavalli, F.; Libutti, M.; Misso, S.; Cabaro, S.; Ambrosio, M.R.; Parascandolo, A.; Covelli, B.; et al. Lifestyle and Dietary Habits Affect Plasma Levels of Specific Cytokines in Healthy Subjects. Front. Nutr. 2022, 9, 913176. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Brzozowski, B.; Mazur-Bialy, A.; Sliwowski, Z.; Brzozowski, T. The Role of Physical Exercise in Inflammatory Bowel Disease. Biomed. Res. Int. 2014, 2014, 429031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohail, M.U.; Al-Mansoori, L.; Al-Jaber, H.; Georgakopoulos, C.; Donati, F.; Botrè, F.; Sellami, M.; Elrayess, M.A. Assessment of Serum Cytokines and Oxidative Stress Markers in Elite Athletes Reveals Unique Profiles Associated with Different Sport Disciplines. Front. Physiol. 2020, 11, 600888. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef]
- Zhou, X.; Fragala, M.S.; McElhaney, J.E.; Kuchel, G.A. Conceptual and Methodological Issues Relevant to Cytokine and Inflammatory Marker Measurements in Clinical Research. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 541–547. [Google Scholar] [CrossRef] [Green Version]

| Cytokine | Physical Activity | Training Level of the Subjects | Response | References |
|---|---|---|---|---|
| IL-1β | Running 20 km | Trained runners | Significantly increased levels of IL-1β are found 1 h after running and persist until the next day | [26] |
| IL-1ra | Treadmill test | Trained runners | Increased level of IL-1ra by 7.8–33% after test and 1 h after the test it dropped significantly | [27] |
| Ultramarathon 160 km | Trained runners | Increased level of IL-1ra 6.1-fold after ultramarathon | [28] | |
| Cycling ergometer tests | Trained endurance athletes | Increased level of IL-1ra after 1 h of recovery, where it reaches a peak concentration | [29] | |
| IL-18 | Cycling 230 km | Trained cyclists | Significantly lower levels of IL-18 24 h after test | [30] |
| Aerobic exercise training | Untrained men and women | Decreased level of IL-18 by 43% after training | [31] | |
| Exercise training | Obese individuals | Decreased IL-18 mRNA by 20% in adipose tissue after training | [32] | |
| IL-2 | Running 5 km | Trained runners | Significantly decreased IL-2 concentrations directly after race and significantly increased after 24 h | [34] |
| Marathon 42 km | Trained runners | Decreased IL-2 concentrations by 32% or 55% directly after the race | [35,36] | |
| 6-week program of aerobic dance exercise | Untrained women | Significantly increased level of IL-2 in after training program | [37] | |
| 2 km rowing ergometer test | Trained rowers | Significant (about 4-fold) increased level of IL-2 after test and a return to normal after 24 h | [38] | |
| IL-4 | Running | Trained runners | No significant differences in IL-4 levels | [35,36,39,40,41] |
| Rowing ergometer test | Trained rowers | No significant differences in IL-4 levels | [42] | |
| Running 40 km | Trained runners | No significant differences in IL-4 levels | [43] | |
| Running 171 km | Trained runners | Significantly increased level of IL-4 after running | [43] | |
| IL-6 | Marathon 42 km | Trained runners | Increased level of IL-6 45-fold after running and 1 h afterward | [49] |
| Marathon 42 km | Trained runners | Increased level of IL-6 100-fold after running | [35] | |
| Ultramarathon 160 km | Trained runners | Increased level of IL-6 50.2-fold after running | [28] | |
| 1 h rowing ergometer test | Trained rowers | Significantly increased mean level of IL-6 after test and after a 30 min rest | [42] | |
| 90-min session on the ergometer | Trained rowers | Increased level of IL-6 7.5-fold 2 and 3 h after the test | [50] | |
| 4 months of dance training | Untrained women | Decreased resting IL-6 concentrations by 60% after training program | [51] | |
| 6-week program on a bicycle ergometer | Untrained men | No significant differences in IL-6 levels | [52] | |
| IL-10 | Ultramarathon 160 km | Trained runners | Increased level of IL-10 9.5-fold after running | [28] |
| Marathon 42 km | Trained runners | Increased level of IL-10 3.5-fold after running | [35] | |
| 1 h rowing ergometer test | Trained rowers | Non-significantly reduced level of IL-10 after test and after a 30 min rest | [42] | |
| IL-12 | Treadmill test | Trained runners | Significantly increased level of IL-12 p40 after a test | [66] |
| Cytokine | Physical Activity | Training Level of the Subjects | Response | References |
|---|---|---|---|---|
| IL-8 | Marathon 42 km | Trained runners | Increased level of IL-8 6.7-fold 30 min after running | [72] |
| Ultramarathon 160 km | Trained runners | Increased level of IL-8 2.5-fold after running | [28] | |
| 1 h cycling | Trained cyclists | Significantly increased level of IL-8 after cycling | [73] | |
| 1 h cycling | Trained cyclists | 30-fold increased IL-8 mRNA in skeletal muscles immediately after exercise | [73] | |
| MCP-1 | 1 h rowing ergometer test | Trained rowers | Significantly increased level of MCP-1 after test | [42] |
| Marathon 42 km | Trained runners | Significantly increased level of MCP-1 after running | [74] | |
| Wingate Anerobic Test | Trained soccer players | Minimal MCP-1 mRNA expression after the test and continuous increase over time with a maximum at the sixth hour after test | [75] | |
| Wingate Anerobic Test | Untrained individuals | Significantly increased MCP-1 mRNA expression immediately after the test and then decreased to a lower level | [75] | |
| Treadmill test | Trained runners | Significantly increased level of MCP-1 after running | [66] | |
| MIP-1α | Marathon 42 km | Trained runners | Increased level of MIP-1α 3.5-fold 30 min after running | [72] |
| MIP-1β | Marathon 42 km | Trained runners | Increased level of MIP-1β 4.1-fold 30 min after running | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małkowska, P.; Sawczuk, M. Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11156. https://doi.org/10.3390/ijms241311156
Małkowska P, Sawczuk M. Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(13):11156. https://doi.org/10.3390/ijms241311156
Chicago/Turabian StyleMałkowska, Paulina, and Marek Sawczuk. 2023. "Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review" International Journal of Molecular Sciences 24, no. 13: 11156. https://doi.org/10.3390/ijms241311156
APA StyleMałkowska, P., & Sawczuk, M. (2023). Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review. International Journal of Molecular Sciences, 24(13), 11156. https://doi.org/10.3390/ijms241311156






