Formation of Transient Protein Aggregate-like Centers Is a General Strategy Postponing Degradation of Misfolded Intermediates
Abstract
:1. Introduction
2. Results
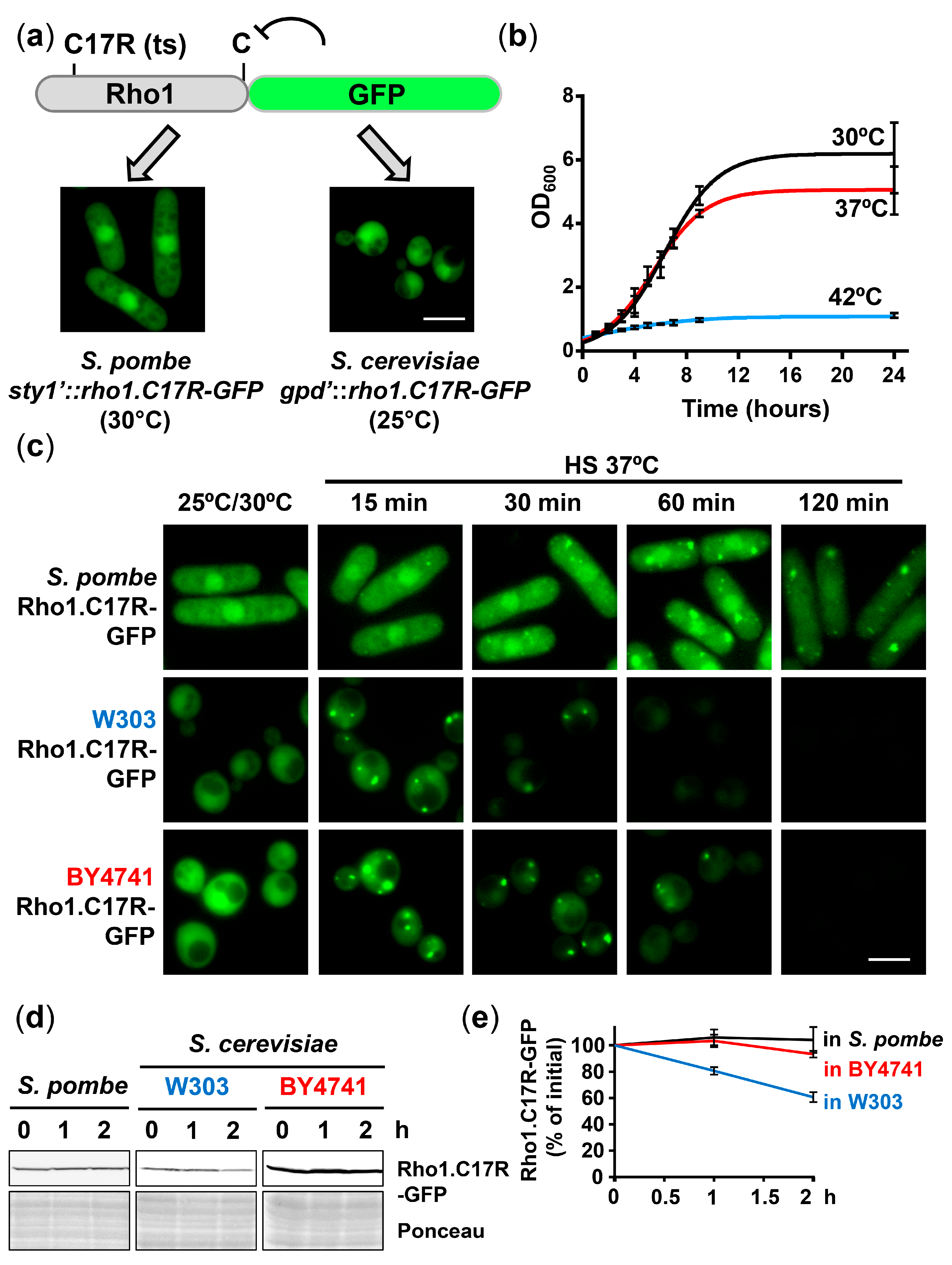
2.1. Rho1.C17R-GFP Collapses into Protein Aggregate-like Centers (PACs) upon Mild Heat Stress in Budding Yeast
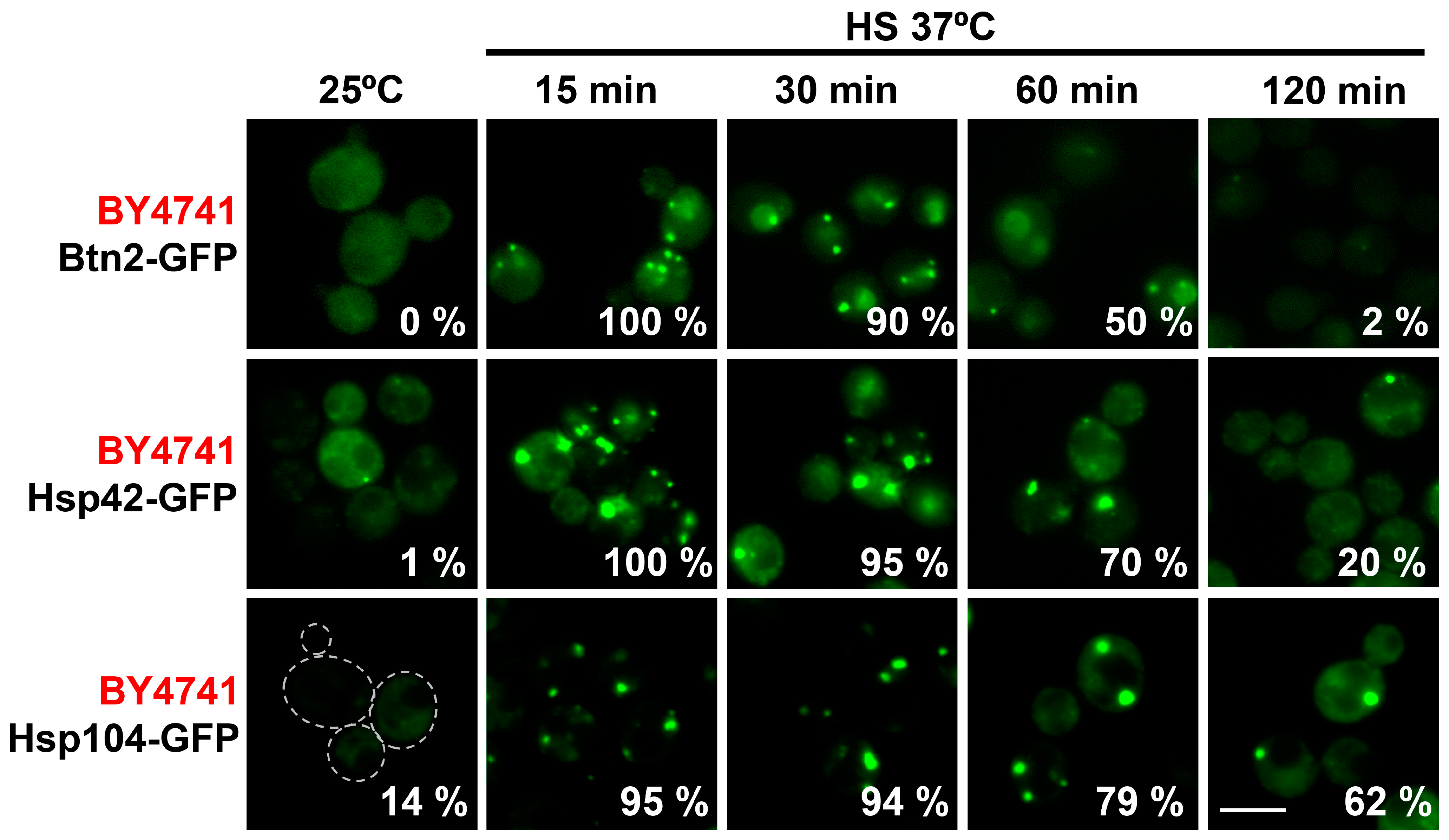
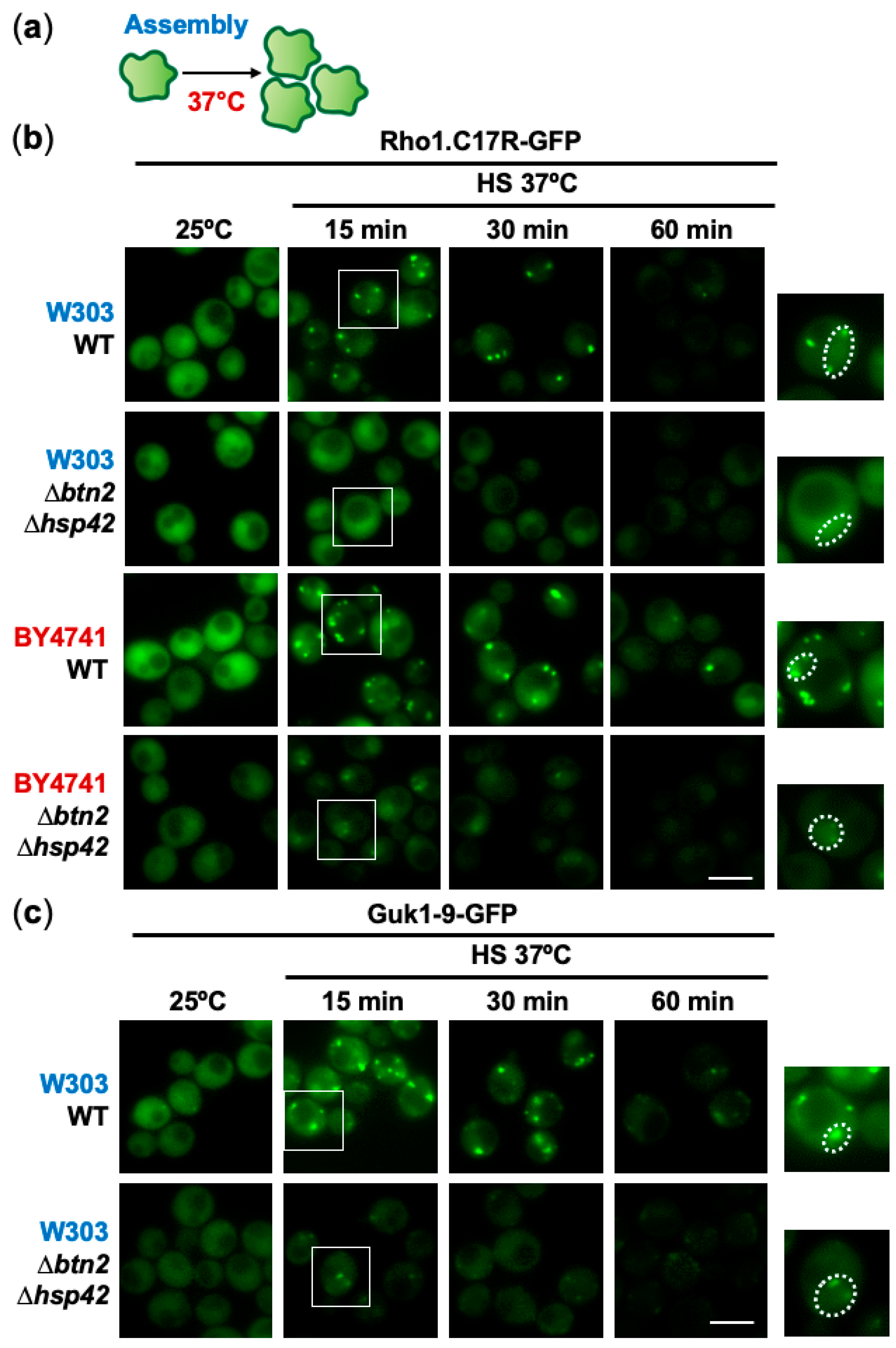
2.2. Role of the Chaperones Hsp42 and Btn2 in Sorting Misfolded Intermediates to PACs in Different Budding Yeast Backgrounds
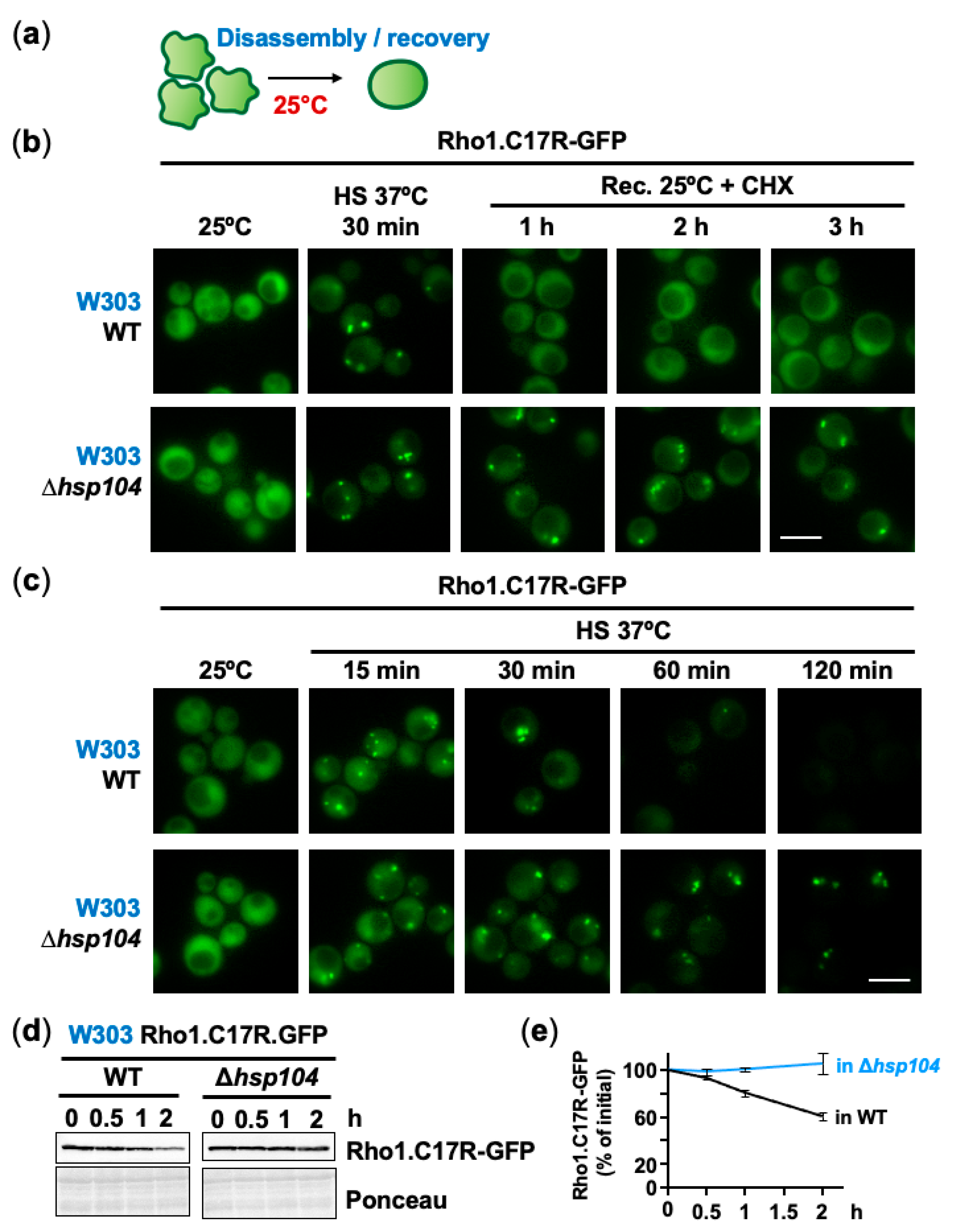
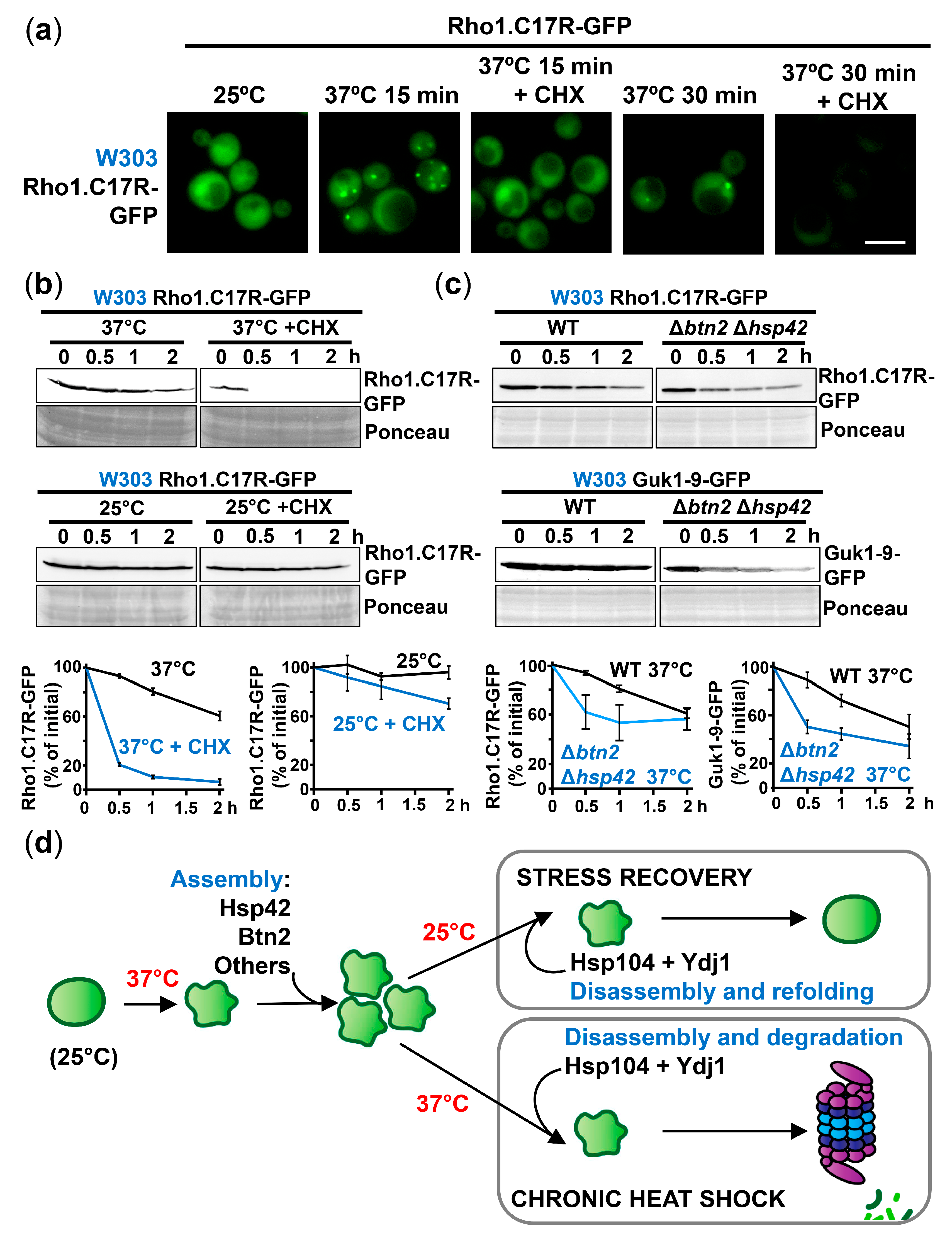
2.3. The Disaggregase Hsp104 Participates in PAC Clearance during Heat Stress and Recovery
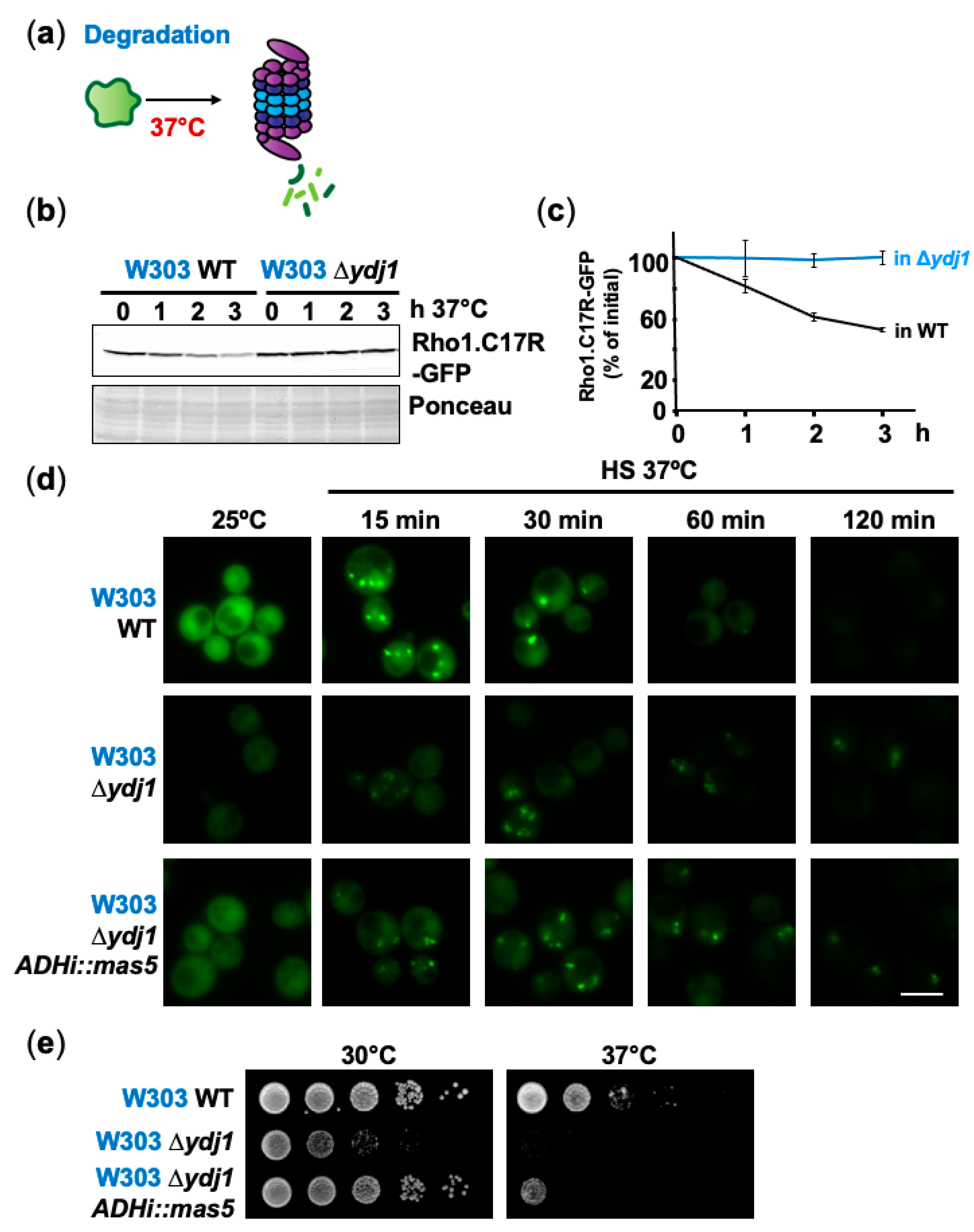
2.4. Degradation Mediated by the Hsp40 Ydj1 Can Be Prevented by Formation of PACs
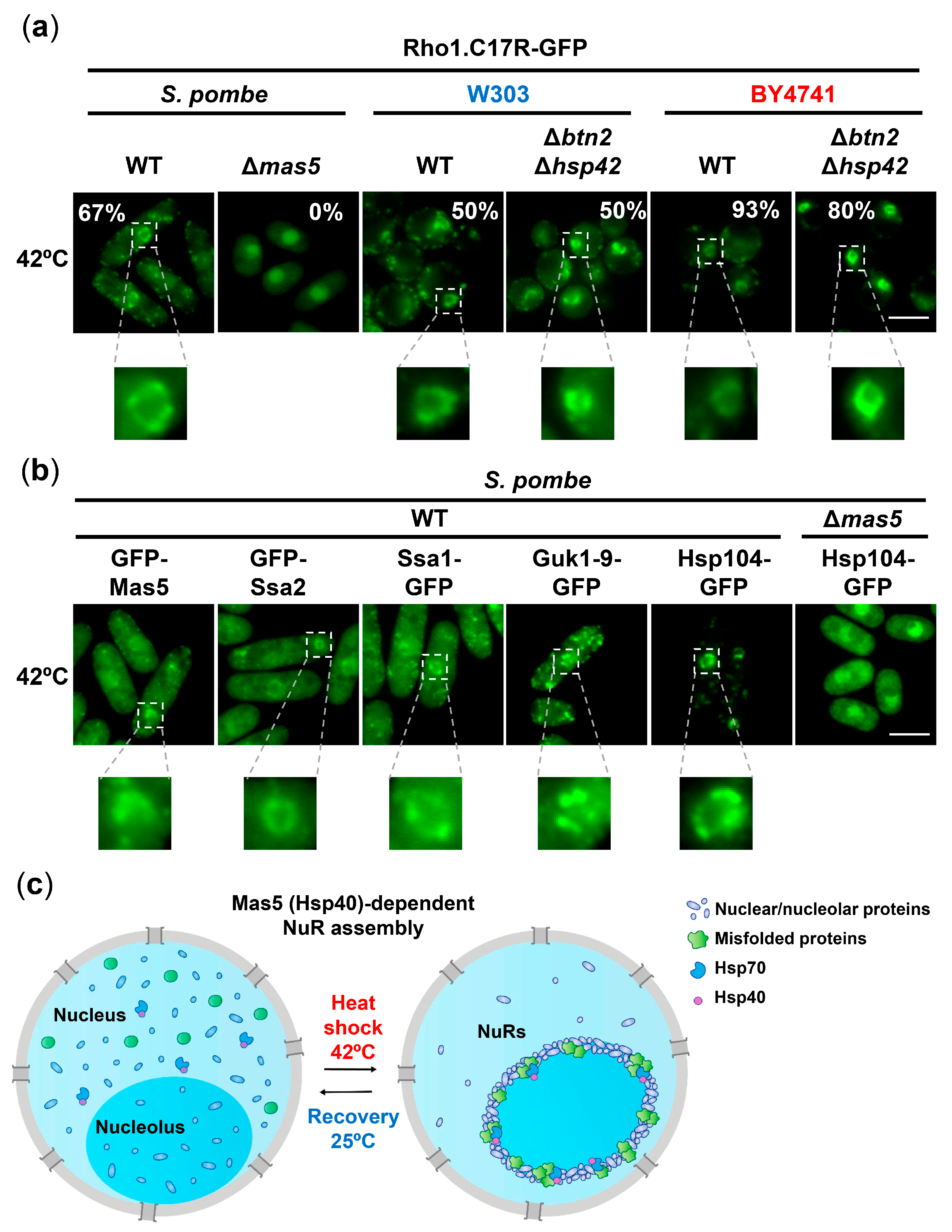
2.5. Formation of Nucleolar Rings, NuRs, upon Severe Heat Shock Occurs in Both Yeasts and Is Chaperone-Mediated
3. Discussion
4. Materials and Methods
4.1. Growth Conditions, Yeast Strains and Plasmids
4.2. Native and TCA Extracts and Western Blot
4.3. Fluorescence Microscopy
4.4. Sensitivity and Survival Assays on Plates
4.5. Growth Curves
4.6. Immunofluorescence Microscopy
4.7. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In vivo aspects of protein folding and quality control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Pilla, E.; Schneider, K.; Bertolotti, A. Coping with Protein Quality Control Failure. Annu. Rev. Cell Dev. Biol. 2017, 33, 439–465. [Google Scholar] [CrossRef]
- Kleiger, G.; Mayor, T. Perilous journey: A tour of the ubiquitin-proteasome system. Trends Cell Biol. 2014, 24, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Dikic, I. Proteasomal and Autophagic Degradation Systems. Annu. Rev. Biochem. 2017, 86, 193–224. [Google Scholar] [CrossRef]
- Kainth, A.S.; Chowdhary, S.; Pincus, D.; Gross, D.S. Primordial super-enhancers: Heat shock-induced chromatin organization in yeast. Trends Cell Biol. 2021, 31, 801–813. [Google Scholar] [CrossRef]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Vihervaara, A.; Duarte, F.M.; Lis, J.T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet. 2018, 19, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Veri, A.O.; Robbins, N.; Cowen, L.E. Regulation of the heat shock transcription factor Hsf1 in fungi: Implications for temperature-dependent virulence traits. FEMS Yeast Res. 2018, 18, foy041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Toone, W.M.; Mata, J.; Lyne, R.; Burns, G.; Kivinen, K.; Brazma, A.; Jones, N.; Bahler, J. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 2003, 14, 214–229. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Labbadia, J.; Morimoto, R.I. Rethinking HSF1 in Stress, Development, and Organismal Health. Trends Cell Biol. 2017, 27, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.B. Stress-activated MAP kinase (mitogen-activated protein kinase) pathways of budding and fission yeasts. Biochem. Soc. Symp. 1999, 64, 49–62. [Google Scholar] [PubMed]
- Sontag, E.M.; Samant, R.S.; Frydman, J. Mechanisms and Functions of Spatial Protein Quality Control. Annu. Rev. Biochem. 2017, 86, 97–122. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.B.; Mogk, A.; Bukau, B. Spatially Organized Aggregation of Misfolded Proteins as Cellular Stress Defense Strategy. J. Mol. Biol. 2015, 427, 1564–1574. [Google Scholar] [CrossRef]
- Reinle, K.; Mogk, A.; Bukau, B. The Diverse Functions of Small Heat Shock Proteins in the Proteostasis Network. J. Mol. Biol 2022, 434, 167157. [Google Scholar] [CrossRef]
- Mogk, A.; Ruger-Herreros, C.; Bukau, B. Cellular Functions and Mechanisms of Action of Small Heat Shock Proteins. Annu. Rev. Microbiol. 2019, 73, 89–110. [Google Scholar] [CrossRef]
- Escusa-Toret, S.; Vonk, W.I.M.; Frydman, J. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat. Cell Biol. 2013, 15, 1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, S.B.; Ho, C.T.; Winkler, J.; Khokhrina, M.; Neuner, A.; Mohamed, M.Y.; Guilbride, D.L.; Richter, K.; Lisby, M.; Schiebel, E.; et al. Compartment-specific aggregases direct distinct nuclear and cytoplasmic aggregate deposition. EMBO J. 2015, 34, 778–797. [Google Scholar] [CrossRef] [Green Version]
- Gallina, I.; Colding, C.; Henriksen, P.; Beli, P.; Nakamura, K.; Offman, J.; Mathiasen, D.P.; Silva, S.; Hoffmann, E.; Groth, A.; et al. Cmr1/WDR76 defines a nuclear genotoxic stress body linking genome integrity and protein quality control. Nat. Commun. 2015, 6, 6533. [Google Scholar] [CrossRef] [Green Version]
- Kaganovich, D.; Kopito, R.; Frydman, J. Misfolded proteins partition between two distinct quality control compartments. Nature 2008, 454, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Saugar, I.; Jimenez-Martin, A.; Tercero, J.A. Subnuclear Relocalization of Structure-Specific Endonucleases in Response to DNA Damage. Cell Rep. 2017, 20, 1553–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, M.; Boronat, S.; Marte, L.; Vega, M.; Perez, P.; Ayte, J.; Hidalgo, E. Chaperone-Facilitated Aggregation of Thermo-Sensitive Proteins Shields Them from Degradation during Heat Stress. Cell Rep. 2020, 30, 2430–2443.e4. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.T.; Grousl, T.; Shatz, O.; Jawed, A.; Ruger-Herreros, C.; Semmelink, M.; Zahn, R.; Richter, K.; Bukau, B.; Mogk, A. Cellular sequestrases maintain basal Hsp70 capacity ensuring balanced proteostasis. Nat. Commun. 2019, 10, 4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specht, S.; Miller, S.B.; Mogk, A.; Bukau, B. Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J. Cell Biol. 2011, 195, 617–629. [Google Scholar] [CrossRef]
- Wallace, E.W.; Kear-Scott, J.L.; Pilipenko, E.V.; Schwartz, M.H.; Laskowski, P.R.; Rojek, A.E.; Katanski, C.D.; Riback, J.A.; Dion, M.F.; Franks, A.M.; et al. Reversible, Specific, Active Aggregates of Endogenous Proteins Assemble upon Heat Stress. Cell 2015, 162, 1286–1298. [Google Scholar] [CrossRef] [Green Version]
- den Brave, F.; Cairo, L.V.; Jagadeesan, C.; Ruger-Herreros, C.; Mogk, A.; Bukau, B.; Jentsch, S. Chaperone-Mediated Protein Disaggregation Triggers Proteolytic Clearance of Intra-nuclear Protein Inclusions. Cell Rep. 2020, 31, 107680. [Google Scholar] [CrossRef] [PubMed]
- Kaimal, J.M.; Kandasamy, G.; Gasser, F.; Andreasson, C. Coordinated Hsp110 and Hsp104 Activities Power Protein Disaggregation in Saccharomyces cerevisiae. Mol. Cell Biol. 2017, 37, e00027-17. [Google Scholar] [CrossRef] [Green Version]
- Saad, S.; Cereghetti, G.; Feng, Y.; Picotti, P.; Peter, M.; Dechant, R. Reversible protein aggregation is a protective mechanism to ensure cell cycle restart after stress. Nat. Cell Biol. 2017, 19, 1202–1213. [Google Scholar] [CrossRef]
- Zhou, C.; Slaughter, B.D.; Unruh, J.R.; Guo, F.; Yu, Z.; Mickey, K.; Narkar, A.; Ross, R.T.; McClain, M.; Li, R. Organelle-based aggregation and retention of damaged proteins in asymmetrically dividing cells. Cell 2014, 159, 530–542. [Google Scholar] [CrossRef] [Green Version]
- Weids, A.J.; Grant, C.M. The yeast peroxiredoxin Tsa1 protects against protein-aggregate-induced oxidative stress. J. Cell Sci. 2014, 127, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Ralser, M.; Kuhl, H.; Ralser, M.; Werber, M.; Lehrach, H.; Breitenbach, M.; Timmermann, B. The Saccharomyces cerevisiae W303-K6001 cross-platform genome sequence: Insights into ancestry and physiology of a laboratory mutt. Open Biol. 2012, 2, 120093. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, L.N.; Brem, R.B.; Kruglyak, L.; Gottschling, D.E. Polymorphisms in multiple genes contribute to the spontaneous mitochondrial genome instability of Saccharomyces cerevisiae S288C strains. Genetics 2009, 183, 365–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, S.; Naganuma, A.; Kuge, S. Peroxiredoxin-mediated redox regulation of the nuclear localization of Yap1, a transcription factor in budding yeast. Antioxid. Redox Signal. 2005, 7, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Petrezselyova, S.; Zahradka, J.; Sychrova, H. Saccharomyces cerevisiae BY4741 and W303-1A laboratory strains differ in salt tolerance. Fungal Biol. 2010, 114, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zadrag-Tecza, R.; Kwolek-Mirek, M.; Bartosz, G.; Bilinski, T. Cell volume as a factor limiting the replicative lifespan of the yeast Saccharomyces cerevisiae. Biogerontology 2009, 10, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Fang, N.N.; Ng, A.H.; Winget, J.M.; Comyn, S.A.; Mayor, T. The yeast ubr1 ubiquitin ligase participates in a prominent pathway that targets cytosolic thermosensitive mutants for degradation. G3 2012, 2, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, W.K.; Falvo, J.V.; Gerke, L.C.; Carroll, A.S.; Howson, R.W.; Weissman, J.S.; O’Shea, E.K. Global analysis of protein localization in budding yeast. Nature 2003, 425, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Malinovska, L.; Kroschwald, S.; Munder, M.C.; Richter, D.; Alberti, S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol. Biol. Cell 2012, 23, 3041–3056. [Google Scholar] [CrossRef]
- Ungelenk, S.; Moayed, F.; Ho, C.T.; Grousl, T.; Scharf, A.; Mashaghi, A.; Tans, S.; Mayer, M.P.; Mogk, A.; Bukau, B. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat. Commun. 2016, 7, 13673. [Google Scholar] [CrossRef] [Green Version]
- Nillegoda, N.B.; Wentink, A.S.; Bukau, B. Protein Disaggregation in Multicellular Organisms. Trends Biochem. Sci. 2018, 43, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Sherman, M.Y.; Goldberg, A.L. Involvement of the molecular chaperone Ydj1 in the ubiquitin-dependent degradation of short-lived and abnormal proteins in Saccharomyces cerevisiae. Mol. Cell Biol. 1996, 16, 4773–4781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, N.N.; Chan, G.T.; Zhu, M.; Comyn, S.A.; Persaud, A.; Deshaies, R.J.; Rotin, D.; Gsponer, J.; Mayor, T. Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress. Nat. Cell Biol. 2014, 16, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Boronat, S.; Marte, L.; Vega, M.; Garcia-Santamarina, S.; Cabrera, M.; Ayte, J.; Hidalgo, E. The Hsp40 Mas5 Connects Protein Quality Control and the General Stress Response through the Thermo-sensitive Pyp1. iScience 2020, 23, 101725. [Google Scholar] [CrossRef] [PubMed]
- Boronat, S.; Cabrera, M.; Hidalgo, E. Spatial sequestration of misfolded proteins as an active chaperone-mediated process during heat stress. Curr. Genet. 2021, 67, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, H.; Janta, A.; Sztangierska, W.; Obuchowski, I.; Chamera, T.; Klosowska, A.; Liberek, K. Class-specific interactions between Sis1 J-domain protein and Hsp70 chaperone potentiate disaggregation of misfolded proteins. Proc. Natl. Acad. Sci USA 2021, 118, e2108163118. [Google Scholar] [CrossRef]
- Gallardo, P.; Real-Calderon, P.; Flor-Parra, I.; Salas-Pino, S.; Daga, R.R. Acute Heat Stress Leads to Reversible Aggregation of Nuclear Proteins into Nucleolar Rings in Fission Yeast. Cell Rep. 2020, 33, 108377. [Google Scholar] [CrossRef]
- Kryndushkin, D.S.; Shewmaker, F.; Wickner, R.B. Curing of the [URE3] prion by Btn2p, a Batten disease-related protein. EMBO J. 2008, 27, 2725–2735. [Google Scholar] [CrossRef] [Green Version]
- Sherman, F. Getting started with yeast. Methods Enzym. 2002, 350, 3–41. [Google Scholar]
- Bahler, J.; Wu, J.Q.; Longtine, M.S.; Shah, N.G.; McKenzie, A., III; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Alfa, C.; Fantes, P.; Hyams, J.; McLeod, M.; Warbrick, E. Experiments with Fission Yeast: A Laboratory Course Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1993. [Google Scholar]
- Vivancos, A.P.; Castillo, E.A.; Biteau, B.; Nicot, C.; Ayte, J.; Toledano, M.B.; Hidalgo, E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 8875–8880. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker Brachmann, C.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Rothstein, R.J. One-step gene disruption in yeast. Methods Enzym. 1983, 101, 202–211. [Google Scholar]
- Leupold, U. Genetical methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970, 4, 169–177. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boronat, S.; Cabrera, M.; Vega, M.; Alcalá, J.; Salas-Pino, S.; Daga, R.R.; Ayté, J.; Hidalgo, E. Formation of Transient Protein Aggregate-like Centers Is a General Strategy Postponing Degradation of Misfolded Intermediates. Int. J. Mol. Sci. 2023, 24, 11202. https://doi.org/10.3390/ijms241311202
Boronat S, Cabrera M, Vega M, Alcalá J, Salas-Pino S, Daga RR, Ayté J, Hidalgo E. Formation of Transient Protein Aggregate-like Centers Is a General Strategy Postponing Degradation of Misfolded Intermediates. International Journal of Molecular Sciences. 2023; 24(13):11202. https://doi.org/10.3390/ijms241311202
Chicago/Turabian StyleBoronat, Susanna, Margarita Cabrera, Montserrat Vega, Jorge Alcalá, Silvia Salas-Pino, Rafael R. Daga, José Ayté, and Elena Hidalgo. 2023. "Formation of Transient Protein Aggregate-like Centers Is a General Strategy Postponing Degradation of Misfolded Intermediates" International Journal of Molecular Sciences 24, no. 13: 11202. https://doi.org/10.3390/ijms241311202




