Innate Immunity in Cancer Biology and Therapy
Abstract
:1. Introduction
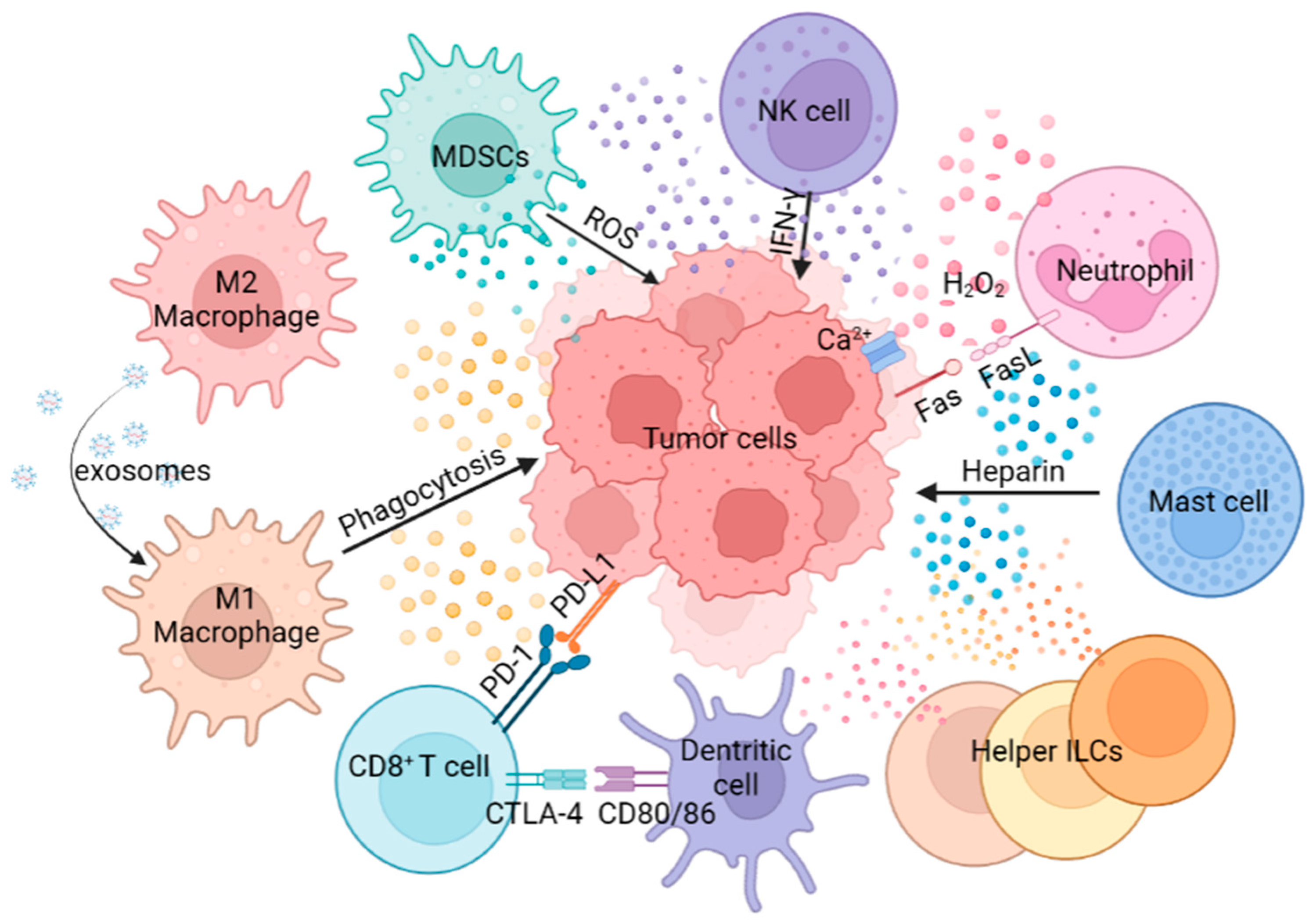
2. Innate Immunity in TME
2.1. MDSCs
2.2. Macrophages
2.3. Neutrophils
2.4. NK Cells
2.5. DCs
2.6. MCs
2.7. Helper ILCs
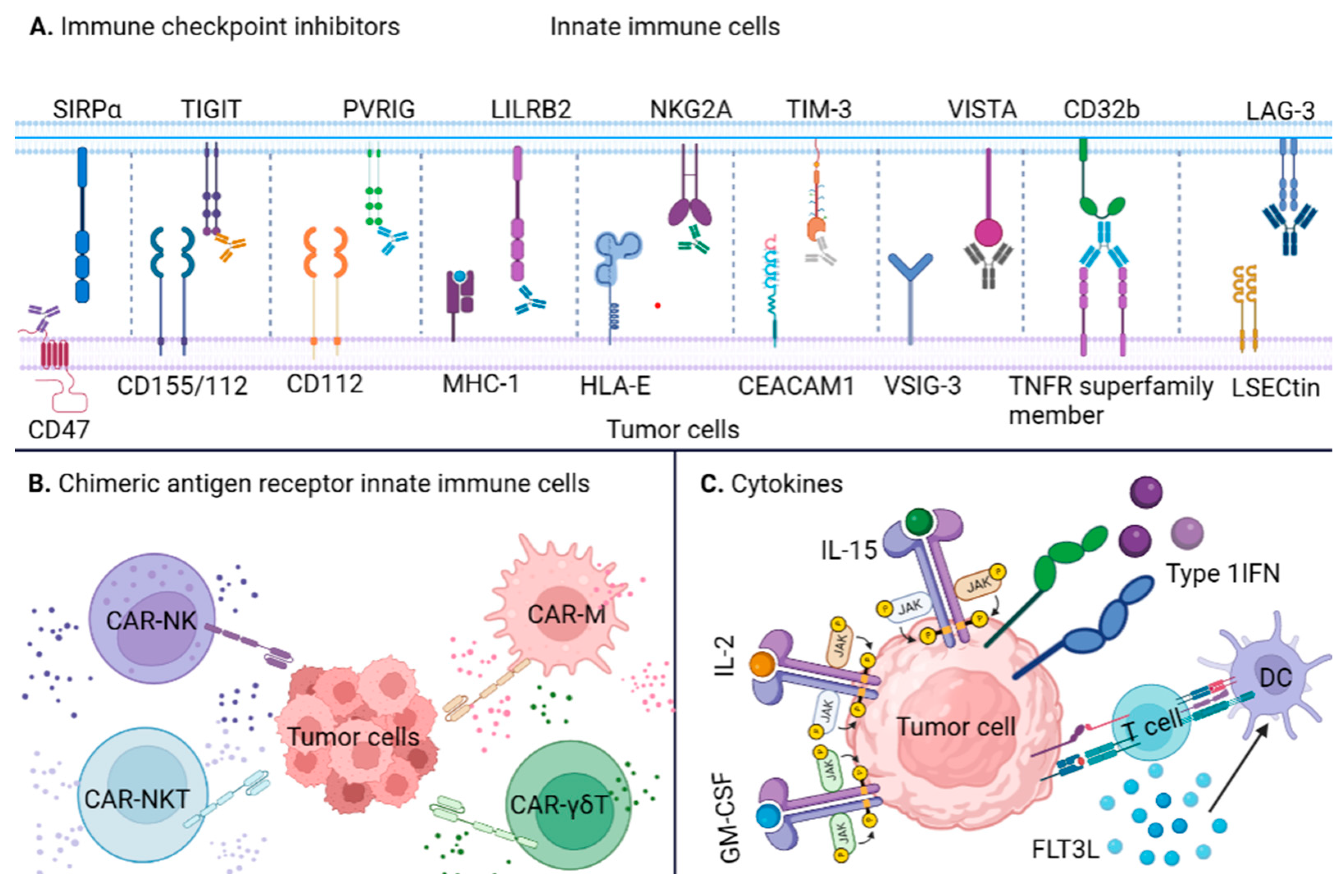
3. Immunotherapies Targeting Innate Immune System
3.1. Innate ICIs
3.2. Innate Immune Cells Engineered with CARs
3.3. Cytokines
4. Challenges and Future Directions of Innate Immunotherapy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anwar, S.; Almatroudi, A.; Alsahli, A.M.; Khan, A.M.; Khan, A.A.; Rahmani, H.A. Natural Products: Implication in Cancer Prevention and Treatment through Modulating Various Biological Activities. Anti-Cancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.K.; Khan, N.; Meghwanshi, G.K.; Ponne, S.; Althobiti, M.; Kumar, R. Current research status of anti-cancer peptides: Mechanism of action, production, and clinical applications. Biomed. Pharmacother. 2023, 164, 114996. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Seager, R.J.; Hajal, C.; Spill, F.; Kamm, R.D.; Zaman, M.H. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg. Sci. Phys. Oncol. 2017, 3, 034002. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [Green Version]
- Alderton, G.K.; Bordon, Y. Tumour immunotherapy—Leukocytes take up the fight. Nat. Rev. Immunol. 2012, 12, 237. [Google Scholar] [CrossRef]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv. Mater. 2022, 34, 2206121. [Google Scholar] [CrossRef]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Song, W.; Shao, C.; Shi, Y.; Han, W. Emerging predictors of the response to the blockade of immune checkpoints in cancer therapy. Cell. Mol. Immunol. 2019, 16, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Guo, Y.; Wang, Y.; Wu, Z.; Bo, J.; Zhang, B.; Zhu, J.; Han, W. Clinical development of CAR T cell therapy in China: 2020 update. Cell. Mol. Immunol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Appl. Sci. 2022, 12, 9175. [Google Scholar] [CrossRef]
- Liu, S.W.; Song, W.J.; Ma, G.K.; Wang, H.; Yang, L. Pyroptosis and its role in cancer. World J. Clin. Cases 2023, 11, 2386–2395. [Google Scholar] [CrossRef]
- Xu, X.; Fan, H.; Yang, Y.; Yao, S.; Yu, W.; Guo, Z.; Tan, W. Virus-Like Particle-Induced cGAS-STING Activation and AIM2 Inflammasome-Mediated Pyroptosis for Robust Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303010. [Google Scholar] [CrossRef]
- Li, X.; Dai, H.; Wang, H.; Han, W. Exploring innate immunity in cancer immunotherapy: Opportunities and challenges. Cell. Mol. Immunol. 2021, 18, 1607–1609. [Google Scholar] [CrossRef]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, H.; Vesely, M.D.; Koboldt, D.C.; Rickert, C.G.; Uppaluri, R.; Magrini, V.J.; Arthur, C.D.; White, J.M.; Chen, Y.S.; Shea, L.K.; et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012, 482, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Muntjewerff, E.M.; Meesters, L.D.; van den Bogaart, G. Antigen Cross-Presentation by Macrophages. Front. Immunol. 2020, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Furumaya, C.; Martinez-Sanz, P.; Bouti, P.; Kuijpers, T.W.; Matlung, H.L. Plasticity in Pro- and Anti-tumor Activity of Neutrophils: Shifting the Balance. Front. Immunol. 2020, 11, 2100. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Li, Z.; Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 2021, 11, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Dolcetti, L.; Peranzoni, E.; Ugel, S.; Marigo, I.; Fernandez Gomez, A.; Mesa, C.; Geilich, M.; Winkels, G.; Traggiai, E.; Casati, A.; et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 2010, 40, 22–35. [Google Scholar] [CrossRef]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, development, states, and unaddressed complexity. Immunity 2021, 54, 875–884. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, Z.; Zhong, H.; Ren, Z.; Li, Y.; Wang, H.; Qiu, Y. The role of myeloid-derived suppressor cells in liver cancer. Discov. Oncol. 2023, 14, 77. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Van Vlerken-Ysla, L.; Tyurina, Y.Y.; Kagan, V.E.; Gabrilovich, D.I. Functional states of myeloid cells in cancer. Cancer Cell 2023, 41, 490–504. [Google Scholar] [CrossRef]
- Mohamed, A.G.; Salome, V.I.; Abdelmetalab, F.T.; Youssef, E.; Hanh, H.L.; Matthew, J.D.; Ali, H.E.-B.; Dorota, W.; Ilyes, A.B.; Luis Del, V.; et al. Targeting PARP-1 with metronomic therapy modulates MDSC suppressive function and enhances anti-PD-1 immunotherapy in colon cancer. J. Immunother. Cancer 2021, 9, e001643. [Google Scholar] [CrossRef]
- Francesco De, S.; Alessia, L.; Federico, B.; Chiara, M.; Simone, C.; Rosalinda, T.; Alessandra, F.; Cristina, F.; Cristina, A.; Ornella, P.; et al. Interrupting the nitrosative stress fuels tumor-specific cytotoxic T lymphocytes in pancreatic cancer. J. Immunother. Cancer 2022, 10, e003549. [Google Scholar] [CrossRef]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef] [Green Version]
- Lavin, Y.; Merad, M. Macrophages: Gatekeepers of tissue integrity. Cancer Immunol. Res. 2013, 1, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Anderson, N.R.; Minutolo, N.G.; Gill, S.; Klichinsky, M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021, 81, 1201–1208. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Biswas, S.K.; Gangi, L.; Paul, S.; Schioppa, T.; Saccani, A.; Sironi, M.; Bottazzi, B.; Doni, A.; Vincenzo, B.; Pasqualini, F.; et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 2006, 107, 2112–2122. [Google Scholar] [CrossRef] [Green Version]
- Saccani, A.; Schioppa, T.; Porta, C.; Biswas, S.K.; Nebuloni, M.; Vago, L.; Bottazzi, B.; Colombo, M.P.; Mantovani, A.; Sica, A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006, 66, 11432–11440. [Google Scholar] [CrossRef] [Green Version]
- Leach, J.; Morton, J.P.; Sansom, O.J. Neutrophils: Homing in on the myeloid mechanisms of metastasis. Mol. Immunol. 2019, 110, 69–76. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Lang, S.; Brandau, S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin. Cancer Biol. 2013, 23, 141–148. [Google Scholar] [CrossRef]
- Benson, D.D.; Meng, X.; Fullerton, D.A.; Moore, E.E.; Lee, J.H.; Ao, L.; Silliman, C.C.; Barnett, C.C., Jr. Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1067-75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.F.; Huang, Y.; Han, Y.Y.; Lin, L.Y.; Sun, W.H.; Rabson, A.B.; Wang, Y.; Shi, Y.F. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene 2017, 36, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Eomm, M.; Han, A. Usefulness of Pretreatment Neutrophil to Lymphocyte Ratio in Predicting Disease-Specific Survival in Breast Cancer Patients. J. Breast Cancer 2013, 16, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Azab, B.; Bhatt, V.R.; Phookan, J.; Murukutla, S.; Kohn, N.; Terjanian, T.; Widmann, W.D. Usefulness of the Neutrophil-to-Lymphocyte Ratio in Predicting Short- and Long-Term Mortality in Breast Cancer Patients. Ann. Surg. Oncol. 2012, 19, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, Z.; Wen, S.; Zhang, B.; Cao, X.; Wang, X. Prognostic value of chemotherapy-induced neutropenia in early-stage breast cancer. Breast Cancer Res. Treat. 2012, 131, 483–490. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lai, Y.-H.; Chen, H.-Y.; Guo, H.-R.; Su, I.-J.; Chen, H.H.W. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013, 63, 225–233. [Google Scholar] [CrossRef]
- Novitskiy, S.V.; Pickup, M.W.; Gorska, A.E.; Owens, P.; Chytil, A.; Aakre, M.; Wu, H.; Shyr, Y.; Moses, H.L. TGF-β Receptor II Loss Promotes Mammary Carcinoma Progression by Th17-Dependent Mechanisms. Cancer Discov. 2011, 1, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Karre, K. NK cells, MHC class I molecules and the missing self. Scand. J. Immunol. 2002, 55, 221–228. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, Y.; Xin, V.W.; Wang, X.-W.; Peng, X.-C.; Liu, X.-Q.; Wang, D.; Li, N.; Cheng, J.-T.; Lyv, Y.-N.; et al. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020, 13, 107. [Google Scholar] [CrossRef]
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4(+) T Cell Immunity. Cell 2019, 177, 556–571.e516. [Google Scholar] [CrossRef]
- Salio, M.; Palmowski, M.J.; Atzberger, A.; Hermans, I.F.; Cerundolo, V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 2004, 199, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Tel, J.; Aarntzen, E.H.; Baba, T.; Schreibelt, G.; Schulte, B.M.; Benitez-Ribas, D.; Boerman, O.C.; Croockewit, S.; Oyen, W.J.; van Rossum, M.; et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013, 73, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Chiang, M.C.; Tullett, K.M.; Lee, Y.S.; Idris, A.; Ding, Y.; McDonald, K.J.; Kassianos, A.; Leal Rojas, I.M.; Jeet, V.; Lahoud, M.H.; et al. Differential uptake and cross-presentation of soluble and necrotic cell antigen by human DC subsets. Eur. J. Immunol. 2016, 46, 329–339. [Google Scholar] [CrossRef]
- Russo, E.; Teijeira, A.; Vaahtomeri, K.; Willrodt, A.-H.; Bloch, J.S.; Nitschké, M.; Santambrogio, L.; Kerjaschki, D.; Sixt, M.; Halin, C. Intralymphatic CCL21 Promotes Tissue Egress of Dendritic Cells through Afferent Lymphatic Vessels. Cell Rep. 2016, 14, 1723–1734. [Google Scholar] [CrossRef] [Green Version]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723 e714. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lopez, M.; Iborra, S.; Conde-Garrosa, R.; Sancho, D. Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur. J. Immunol. 2015, 45, 119–129. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Parker, B.S.; Rautela, J.; Hertzog, P.J. Antitumour actions of interferons: Implications for cancer therapy. Nat. Rev. Cancer 2016, 16, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Eissmann, M.F.; Dijkstra, C.; Jarnicki, A.; Phesse, T.; Brunnberg, J.; Poh, A.R.; Etemadi, N.; Tsantikos, E.; Thiem, S.; Huntington, N.D.; et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 2019, 10, 2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukic, A.; Wahlund, C.J.E.; Gómez, C.; Brodin, D.; Samuelsson, B.; Wheelock, C.E.; Gabrielsson, S.; Rådmark, O. Exosomes and cells from lung cancer pleural exudates transform LTC4 to LTD4, promoting cell migration and survival via CysLT1. Cancer Lett. 2019, 444, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lukic, A.; Ji, J.; Idborg, H.; Samuelsson, B.; Palmberg, L.; Gabrielsson, S.; Rådmark, O. Pulmonary epithelial cancer cells and their exosomes metabolize myeloid cell-derived leukotriene C4 to leukotriene D4. J. Lipid Res. 2016, 57, 1659–1669. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yao, K.; Li, D.; Liu, K.; Jin, G.; Yan, M.; Wu, Q.; Chen, H.; Shin, S.H.; Bai, R.; et al. Inhibition of LTA4H by bestatin in human and mouse colorectal cancer. eBioMedicine 2019, 44, 361–374. [Google Scholar] [CrossRef]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salome, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C.; et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 2017, 8, 593. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Bie, Q.; Wu, P.; Zhang, J.; You, B.; Shi, H.; Qian, H.; Xu, W. PGD2/PTGDR2 Signaling Restricts the Self-Renewal and Tumorigenesis of Gastric Cancer. Stem Cells 2018, 36, 990–1003. [Google Scholar] [CrossRef] [Green Version]
- Grauers Wiktorin, H.; Nilsson, M.S.; Kiffin, R.; Sander, F.E.; Lenox, B.; Rydstrom, A.; Hellstrand, K.; Martner, A. Histamine targets myeloid-derived suppressor cells and improves the anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade. Cancer Immunol. Immunother. 2019, 68, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Parmiani, G.; Castelli, C.; Pilla, L.; Santinami, M.; Colombo, M.P.; Rivoltini, L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann. Oncol. 2007, 18, 226–232. [Google Scholar] [CrossRef]
- Jacquelot, N.; Seillet, C.; Vivier, E.; Belz, G.T. Innate lymphoid cells and cancer. Nat. Immunol. 2022, 23, 371–379. [Google Scholar] [CrossRef]
- Cuff, A.O.; Sillito, F.; Dertschnig, S.; Hall, A.; Luong, T.V.; Chakraverty, R.; Male, V. The Obese Liver Environment Mediates Conversion of NK Cells to a Less Cytotoxic ILC1-Like Phenotype. Front. Immunol. 2019, 10, 2180. [Google Scholar] [CrossRef] [Green Version]
- Ricardo-Gonzalez, R.R.; Van Dyken, S.J.; Schneider, C.; Lee, J.; Nussbaum, J.C.; Liang, H.-E.; Vaka, D.; Eckalbar, W.L.; Molofsky, A.B.; Erle, D.J.; et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 2018, 19, 1093–1099. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Moon, U.J.; Kim, H.J.; Choi, H.J.; Sin, J.I.; Park, N.H.; Cho, H.R.; Kwon, B. Intratumorally Establishing Type 2 Innate Lymphoid Cells Blocks Tumor Growth. J. Immunol. 2016, 196, 2410–2423. [Google Scholar] [CrossRef] [Green Version]
- Jacquelot, N.; Seillet, C.; Wang, M.; Pizzolla, A.; Liao, Y.; Hediyeh-Zadeh, S.; Grisaru-Tal, S.; Louis, C.; Huang, Q.; Schreuder, J.; et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 2021, 22, 851–864. [Google Scholar] [CrossRef]
- Wang, S.; Qu, Y.; Xia, P.; Chen, Y.; Zhu, X.; Zhang, J.; Wang, G.; Tian, Y.; Ying, J.; Fan, Z. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020, 30, 610–622. [Google Scholar] [CrossRef]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef]
- Chan, I.H.; Jain, R.; Tessmer, M.S.; Gorman, D.; Mangadu, R.; Sathe, M.; Vives, F.; Moon, C.; Penaflor, E.; Turner, S.; et al. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 2014, 7, 842–856. [Google Scholar] [CrossRef]
- Yang, Y.H.; Liu, J.W.; Lu, C.; Wei, J.F. CAR-T Cell Therapy for Breast Cancer: From Basic Research to Clinical Application. Int. J. Biol. Sci. 2022, 18, 2609–2626. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Qiao, M.; Zhou, C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol. Immunol. 2021, 18, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Kesmir, C.; van Buuren, M.M. Biomarkers in cancer immunotherapy. Cancer Cell 2015, 27, 12–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, J. Chimeric antigen receptor engineered innate immune cells in cancer immunotherapy. Sci. China Life Sci. 2019, 62, 633–639. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Jia, X.; Yan, B.; Tian, X.; Liu, Q.; Jin, J.; Shi, J.; Hou, Y. CD47/SIRPalpha pathway mediates cancer immune escape and immunotherapy. Int. J. Biol. Sci. 2021, 17, 3281–3287. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Gao, P.; Yao, Z. Targeting CD47: The achievements and concerns of current studies on cancer immunotherapy. J. Thorac. Dis. 2017, 9, E168–E174. [Google Scholar] [CrossRef] [Green Version]
- Folkes, A.S.; Feng, M.; Zain, J.M.; Abdulla, F.; Rosen, S.T.; Querfeld, C. Targeting CD47 as a cancer therapeutic strategy: The cutaneous T-cell lymphoma experience. Curr. Opin. Oncol. 2018, 30, 332–337. [Google Scholar] [CrossRef]
- Querfeld, C.; Thompson, J.; Taylor, M.; Pillai, R.; Johnson, L.D.S.; Catalano, T.; Petrova, P.S.; Uger, R.A.; Irwin, M.; Sievers, E.L.; et al. A Single Direct Intratumoral Injection of TTI-621 (SIRPαFc) Induces Antitumor Activity in Patients with Relapsed/Refractory Mycosis Fungoides and Sézary Syndrome: Preliminary Findings Employing an Immune Checkpoint Inhibitor Blocking the CD47 “Do Not Eat” Signal. Blood 2017, 130, 4076. [Google Scholar] [CrossRef]
- Hodgins, J.J.; Khan, S.T.; Park, M.M.; Auer, R.C.; Ardolino, M. Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Investig. 2019, 129, 3499–3510. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Whelan, S.; Ophir, E.; Kotturi, M.F.; Levy, O.; Ganguly, S.; Leung, L.; Vaknin, I.; Kumar, S.; Dassa, L.; Hansen, K.; et al. PVRIG and PVRL2 Are Induced in Cancer and Inhibit CD8(+) T-cell Function. Cancer Immunol. Res. 2019, 7, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Harjunpaa, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Correa, B.; Valhondo, I.; Hassouneh, F.; Lopez-Sejas, N.; Pera, A.; Bergua, J.M.; Arcos, M.J.; Banas, H.; Casas-Aviles, I.; Duran, E.; et al. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers 2019, 11, 877. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Sunderland, A.; Zhou, Y.; Schulick, R.D.; Edil, B.H.; Zhu, Y. Blockade of CD112R and TIGIT signaling sensitizes human natural killer cell functions. Cancer Immunol. Immunother. 2017, 66, 1367–1375. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.X.; Weissman, I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef]
- Yu, T.; Gan, S.; Zhu, Q.; Dai, D.; Li, N.; Wang, H.; Chen, X.; Hou, D.; Wang, Y.; Pan, Q.; et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat. Commun. 2019, 10, 4353. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.M.; van der Touw, W.; Wang, Y.S.; Kang, K.; Mai, S.; Zhang, J.; Alsina-Beauchamp, D.; Duty, J.A.; Mungamuri, S.K.; Zhang, B.; et al. Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J. Clin. Investig. 2018, 128, 5647–5662. [Google Scholar] [CrossRef] [Green Version]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulal, D.; Boring, A.; Terrero, D.; Johnson, T.; Tiwari, A.K.; Raman, D. Tackling of Immunorefractory Tumors by Targeting Alternative Immune Checkpoints. Cancers 2023, 15, 2774. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Xu, C.; Ju, D.; Zhang, X. Chimeric antigen receptor T-cell therapy: Challenges and opportunities in lung cancer. Antib. Ther. 2022, 5, 73–83. [Google Scholar] [CrossRef]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.; Xu, H.; Pan, C.X.; Zhu, Z. CAR race to cancer immunotherapy: From CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
- Bollino, D.; Webb, T.J. Chimeric antigen receptor-engineered natural killer and natural killer T cells for cancer immunotherapy. Transl. Res. 2017, 187, 32–43. [Google Scholar] [CrossRef]
- Heczey, A.; Liu, D.; Tian, G.; Courtney, A.N.; Wei, J.; Marinova, E.; Gao, X.; Guo, L.; Yvon, E.; Hicks, J.; et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 2014, 124, 2824–2833. [Google Scholar] [CrossRef] [Green Version]
- Tian, G.; Courtney, A.N.; Jena, B.; Heczey, A.; Liu, D.; Marinova, E.; Guo, L.; Xu, X.; Torikai, H.; Mo, Q.; et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J. Clin. Investig. 2016, 126, 2341–2355. [Google Scholar] [CrossRef] [Green Version]
- Morandi, F.; Yazdanifar, M.; Cocco, C.; Bertaina, A.; Airoldi, I. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on gammadelta T and NK Cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef]
- Rozenbaum, M.; Meir, A.; Aharony, Y.; Itzhaki, O.; Schachter, J.; Bank, I.; Jacoby, E.; Besser, M.J. Gamma-Delta CAR-T Cells Show CAR-Directed and Independent Activity Against Leukemia. Front. Immunol. 2020, 11, 1347. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Song, X.; Peng, Y.; Wang, X.; Chen, Q.; Lan, X.; Shi, F. The stimulator of interferon genes (STING) agonists for treating acute myeloid leukemia (AML): Current knowledge and future outlook. Clin. Transl. Oncol. 2023, 25, 1545–1553. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, W.; Guo, H.; Wang, L.; Wu, H.; Ding, L.; Yang, B. Strategies involving STING pathway activation for cancer immunotherapy: Mechanism and agonists. Biochem. Pharmacol. 2023, 213, 115596. [Google Scholar] [CrossRef]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef]
- Hopfner, K.-P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef]
- Wennerberg, E.; Kremer, V.; Childs, R.; Lundqvist, A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol. Immunother. 2015, 64, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Xin, S.; Yang, L.; Jiang, M.; Xin, Y.; Wang, Y.; Cao, P.; Zhang, S.; Yang, Y.; et al. The emerging roles of IFIT3 in antiviral innate immunity and cellular biology. J. Med. Virol. 2023, 95, e28259. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, S.R.; Barnett, B.B.; Pandeswara, S.; Wall, S.R.; Hurez, V.; Dao, V.; Sun, L.; Daniel, B.J.; Brumlik, M.J.; Drerup, J.; et al. IFNalpha Augments Clinical Efficacy of Regulatory T-cell Depletion with Denileukin Diftitox in Ovarian Cancer. Clin. Cancer Res. 2021, 27, 3661–3673. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chintalacharuvu, K.R.; Morrison, S.L. Targeting IFN-alpha to B cell lymphoma by a tumor-specific antibody elicits potent antitumor activities. J. Immunol. 2007, 179, 6881–6888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, E.A.; Rossi, D.L.; Cardillo, T.M.; Stein, R.; Goldenberg, D.M.; Chang, C.H. Preclinical studies on targeted delivery of multiple IFNalpha2b to HLA-DR in diverse hematologic cancers. Blood 2011, 118, 1877–1884. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, X.; Fu, M.L.; Weichselbaum, R.R.; Gajewski, T.F.; Guo, Y.; Fu, Y.X. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell 2014, 25, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Cueto, F.J.; Sancho, D. The Flt3L/Flt3 Axis in Dendritic Cell Biology and Cancer Immunotherapy. Cancers 2021, 13, 1525. [Google Scholar] [CrossRef]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [Green Version]
- Hammerich, L.; Marron, T.U.; Upadhyay, R.; Svensson-Arvelund, J.; Dhainaut, M.; Hussein, S.; Zhan, Y.; Ostrowski, D.; Yellin, M.; Marsh, H.; et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 2019, 25, 814–824. [Google Scholar] [CrossRef]
- Sim, G.C.; Martin-Orozco, N.; Jin, L.; Yang, Y.; Wu, S.; Washington, E.; Sanders, D.; Lacey, C.; Wang, Y.; Vence, L.; et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J. Clin. Investig. 2014, 124, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Stein-Merlob, A.F.; Rothberg, M.V.; Ribas, A.; Yang, E.H. Cardiotoxicities of novel cancer immunotherapies. Heart 2021, 107, 1694–1703. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef]
- Zhang, Y.; Wallace, D.L.; de Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.; et al. In vivo kinetics of human natural killer cells: The effects of ageing and acute and chronic viral infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef]
- Wang, X.; Jasinski, D.L.; Medina, J.L.; Spencer, D.M.; Foster, A.E.; Bayle, J.H. Inducible MyD88/CD40 synergizes with IL-15 to enhance antitumor efficacy of CAR-NK cells. Blood Adv. 2020, 4, 1950–1964. [Google Scholar] [CrossRef]
- Mishra, A.K.; Malonia, S.K. Advancing cellular immunotherapy with macrophages. Life Sci. 2023, 328, 121857. [Google Scholar] [CrossRef]
- Hutmacher, C.; Neri, D. Antibody-cytokine fusion proteins: Biopharmaceuticals with immunomodulatory properties for cancer therapy. Adv. Drug Deliv. Rev. 2019, 141, 67–91. [Google Scholar] [CrossRef]
- Holder, P.G.; Lim, S.A.; Huang, C.S.; Sharma, P.; Dagdas, Y.S.; Bulutoglu, B.; Sockolosky, J.T. Engineering interferons and interleukins for cancer immunotherapy. Adv. Drug Deliv. Rev. 2022, 182, 114112. [Google Scholar] [CrossRef]
- Garcin, G.; Paul, F.; Staufenbiel, M.; Bordat, Y.; Van der Heyden, J.; Wilmes, S.; Cartron, G.; Apparailly, F.; De Koker, S.; Piehler, J.; et al. High efficiency cell-specific targeting of cytokine activity. Nat. Commun. 2014, 5, 3016. [Google Scholar] [CrossRef] [Green Version]
- Puskas, J.; Skrombolas, D.; Sedlacek, A.; Lord, E.; Sullivan, M.; Frelinger, J. Development of an attenuated interleukin-2 fusion protein that can be activated by tumour-expressed proteases. Immunology 2011, 133, 206–220. [Google Scholar] [CrossRef]
- Venetz, D.; Koovely, D.; Weder, B.; Neri, D. Targeted Reconstitution of Cytokine Activity upon Antigen Binding using Split Cytokine Antibody Fusion Proteins. J. Biol. Chem. 2016, 291, 18139–18147. [Google Scholar] [CrossRef] [Green Version]
- Quijano-Rubio, A.; Bhuiyan, A.M.; Yang, H.; Leung, I.; Bello, E.; Ali, L.R.; Zhangxu, K.; Perkins, J.; Chun, J.H.; Wang, W.; et al. A split, conditionally active mimetic of IL-2 reduces the toxicity of systemic cytokine therapy. Nat. Biotechnol. 2022, 41, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Slingluff, C.L., Jr.; Petroni, G.R.; Olson, W.C.; Smolkin, M.E.; Ross, M.I.; Haas, N.B.; Grosh, W.W.; Boisvert, M.E.; Kirkwood, J.M.; Chianese-Bullock, K.A. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: Outcome of a multicenter randomized trial. Clin. Cancer Res. 2009, 15, 7036–7044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faries, M.B.; Hsueh, E.C.; Ye, X.; Hoban, M.; Morton, D.L. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin. Cancer Res. 2009, 15, 7029–7035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Target | NCT Number | Status | Phase | Cancer Type | Interventions | Enrollment |
|---|---|---|---|---|---|---|
| SIRPα | NCT05076591 | Recruiting | I | Advanced Breast Cancer, Advanced Gastric Cancer | IMM2902 | 135 |
| NCT05276310 | Recruiting | I | Advanced Cancer | IMC-002 | 24 | |
| NCT05192512 | Recruiting | I | Advanced Cancer | TQB2928 | 180 | |
| NCT05507541 | Not yet recruiting | II | Multiple cancer types | TTI-622 and TTI-621 in combination with Pembrolizumab | 62 | |
| NCT05263271 | Recruiting | I | AML; MDS | Gentulizumab | 58 | |
| TIGIT&PVRIG | NCT04570839 | Recruiting | I/II | Endometrial Neoplasms, Ovarian Cancer, HNC | COM701 in combination with BMS-986207 and Nivolumab | 100 |
| NCT03667716 | Recruiting | I | Ovarian Cancer, Breast Cancer | COM701 and Nivolumab | 140 | |
| LILRB2 | NCT04669899 | Recruiting | I/II | Multiple cancer types | JTX-8064 | 281 |
| NCT05054348 | Recruiting | I | Solid Tumor | IO-108, Pembrolizumab | 36 | |
| NKG2A | NCT05162755 | Recruiting | I | Solid Tumor | S095029 | 129 |
| NCT04914351 | Not yet recruiting | I | Solid Tumor | HY-0102 | 32 | |
| NCT05414032 | Not yet recruiting | II | Advanced HNSCC | Monalizumab, Cetuximab | 200 | |
| LAG-3 | NCT03252938 | Recruiting | I | Peritoneal Carcinomatosis | IMP321 | 45 |
| NCT05101109 | Recruiting | I | Advanced Solid Tumor | ABL501 | 36 | |
| NCT05078593 | Recruiting | I | Solid Tumor, Lymphoma | HLX26 | 11 | |
| NCT04618393 | Recruiting | I/II | Advanced Solid Tumor | EMB-02 | 43 | |
| NCT05577182 | Not yet recruiting | I | Advanced Malignancies | INCA32459-101 | 120 | |
| TIM-3 | NCT05357651 | Not yet recruiting | I | Solid Tumor, Lymphoma | LB1410 | 100 |
| NCT02817633 | Recruiting | I | Multiple cancer types | TSR-022 | 369 | |
| NCT04370704 | Recruiting | I/II | Melanoma | INCAGN02385 in combination with INCAGN02390 and INCMGA00012 | 146 | |
| NCT04931654 | Recruiting | I/II | NSCLC | AZD7789 | 81 | |
| NCT05367401 | Not yet recruiting | I/II | MDS, AML | Sabatolimab, Magrolimab | 63 | |
| VISTA | NCT04475523 | Recruiting | I | Solid Tumor | CI-8993 | 50 |
| NCT05082610 | Recruiting | I | NSCLC | HMBD-002, Pembrolizumab | 240 | |
| CD32B | NCT04219254 | Recruiting | I/II | Solid Tumor | BI-1206 in combination with Pembrolizumab | 90 |
| NCT05555251 | Recruiting | I/II | HER2-positive Breast Cancer, HER2-positive Gastric Cancer | BI-1607 in combination with Trastuzumab | 116 | |
| NCT03571568 | Recruiting | I/II | Indolent B-Cell NHL | BI1206 in combination with Rituximab | 30 | |
| CAR NK | NCT05213195 | Recruiting | I | Refractory Metastatic CRC | NKG2D CAR-NK cells | 38 |
| NCT05507593 | Recruiting | I | SCLC | DLL3-CAR-NK cells | 18 | |
| NCT04662788 | Not yet recruiting | I | Hematological Malignancies | NK cells/Combined Monoclonal Antibodies | 36 | |
| NCT05410717 | Recruiting | I/II | Ovarian cancer, Testis cancer | Claudin6 targeting CAR-NK cells | 40 | |
| CAR M | NCT04660929 | Recruiting | I | Multiple cancer types | CT-0508 | 18 |
| CAR NKT | NCT03294954 | Recruiting | I | Neuroblastoma | GD2 Specific CAR and IL-15 Expressing Autologous NKT Cells | 36 |
| NCT03774654 | Recruiting | I | Multiple cancer types | CD19.CAR-aNKT cells | 48 | |
| NCT05487651 | Not yet recruiting | I | NHL, BCL, DLBCL | KUR-502 | 36 |
| Target | NCT Number | Status | Phase | Cancer Type | Interventions | Enrollment |
|---|---|---|---|---|---|---|
| GM-CSF | NCT05284214 | Not yet recruiting | II | Solid Tumor | Sargramostim, Ipilimumab-containing therapy | 65 |
| NCT05530200 | Not yet recruiting | II | Metastatic Solid Tumor | PD-L1 inhibitor, GM-CSF, IL-2 | 56 | |
| NCT03866525 | Recruiting | I/II | Gastrointestinal Cancer | OH2 | 300 | |
| NCT05292417 | Recruiting | II | CRC | GM-CSF in combination with Sintilimab and Fruquintinib | 71 | |
| NCT04725331 | Recruiting | I/II | Metastatic Cancer | BT-001, Pembrolizumab | 48 | |
| type I IFNs | NCT04053673 | Recruiting | I | Solid Tumor | RBN-2397 | 130 |
| NCT05127590 | Recruiting | I/II | Advanced NSCLC | RBN-2397 | 50 | |
| NCT04544007 | Recruiting | II | Glioma | Poly ICLC | 20 | |
| FLT3L | NCT05029999 | Recruiting | I | Metastatic Triple Negative Breast Cancer | PLD Chemotherapy, CDX-1140, CDX-301 | 45 |
| NCT04491084 | Recruiting | I/II | NSCLC | CDX-301, CDX-1140 | 46 | |
| NCT03789097 | Recruiting | I/II | NHL; HNSCC; Metastatic Breast Cancer | Pembrolizumab, CDX-301, Poly ICLC | 56 | |
| NCT04616248 | Not yet recruiting | I | Multiple cancer types | CDX-301, CDX-1140, Poly ICLC | 18 | |
| IL-2 | NCT05307874 | Recruiting | I/II | Solid Tumor | ICT01 in combination with IL-2 | 75 |
| NCT05267626 | Recruiting | I/II | Advanced Solid Tumor | AU-007 | 69 | |
| NCT04862767 | Recruiting | I | Solid Tumor | TASO-001 in combination with IL-2 | 9 | |
| NCT05493566 | Not yet recruiting | I | Lung Cancer | IL-2 in combination with Pembrolizumab | 15 | |
| NCT05538624 | Not yet recruiting | I/II | Multiple cancer types | AVB-001 | 44 | |
| IL-15 | NCT04294576 | Recruiting | I | Advanced/Metastatic Solid Tumor | BJ-001 | 92 |
| NCT05470283 | Recruiting | I | Metastatic Melanoma | OBX-115 | 30 | |
| NCT05445882 | Not yet recruiting | II | Castration-Resistant PCA | N-803TET | 28 | |
| NCT05266612 | Not yet recruiting | I | Solid Tumor | VG2025 | 12 | |
| NCT05359211 | Recruiting | I | Multiple cancer types | NKTR-255 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xue, W.; Xu, C.; Nan, Y.; Mei, S.; Ju, D.; Wang, S.; Zhang, X. Innate Immunity in Cancer Biology and Therapy. Int. J. Mol. Sci. 2023, 24, 11233. https://doi.org/10.3390/ijms241411233
Zhang Y, Xue W, Xu C, Nan Y, Mei S, Ju D, Wang S, Zhang X. Innate Immunity in Cancer Biology and Therapy. International Journal of Molecular Sciences. 2023; 24(14):11233. https://doi.org/10.3390/ijms241411233
Chicago/Turabian StyleZhang, Yuxia, Wenjing Xue, Caili Xu, Yanyang Nan, Shuang Mei, Dianwen Ju, Shaofei Wang, and Xuyao Zhang. 2023. "Innate Immunity in Cancer Biology and Therapy" International Journal of Molecular Sciences 24, no. 14: 11233. https://doi.org/10.3390/ijms241411233





