Dissecting the Therapeutic Mechanisms of Sphingosine-1-Phosphate Receptor Agonism during Ischaemia and Reperfusion
Abstract
1. Introduction
2. Results
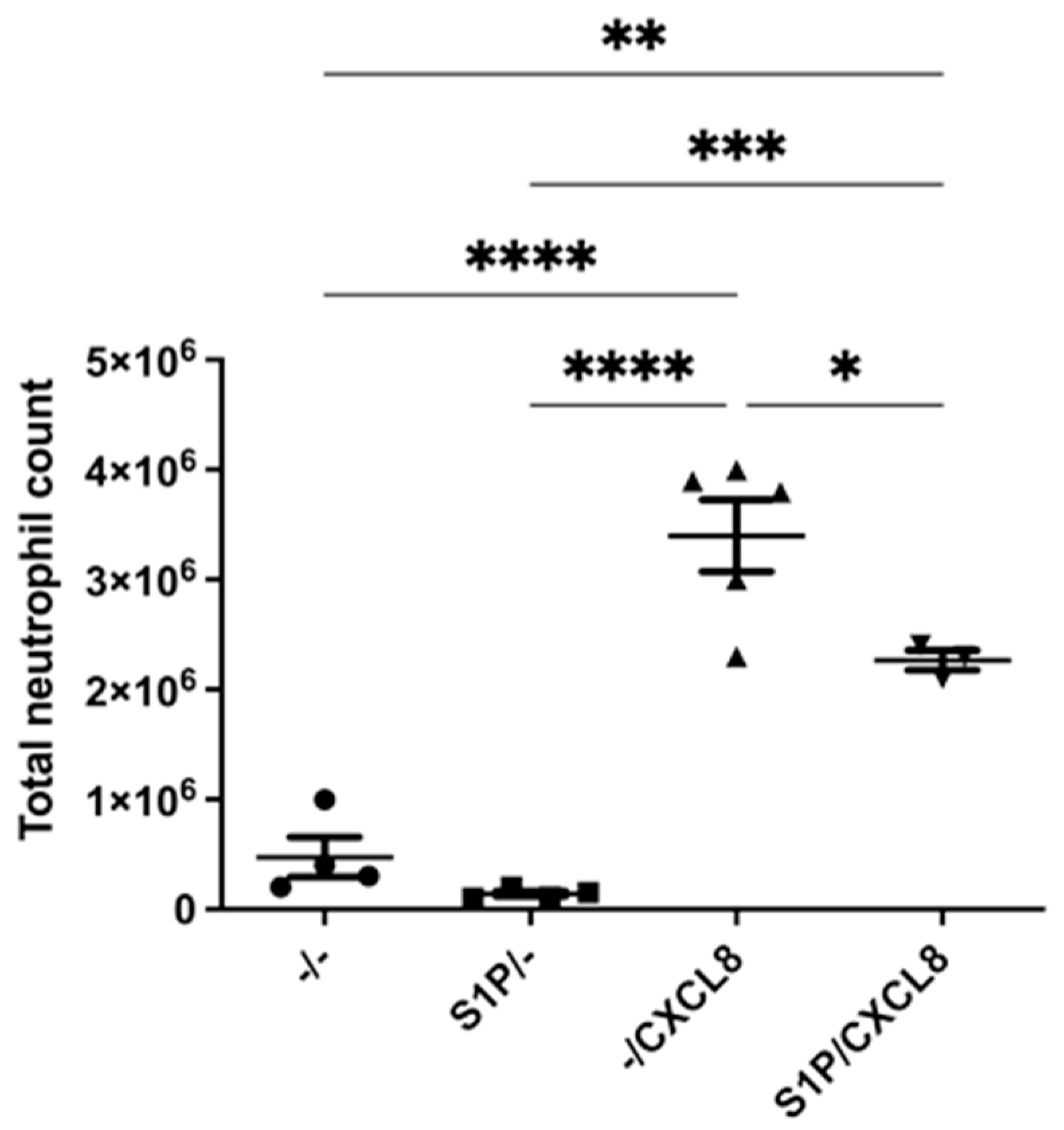
2.1. Intraperitoneal Treatment with S1P Reduces Neutrophil Recruitment In Vivo
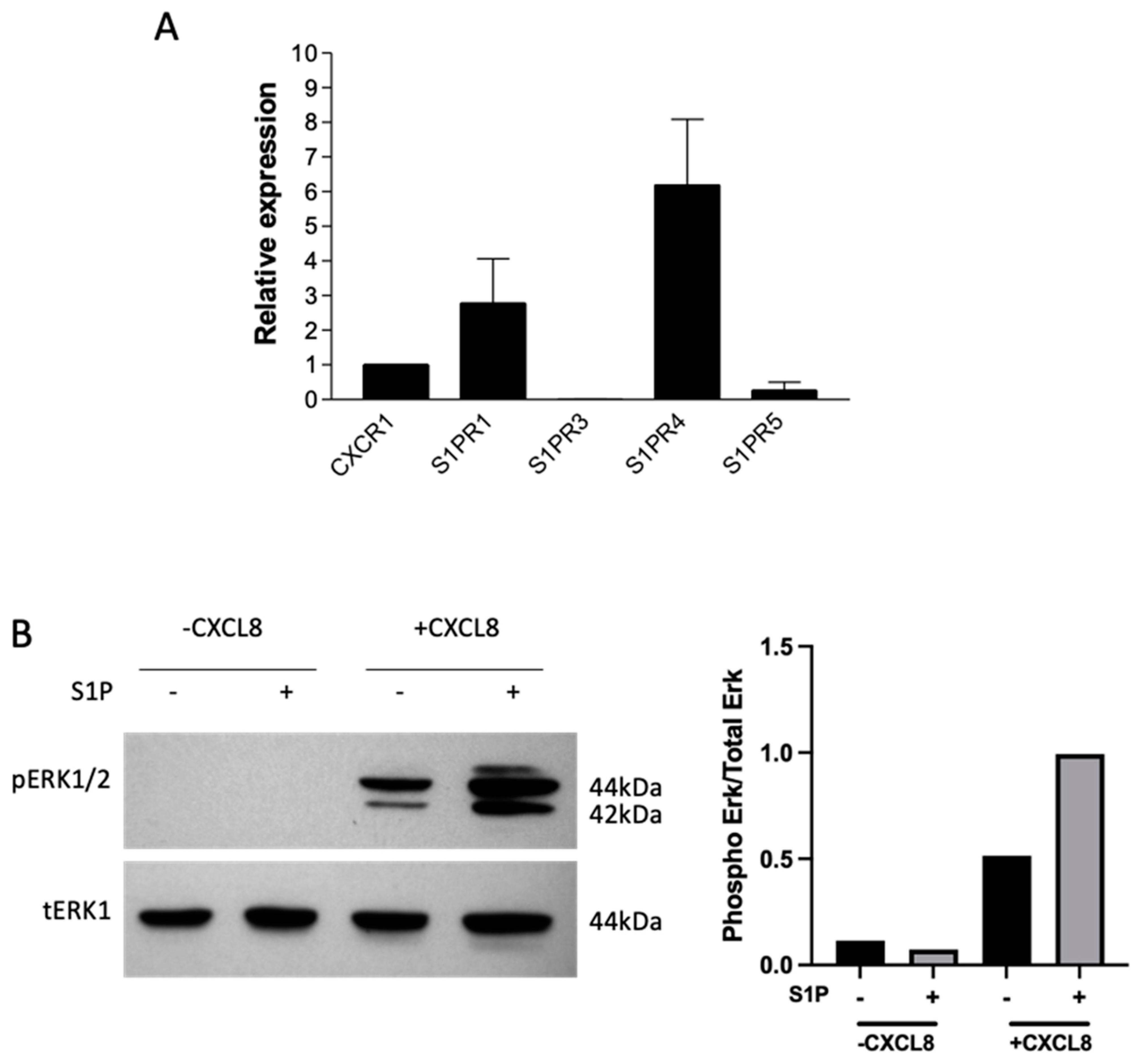
2.2. S1P Signals through S1P Receptors on Neutrophils and Primes Them for CXCL8 Signalling
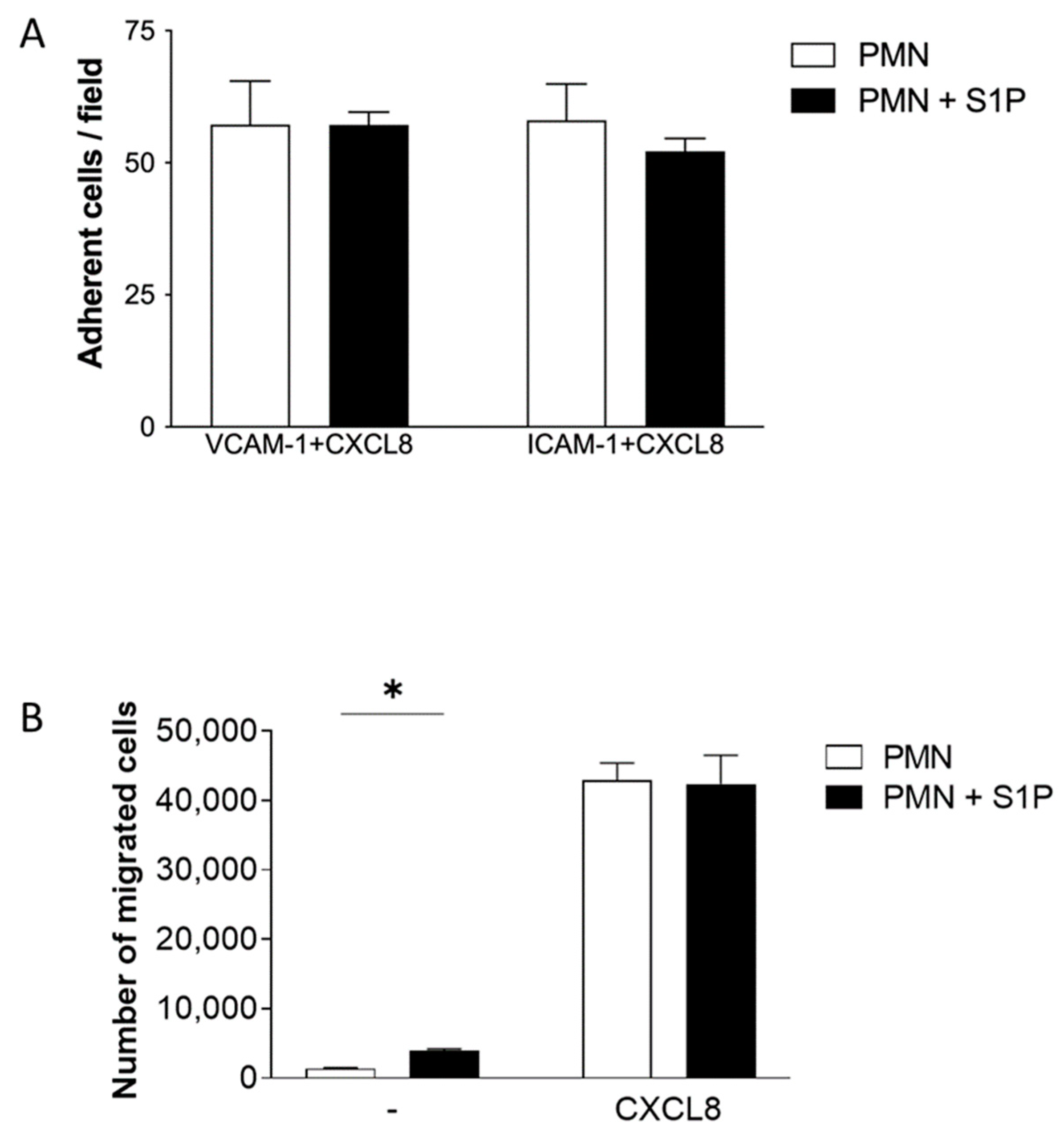
2.3. S1P Treatment of Neutrophils Does Not Significantly Alter ICAM-1 and VCAM-1 Adhesion or Transfilter Chemotaxis towards CXCL8
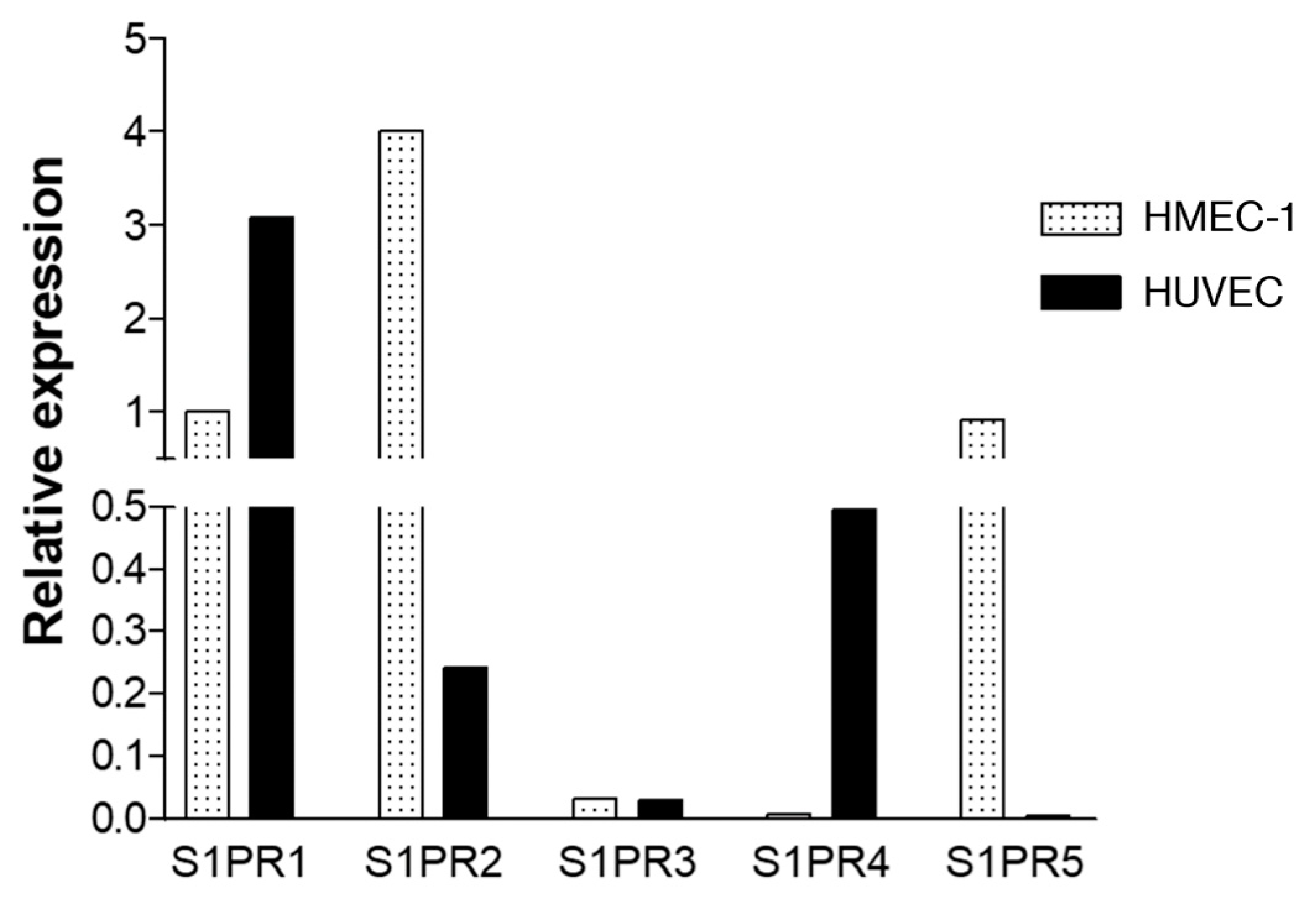
2.4. Expression of S1P Receptors on Endothelial Cells
2.5. S1P and S1PR 1 and 3 Agonists Are Associated with an Increase in Chemokine Expression in Endothelial Cells
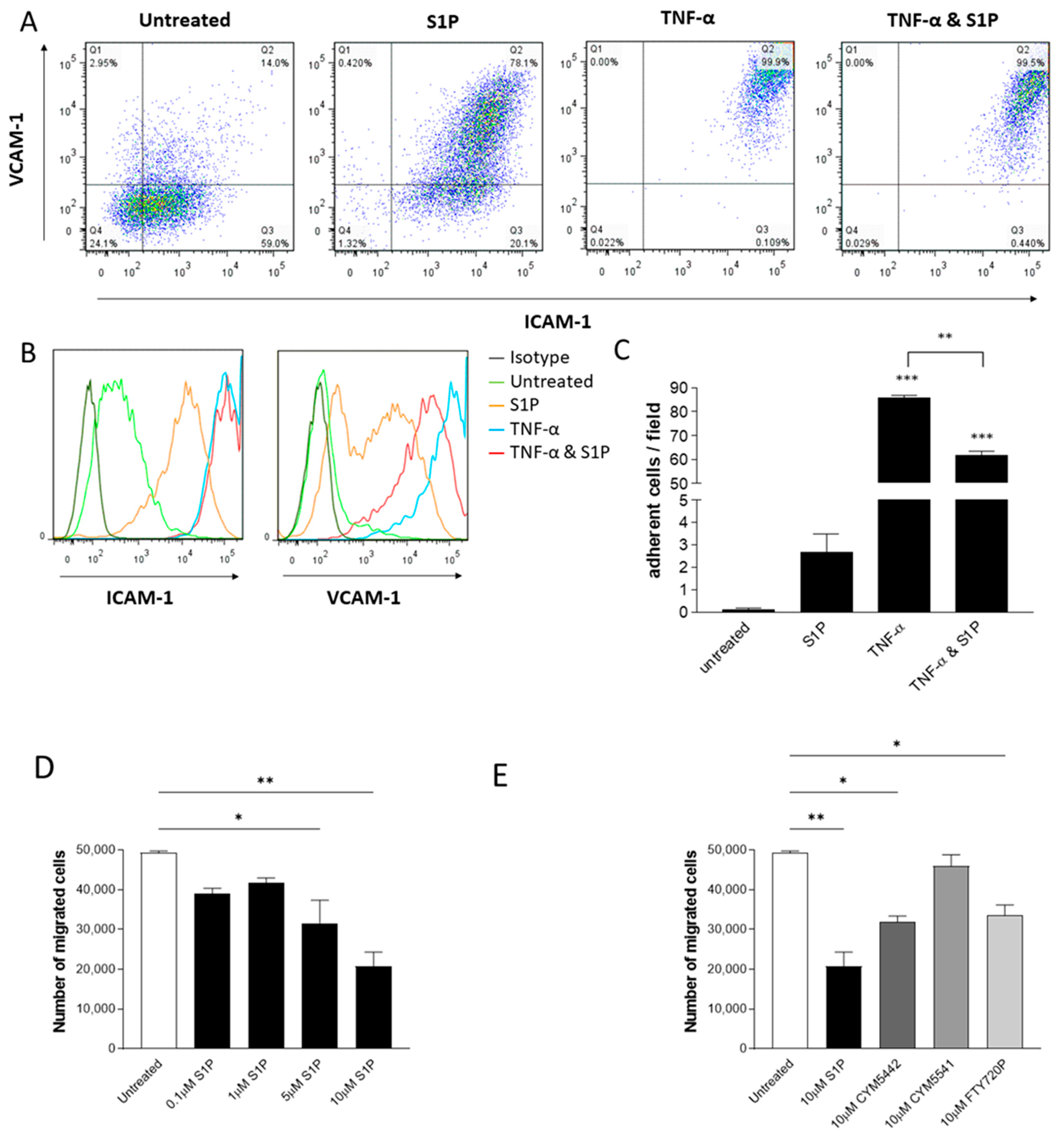
2.6. S1P Treatment of Endothelial Cells Decreases TNF-α-Induced VCAM-1 Expression, Adhesion and Neutrophil Transmigration through S1PR1 Signalling
2.7. Signalling through S1PR1 on Endothelial Cells Reduces Endothelial Barrier Permeability In Vitro and In Vivo and Reduces VE–Cadherin Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Intraperitoneal Recruitment Experiment
4.3. Ischaemia–Reperfusion Injury Model
4.4. Cell Culture
4.5. RT-qPCR
4.6. Immunoprecipitation
4.7. Western Blotting
4.8. Chemotaxis
4.9. Flow-Based Adhesion Assay
4.10. Measurement of Chemokine Concentration
4.11. Flow Cytometry
4.12. Permeability Assays
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swan, D.J.; Kirby, J.A.; Ali, S. Vascular biology: The role of sphingosine 1-phosphate in both the resting state and inflammation. J. Cell. Mol. Med. 2010, 14, 2211–2222. [Google Scholar] [CrossRef]
- Reinhard, N.R.; Mastop, M.; Yin, T.; Wu, Y.; Bosma, E.K.; Gadella, T.W.J., Jr.; Goedhart, J.; Hordijk, P.L. The balance between Galpha(i)-Cdc42/Rac and Galpha(1)(2)/(1)(3)-RhoA pathways determines endothelial barrier regulation by sphingosine-1-phosphate. Mol. Biol. Cell 2017, 28, 3371–3382. [Google Scholar] [CrossRef]
- Li, Q.; Chen, B.; Zeng, C.; Fan, A.; Yuan, Y.; Guo, X.; Huang, X.; Huang, Q. Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Exp. Physiol. 2015, 100, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Galvani, S.; Sanson, M.; Blaho, V.A.; Swendeman, S.L.; Obinata, H.; Conger, H.; Dahlback, B.; Kono, M.; Proia, R.L.; Smith, J.D.; et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 2015, 8, ra79. [Google Scholar] [CrossRef]
- Qiang, G.H.; Wang, Z.X.; Ji, A.L.; Wu, J.Y.; Cao, Y.; Zhang, G.; Zhang, Y.Y.; Jiang, C.P. Sphingosine kinase 1 knockout alleviates hepatic ischemia/reperfusion injury by attenuating inflammation and oxidative stress in mice. Hepatobiliary Pancreat Dis. Int. 2019, 18, 255–265. [Google Scholar] [CrossRef]
- Nitzsche, A.; Poittevin, M.; Benarab, A.; Bonnin, P.; Faraco, G.; Uchida, H.; Favre, J.; Garcia-Bonilla, L.; Garcia, M.C.L.; Leger, P.L.; et al. Endothelial S1P1 Signalling Counteracts Infarct Expansion in Ischemic Stroke. Circ. Res. 2021, 128, 363–382. [Google Scholar] [CrossRef]
- Morel, S.; Christoffersen, C.; Axelsen, L.N.; Montecucco, F.; Rochemont, V.; Frias, M.A.; Mach, F.; James, R.W.; Naus, C.C.; Chanson, M.; et al. Sphingosine-1-phosphate reduces ischaemia-reperfusion injury by phosphorylating the gap junction protein Connexin43. Cardiovasc. Res. 2016, 109, 385–396. [Google Scholar] [CrossRef]
- Hofmann, U.; Burkard, N.; Vogt, C.; Thoma, A.; Frantz, S.; Ertl, G.; Ritter, O.; Bonz, A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc. Res. 2009, 83, 285–293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ichijo, M.; Ishibashi, S.; Li, F.; Yui, D.; Miki, K.; Mizusawa, H.; Yokota, T. Sphingosine-1-Phosphate Receptor-1 Selective Agonist Enhances Collateral Growth and Protects against Subsequent Stroke. PLoS ONE 2015, 10, e0138029. [Google Scholar] [CrossRef]
- Stone, M.L.; Sharma, A.K.; Zhao, Y.; Charles, E.J.; Huerter, M.E.; Johnston, W.F.; Kron, I.L.; Lynch, K.R.; Laubach, V.E. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L1245–L1252. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kuriyama, N.; Kato, H.; Matsuda, A.; Mizuno, S.; Usui, M.; Sakurai, H.; Isaji, S. Sinusoidal protection by sphingosine-1-phosphate receptor 1 agonist in liver ischemia-reperfusion injury. J. Surg. Res. 2018, 222, 139–152. [Google Scholar] [CrossRef]
- Bajwa, A.; Jo, S.K.; Ye, H.; Huang, L.; Dondeti, K.R.; Rosin, D.L.; Haase, V.H.; Macdonald, T.L.; Lynch, K.R.; Okusa, M.D. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2010, 21, 955–965. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, C.; Shen, G.; Yang, S.; Cheng, X.; Cheng, F.; Rao, J.; Wang, X. Hyperglycemia-Triggered Sphingosine-1-Phosphate and Sphingosine-1-Phosphate Receptor 3 Signaling Worsens Liver Ischemia/Reperfusion Injury by Regulating M1/M2 Polarization. Liver Transpl. 2019, 25, 1074–1090. [Google Scholar] [CrossRef]
- Jo, S.K.; Bajwa, A.; Ye, H.; Vergis, A.L.; Awad, A.S.; Kharel, Y.; Lynch, K.R.; Okusa, M.D. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int. 2009, 75, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.; Costello, R.W.; Belmonte, K.E.; Gendy, S.S.; Walsh, M.T. Neutrophil sphingosine 1-phosphate and lysophosphatidic acid receptors in pneumonia. Am. J. Respir. Cell Mol. Biol. 2006, 34, 233–241. [Google Scholar] [CrossRef]
- Hao, J.; Huang, Y.M.; Zhao, M.H.; Chen, M. The interaction between C5a and sphingosine-1-phosphate in neutrophils for antineutrophil cytoplasmic antibody mediated activation. Arthritis Res. 2014, 16, R142. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Singleton, P.A.; Dudek, S.M.; Ma, S.F.; Garcia, J.G. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J. Biol. Chem. 2006, 281, 34381–34393. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.S.; Ye, H.; Huang, L.; Li, L.; Foss, F.W., Jr.; Macdonald, T.L.; Lynch, K.R.; Okusa, M.D. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am. J. Physiol. Ren. Physiol. 2006, 290, F1516–F1524. [Google Scholar] [CrossRef]
- Thompson, S.; Martínez-Burgo, B.; Sepuru, K.M.; Rajarathnam, K.; Kirby, J.A.; Sheerin, N.S.; Ali, S. Regulation of chemokine function: The roles of GAG-binding and post-translational nitration. Int. J. Mol. Sci. 2017, 18, 1692. [Google Scholar] [CrossRef]
- Thompson, S.; Pang, C.Y.; Sepuru, K.M.; Cambier, S.; Hellyer, T.P.; Scott, J.; Simpson, A.J.; Proost, P.; Kirby, J.A.; Rajarathnam, K.; et al. Nitration of chemokine CXCL8 acts as a natural mechanism to limit acute inflammation. Cell. Mol. Life Sci. 2023, 80, 35. [Google Scholar] [CrossRef]
- Okazaki, M.; Kreisel, F.; Richardson, S.B.; Kreisel, D.; Krupnick, A.S.; Patterson, G.A.; Gelman, A.E. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am. J. Transpl. 2007, 7, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Theilmeier, G.; Schmidt, C.; Herrmann, J.; Keul, P.; Schafers, M.; Herrgott, I.; Mersmann, J.; Larmann, J.; Hermann, S.; Stypmann, J.; et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006, 114, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Fuxman Bass, J.I.; Geffner, J.R.; Fernandez Calotti, P.X.; Costas, M.; Coso, O.A.; Gamberale, R.; Vermeulen, M.E.; Salamone, G.; Martinez, D.; et al. Neutrophil signaling pathways activated by bacterial DNA stimulation. J. Immunol. 2006, 177, 4037–4046. [Google Scholar] [CrossRef]
- Kawa, S.; Kimura, S.; Hakomori, S.; Igarashi, Y. Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1-phosphate. FEBS Lett. 1997, 420, 196–200. [Google Scholar] [CrossRef]
- Florey, O.; Haskard, D.O. Sphingosine 1-phosphate enhances Fc gamma receptor-mediated neutrophil activation and recruitment under flow conditions. J. Immunol. 2009, 183, 2330–2336. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, L.; Chang, N.; Hou, L.; Zhou, X.; Dong, C.; Liu, F.; Yang, L.; Li, L. Neutrophil recruitment mediated by sphingosine 1-phosphate (S1P)/S1P receptors during chronic liver injury. Cell. Immunol. 2021, 359, 104243. [Google Scholar] [CrossRef]
- Fettel, J.; Kuhn, B.; Guillen, N.A.; Surun, D.; Peters, M.; Bauer, R.; Angioni, C.; Geisslinger, G.; Schnutgen, F.; Meyer Zu Heringdorf, D.; et al. Sphingosine-1-phosphate (S1P) induces potent anti-inflammatory effects in vitro and in vivo by S1P receptor 4-mediated suppression of 5-lipoxygenase activity. FASEB J. 2019, 33, 1711–1726. [Google Scholar] [CrossRef] [PubMed]
- Feuerborn, R.; Besser, M.; Poti, F.; Burkhardt, R.; Weissen-Plenz, G.; Ceglarek, U.; Simoni, M.; Proia, R.L.; Freise, H.; Nofer, J.R. Elevating Endogenous Sphingosine-1-Phosphate (S1P) Levels Improves Endothelial Function and Ameliorates Atherosclerosis in Low Density Lipoprotein Receptor-Deficient (LDL-R−/−) Mice. Thromb. Haemost. 2018, 118, 1470–1480. [Google Scholar] [CrossRef]
- Doggett, T.M.; Alves, N.G.; Yuan, S.Y.; Breslin, J.W. Sphingosine-1-Phosphate Treatment Can Ameliorate Microvascular Leakage Caused by Combined Alcohol Intoxication and Hemorrhagic Shock. Sci. Rep. 2017, 7, 4078. [Google Scholar] [CrossRef]
- Schaphorst, K.L.; Chiang, E.; Jacobs, K.N.; Zaiman, A.; Natarajan, V.; Wigley, F.; Garcia, J.G. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 285, L258–L267. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, D.H.; Sung, S.A.; Kim, M.G.; Cho, W.Y.; Kim, H.K.; Jo, S.K. Sphingosine-1-phosphate reduces hepatic ischaemia/reperfusion-induced acute kidney injury through attenuation of endothelial injury in mice. Nephrology 2011, 16, 163–173. [Google Scholar] [CrossRef]
- Miyabe, C.; Miyabe, Y.; Komiya, T.; Shioya, H.; Miura, N.N.; Takahashi, K.; Ohno, N.; Tsuboi, R.; Luster, A.D.; Kawai, S.; et al. A sphingosine 1-phosphate receptor agonist ameliorates animal model of vasculitis. Inflamm. Res. 2017, 66, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Alkhouri, H.; Tang, F.; Che, W.; Ge, Q.; Ammit, A.J. Sphingosine 1-phosphate induces neutrophil chemoattractant IL-8: Repression by steroids. PLoS ONE 2014, 9, e92466. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.I.; Chen, C.N.; Lin, P.W.; Lee, H. Sphingosine 1-phosphate regulates inflammation-related genes in human endothelial cells through S1P1 and S1P3. Biochem. Biophys. Res. Commun. 2007, 355, 895–901. [Google Scholar] [CrossRef]
- Shimamura, K.; Takashiro, Y.; Akiyama, N.; Hirabayashi, T.; Murayama, T. Expression of adhesion molecules by sphingosine 1-phosphate and histamine in endothelial cells. Eur. J. Pharm. 2004, 486, 141–150. [Google Scholar] [CrossRef]
- Kimura, T.; Tomura, H.; Mogi, C.; Kuwabara, A.; Ishiwara, M.; Shibasawa, K.; Sato, K.; Ohwada, S.; Im, D.S.; Kurose, H.; et al. Sphingosine 1-phosphate receptors mediate stimulatory and inhibitory signalings for expression of adhesion molecules in endothelial cells. Cell. Signal. 2006, 18, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Burg, N.; Swendeman, S.; Worgall, S.; Hla, T.; Salmon, J.E. Sphingosine 1-Phosphate Receptor 1 Signaling Maintains Endothelial Cell Barrier Function and Protects Against Immune Complex-Induced Vascular Injury. Arthritis Rheumatol. 2018, 70, 1879–1889. [Google Scholar] [CrossRef]
- Tauseef, M.; Kini, V.; Knezevic, N.; Brannan, M.; Ramchandaran, R.; Fyrst, H.; Saba, J.; Vogel, S.M.; Malik, A.B.; Mehta, D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 2008, 103, 1164–1172. [Google Scholar] [CrossRef]
- Sanchez, T.; Skoura, A.; Wu, M.T.; Casserly, B.; Harrington, E.O.; Hla, T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arter. Thromb. Vasc. Biol. 2007, 27, 1312–1318. [Google Scholar] [CrossRef]
- Perry, H.M.; Huang, L.; Ye, H.; Liu, C.; Sung, S.J.; Lynch, K.R.; Rosin, D.L.; Bajwa, A.; Okusa, M.D. Endothelial Sphingosine 1-Phosphate Receptor-1 Mediates Protection and Recovery from Acute Kidney Injury. J. Am. Soc. Nephrol. 2016, 27, 3383–3393. [Google Scholar] [CrossRef]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef]
- Mehta, D.; Konstantoulaki, M.; Ahmmed, G.U.; Malik, A.B. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J. Biol. Chem. 2005, 280, 17320–17328. [Google Scholar] [CrossRef]
- Wessel, F.; Winderlich, M.; Holm, M.; Frye, M.; Rivera-Galdos, R.; Vockel, M.; Linnepe, R.; Ipe, U.; Stadtmann, A.; Zarbock, A.; et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat. Immunol. 2014, 15, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Monaghan-Benson, E.; Burridge, K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J. Biol. Chem. 2009, 284, 25602–25611. [Google Scholar] [CrossRef]
- Orsenigo, F.; Giampietro, C.; Ferrari, A.; Corada, M.; Galaup, A.; Sigismund, S.; Ristagno, G.; Maddaluno, L.; Koh, G.Y.; Franco, D.; et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun. 2012, 3, 1208. [Google Scholar] [CrossRef]
- Scotti, L.; Di Pietro, M.; Pascuali, N.; Irusta, G.; Zuniga, I.d.; Gomez Pena, M.; Pomilio, C.; Saravia, F.; Tesone, M.; Abramovich, D.; et al. Sphingosine-1-phosphate restores endothelial barrier integrity in ovarian hyperstimulation syndrome. Mol. Hum. Reprod. 2016, 22, 852–866. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.P.; Lee, J.E.; Lee, A.S.; Jung, Y.J.; Kim, D.; Lee, S.; Hwang, H.P.; Kim, W.; Park, S.K. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol. Med. Rep. 2014, 9, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.J.; Kirby, J.A.; Ali, S. Post-transplant immunosuppression: Regulation of the efflux of allospecific effector T cells from lymphoid tissues. PLoS ONE 2012, 7, e45548. [Google Scholar] [CrossRef] [PubMed]
- Cocchiaro, P.; Fox, C.; Tregidgo, N.W.; Howarth, R.; Wood, K.M.; Situmorang, G.R.; Pavone, L.M.; Sheerin, N.S.; Moles, A. Lysosomal protease cathepsin D; a new driver of apoptosis during acute kidney injury. Sci. Rep. 2016, 6, 27112. [Google Scholar] [CrossRef]
- Martinez-Burgo, B.; Cobb, S.L.; Pohl, E.; Kashanin, D.; Paul, T.; Kirby, J.A.; Sheerin, N.S.; Ali, S. A C-terminal CXCL8 peptide based on chemokine-glycosaminoglycan interactions reduces neutrophil adhesion and migration during inflammation. Immunology 2019, 157, 173–184. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkins, G.C.; Gilmour, J.; Giannoudaki, E.; Kirby, J.A.; Sheerin, N.S.; Ali, S. Dissecting the Therapeutic Mechanisms of Sphingosine-1-Phosphate Receptor Agonism during Ischaemia and Reperfusion. Int. J. Mol. Sci. 2023, 24, 11192. https://doi.org/10.3390/ijms241311192
Wilkins GC, Gilmour J, Giannoudaki E, Kirby JA, Sheerin NS, Ali S. Dissecting the Therapeutic Mechanisms of Sphingosine-1-Phosphate Receptor Agonism during Ischaemia and Reperfusion. International Journal of Molecular Sciences. 2023; 24(13):11192. https://doi.org/10.3390/ijms241311192
Chicago/Turabian StyleWilkins, Georgina C., Jenny Gilmour, Eirini Giannoudaki, John A. Kirby, Neil S. Sheerin, and Simi Ali. 2023. "Dissecting the Therapeutic Mechanisms of Sphingosine-1-Phosphate Receptor Agonism during Ischaemia and Reperfusion" International Journal of Molecular Sciences 24, no. 13: 11192. https://doi.org/10.3390/ijms241311192
APA StyleWilkins, G. C., Gilmour, J., Giannoudaki, E., Kirby, J. A., Sheerin, N. S., & Ali, S. (2023). Dissecting the Therapeutic Mechanisms of Sphingosine-1-Phosphate Receptor Agonism during Ischaemia and Reperfusion. International Journal of Molecular Sciences, 24(13), 11192. https://doi.org/10.3390/ijms241311192





