Pulmonary Sarcoidosis: Experimental Models and Perspectives of Molecular Diagnostics Using Quantum Dots
Abstract
1. Introduction
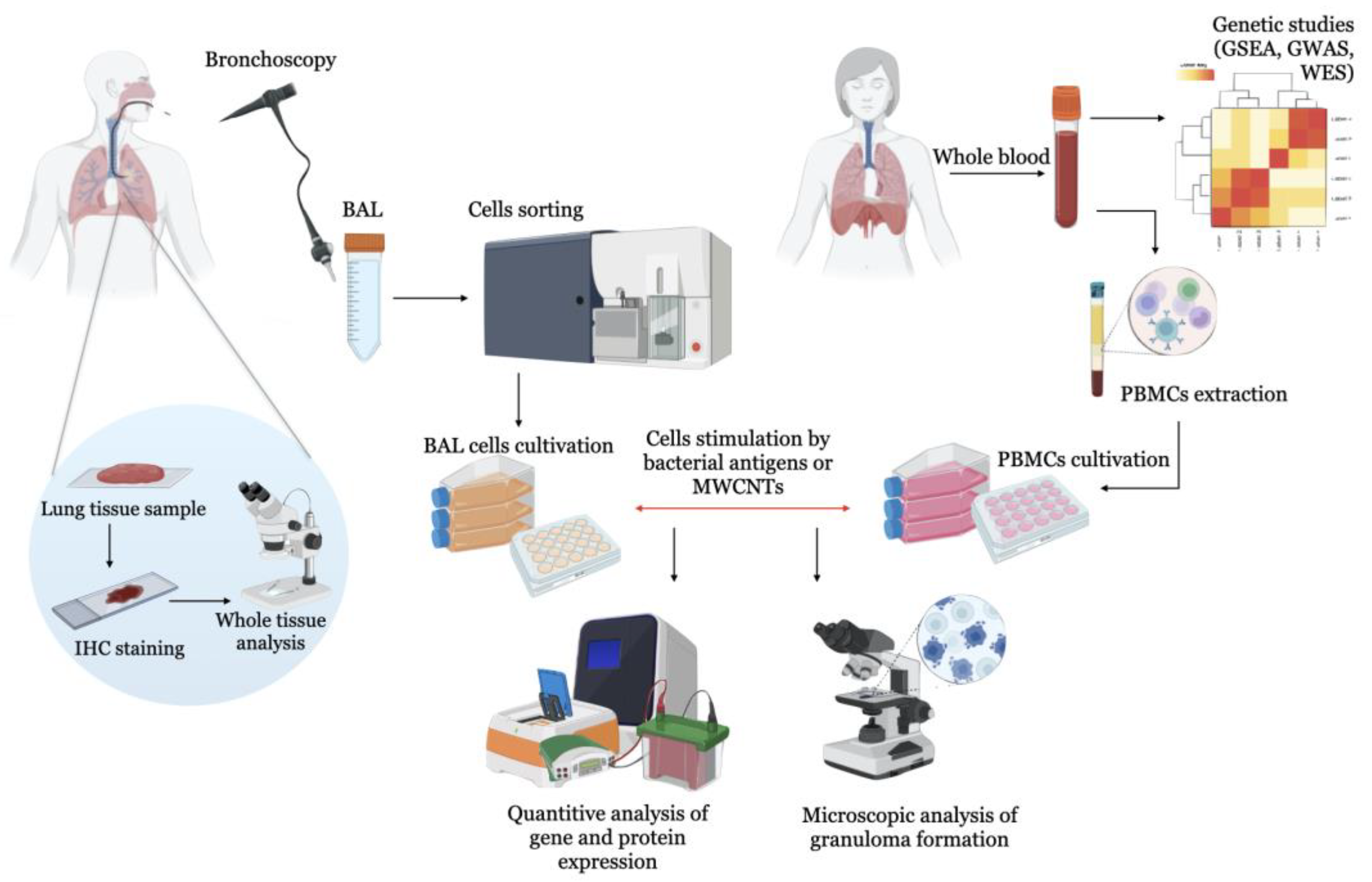
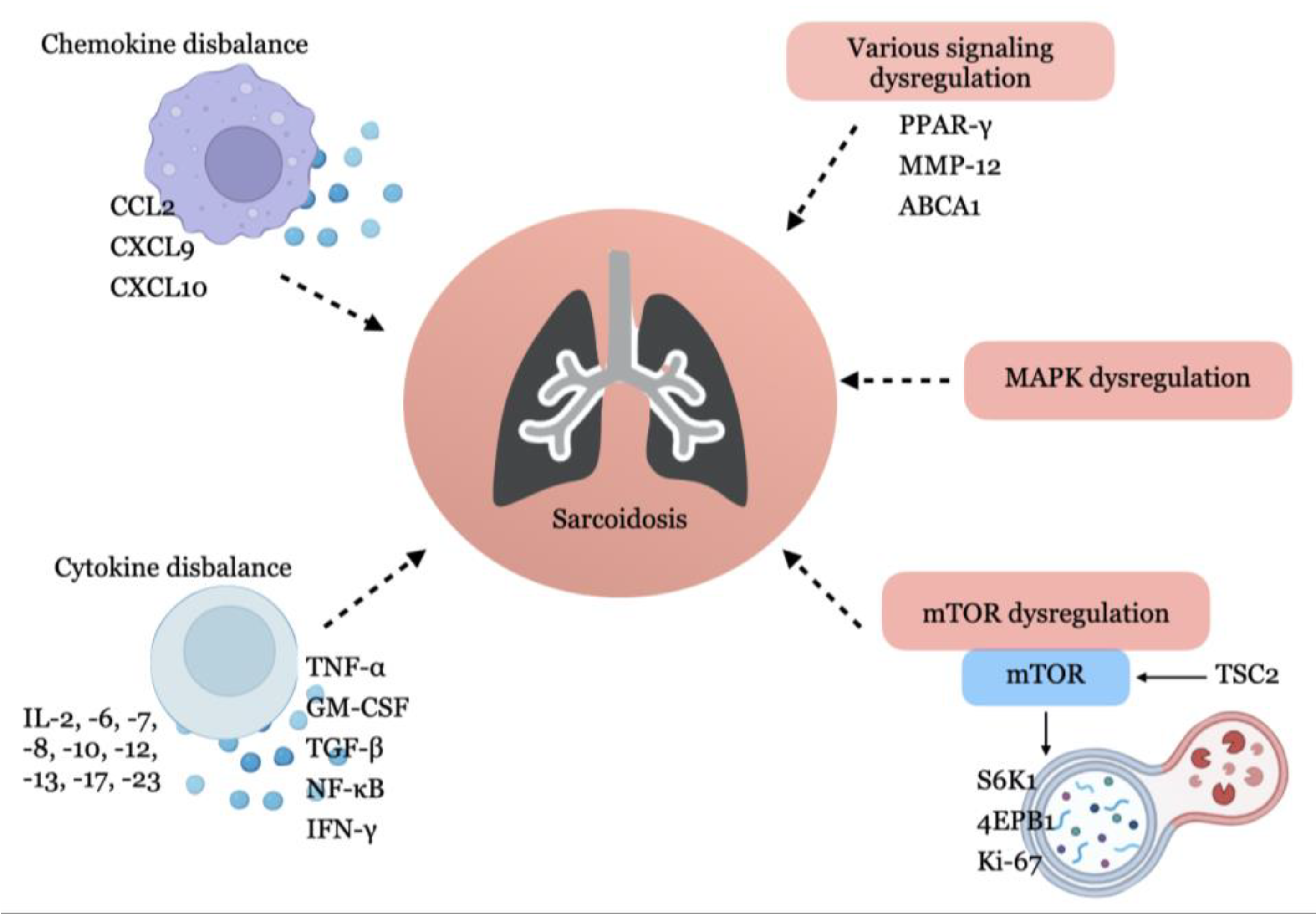
2. Experimental Models of Sarcoidosis
2.1. BAL Cell-Based Models
2.2. Combination of BAL Cell-Based Models and Computational Analysis Methods
2.3. Investigation of Peripheral Blood Mononuclears (PBMC)
2.4. Animal and Experimental Models of Sarcoidosis
2.5. Computational Models of Sarcoidosis
3. The Prerequisites for the Diagnosis of Sarcoidosis with Quantum Dots
3.1. In Vitro Diagnostics Using QDs
3.2. In Vivo Cell Imaging
3.3. Prospectivities for the Use of Quantum Dots in the Diagnosis of Sarcoidosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birnbaum, A.D.; Rifkin, L.M. Sarcoidosis: Sex-Dependent Variations in Presentation and Management. J. Ophthalmol. 2014, 2014, 236905. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A. Environmental Risk Factors for Sarcoidosis. Front. Immunol. 2020, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Sève, P.; Pacheco, Y.; Durupt, F.; Jamilloux, Y.; Gerfaud-Valentin, M.; Isaac, S.; Boussel, L.; Calender, A.; Androdias, G.; Valeyre, D.; et al. Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis. Cells 2021, 10, 766. [Google Scholar] [CrossRef]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Agarwal, R.; Aggarwal, A.N.; Jindal, S.K. Molecular Evidence for the Role of Mycobacteria in Sarcoidosis: A Meta-Analysis. Eur. Respir. J. 2007, 30, 508–516. [Google Scholar] [CrossRef]
- Fang, C.; Huang, H.; Xu, Z. Immunological Evidence for the Role of Mycobacteria in Sarcoidosis: A Meta-Analysis. PLoS ONE 2016, 11, e0154716. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Holownia, A.; Kalinowski, L.; Wybieralska, M.; Dobrucki, I.T.; Singh, M. Is Mycobacterial Heat Shock Protein 16 KDa, a Marker of the Dormant Stage of Mycobacterium Tuberculosis, a Sarcoid Antigen? Hum. Immunol. 2013, 74, 45–51. [Google Scholar] [CrossRef]
- Oswald-Richter, K.A.; Beachboard, D.C.; Zhan, X.; Gaskill, C.F.; Abraham, S.; Jenkins, C.; Culver, D.A.; Drake, W. Multiple Mycobacterial Antigens Are Targets of the Adaptive Immune Response in Pulmonary Sarcoidosis. Respir. Res. 2010, 11, 161. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Costabel, U.; McDowell, A.; Guzman, J.; Uchida, K.; Ohashi, K.; Eishi, Y. Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis. J. Clin. Med. 2021, 10, 983. [Google Scholar] [CrossRef]
- Starshinova, A.; Zinchenko, Y.; Malkova, A.; Kudlay, D.; Kudryavtsev, I.; Yablonskiy, P. Sarcoidosis and Autoimmune Inflammatory Syndrome Induced by Adjuvants. Life 2023, 13, 1047. [Google Scholar] [CrossRef]
- Iannuzzi, M.C. Genetics of Sarcoidosis. Semin. Respir. Crit. Care Med. 2007, 28, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Allebeck, P.; Mishra, G.D.; Koupil, I. Developmental Origins of Endometriosis: A Swedish Cohort Study. J. Epidemiol. Community Health 2019, 73, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, N.Y.; Maier, L.A.; Hamzeh, N.Y.; Maier, L.A. Genetics of Sarcoidosis; IntechOpen: London, UK, 2013; ISBN 978-953-51-1027-9. [Google Scholar]
- Jeny, F.; Pacheco, Y.; Besnard, V.; Valeyre, D.; Bernaudin, J.-F. Experimental Models of Sarcoidosis. Curr. Opin. Pulm. Med. 2016, 22, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Besnard, V.; Jeny, F. Models Contribution to the Understanding of Sarcoidosis Pathogenesis: “Are There Good Models of Sarcoidosis?”. J. Clin. Med. 2020, 9, 2445. [Google Scholar] [CrossRef]
- Wikén, M.; Idali, F.; Al Hayja, M.A.; Grunewald, J.; Eklund, A.; Wahlström, J. No Evidence of Altered Alveolar Macrophage Polarization, but Reduced Expression of TLR2, in Bronchoalveolar Lavage Cells in Sarcoidosis. Respir. Res. 2010, 11, 121. [Google Scholar] [CrossRef]
- Wojtan, P.; Mierzejewski, M.; Osińska, I.; Domagała-Kulawik, J. Macrophage Polarization in Interstitial Lung Diseases. Cent. Eur. J. Immunol. 2016, 41, 159–164. [Google Scholar] [CrossRef]
- Barna, B.P.; Huizar, I.; Malur, A.; McPeek, M.; Marshall, I.; Jacob, M.; Dobbs, L.; Kavuru, M.S.; Thomassen, M.J. Carbon Nanotube-Induced Pulmonary Granulomatous Disease: Twist1 and Alveolar Macrophage M1 Activation. Int. J. Mol. Sci. 2013, 14, 23858–23871. [Google Scholar] [CrossRef]
- McClain Caldwell, I.; Hogden, C.; Nemeth, K.; Boyajian, M.; Krepuska, M.; Szombath, G.; MacDonald, S.; Abshari, M.; Moss, J.; Vitale-Cross, L.; et al. Bone Marrow-Derived Mesenchymal Stromal Cells (MSCs) Modulate the Inflammatory Character of Alveolar Macrophages from Sarcoidosis Patients. J. Clin. Med. 2020, 9, 278. [Google Scholar] [CrossRef]
- Gabrilovich, M.I.; Walrath, J.; van Lunteren, J.; Nethery, D.; Seifu, M.; Kern, J.A.; Harding, C.V.; Tuscano, L.; Lee, H.; Williams, S.D.; et al. Disordered Toll-like Receptor 2 Responses in the Pathogenesis of Pulmonary Sarcoidosis. Clin. Exp. Immunol. 2013, 173, 512–522. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Janssen Bonás, M.; Vorselaars, A.D.M.; Veltkamp, M. Biomarkers in the Diagnosis and Prognosis of Sarcoidosis: Current Use and Future Prospects. Front. Immunol. 2020, 11, 1443. [Google Scholar] [CrossRef]
- Gharib, S.A.; Malur, A.; Huizar, I.; Barna, B.P.; Kavuru, M.S.; Schnapp, L.M.; Thomassen, M.J. Sarcoidosis Activates Diverse Transcriptional Programs in Bronchoalveolar Lavage Cells. Respir. Res. 2016, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Locke, L.W.; Schlesinger, L.S.; Crouser, E.D. Current Sarcoidosis Models and the Importance of Focusing on the Granuloma. Front. Immunol. 2020, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chery, S.; Lazerson, A.; Altman, N.H.; Jackson, R.; Holt, G.; Campos, M.; Schally, A.V.; Mirsaeidi, M. Anti-Inflammatory Effects of α-MSH through p-CREB Expression in Sarcoidosis like Granuloma Model. Sci. Rep. 2020, 10, 7277. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Sato, H.; Kawasaki, T.; Ishii, D.; Imamoto, T.; Abe, M.; Hasegawa, Y.; Ohara, O.; Tatsumi, K.; Suzuki, T. Transcriptome Analysis of Peripheral Blood Mononuclear Cells in Pulmonary Sarcoidosis. Front. Med. 2022, 9, 822094. [Google Scholar] [CrossRef]
- Newman, K.L.; Newman, L.S. Occupational Causes of Sarcoidosis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 145–150. [Google Scholar] [CrossRef]
- Kala, M.; Casanova, N.G.; Feng, A.; Jacobsen, J.R.; Grischo, G.; Liang, Y.; Wang, T.; Knox, K.S. Carbon Nanotube Stimulation of Human Mononuclear Cells to Model Granulomatous Inflammation. Am. J. Transl. Res. 2023, 15, 1704–1714. [Google Scholar]
- Sloet van Oldruitenborgh-Oosterbaan, M.M.; Grinwis, G.C.M. Equine Sarcoidosis: Clinical Signs, Diagnosis, Treatment and Outcome of 22 Cases. Vet. Dermatol. 2013, 24, 218–224.e48. [Google Scholar] [CrossRef]
- Chen, E.S.; Song, Z.; Willett, M.H.; Heine, S.; Yung, R.C.; Liu, M.C.; Groshong, S.D.; Zhang, Y.; Tuder, R.M.; Moller, D.R. Serum Amyloid A Regulates Granulomatous Inflammation in Sarcoidosis through Toll-like Receptor-2. Am. J. Respir. Crit. Care Med. 2010, 181, 360–373. [Google Scholar] [CrossRef]
- Swaisgood, C.M.; Oswald-Richter, K.; Moeller, S.D.; Klemenc, J.M.; Ruple, L.M.; Farver, C.F.; Drake, J.M.; Culver, D.A.; Drake, W.P. Development of a Sarcoidosis Murine Lung Granuloma Model Using Mycobacterium Superoxide Dismutase A Peptide. Am. J. Respir. Cell Mol. Biol. 2011, 44, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Kishi, J.; Nishioka, Y.; Kuwahara, T.; Kakiuchi, S.; Azuma, M.; Aono, Y.; Makino, H.; Kinoshita, K.; Kishi, M.; Batmunkh, R.; et al. Blockade of Th1 Chemokine Receptors Ameliorates Pulmonary Granulomatosis in Mice. Eur. Respir. J. 2011, 38, 415–424. [Google Scholar] [CrossRef]
- Nishiwaki, T.; Yoneyama, H.; Eishi, Y.; Matsuo, N.; Tatsumi, K.; Kimura, H.; Kuriyama, T.; Matsushima, K. Indigenous Pulmonary Propionibacterium Acnes Primes the Host in the Development of Sarcoid-like Pulmonary Granulomatosis in Mice. Am. J. Pathol. 2004, 165, 631–639. [Google Scholar] [CrossRef]
- Werner, J.L.; Escolero, S.G.; Hewlett, J.T.; Mak, T.N.; Williams, B.P.; Eishi, Y.; Núñez, G. Induction of Pulmonary Granuloma Formation by Propionibacterium Acnes Is Regulated by MyD88 and Nox2. Am. J. Respir. Cell Mol. Biol. 2017, 56, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, P.; Sadeghalvad, M.; Rezaei, N. An Inflammatory Triangle in Sarcoidosis: PPAR-γ, Immune Microenvironment, and Inflammation. Expert. Opin. Biol. Ther. 2021, 21, 1451–1459. [Google Scholar] [CrossRef]
- Huizar, I.; Malur, A.; Patel, J.; McPeek, M.; Dobbs, L.; Wingard, C.; Barna, B.P.; Thomassen, M.J. The Role of PPARγ in Carbon Nanotube-Elicited Granulomatous Lung Inflammation. Respir. Res. 2013, 14, 7. [Google Scholar] [CrossRef]
- McPeek, M.; Malur, A.; Tokarz, D.A.; Murray, G.; Barna, B.P.; Thomassen, M.J. PPAR-Gamma Pathways Attenuate Pulmonary Granuloma Formation in a Carbon Nanotube Induced Murine Model of Sarcoidosis. Biochem. Biophys. Res. Commun. 2018, 503, 684–690. [Google Scholar] [CrossRef] [PubMed]
- McPeek, M.; Malur, A.; Tokarz, D.A.; Lertpiriyapong, K.; Gowdy, K.M.; Murray, G.; Wingard, C.J.; Fessler, M.B.; Barna, B.P.; Thomassen, M.J. Alveolar Macrophage ABCG1 Deficiency Promotes Pulmonary Granulomatous Inflammation. Am. J. Respir. Cell Mol. Biol. 2019, 61, 332–340. [Google Scholar] [CrossRef]
- Barna, B.P.; McPeek, M.; Malur, A.; Fessler, M.B.; Wingard, C.J.; Dobbs, L.; Verbanac, K.M.; Bowling, M.; Judson, M.A.; Thomassen, M.J. Elevated MicroRNA-33 in Sarcoidosis and a Carbon Nanotube Model of Chronic Granulomatous Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Culver, D.A.; Knox, K.S.; Julian, M.W.; Shao, G.; Abraham, S.; Liyanarachchi, S.; Macre, J.E.; Wewers, M.D.; Gavrilin, M.A.; et al. Gene Expression Profiling Identifies MMP-12 and ADAMDEC1 as Potential Pathogenic Mediators of Pulmonary Sarcoidosis. Am. J. Respir. Crit. Care Med. 2009, 179, 929–938. [Google Scholar] [CrossRef]
- Mohan, A.; Neequaye, N.; Malur, A.; Soliman, E.; McPeek, M.; Leffler, N.; Ogburn, D.; Tokarz, D.A.; Knudson, W.; Gharib, S.A.; et al. Matrix Metalloproteinase-12 Is Required for Granuloma Progression. Front. Immunol. 2020, 11, 553949. [Google Scholar] [CrossRef]
- Barna, B.P.; Malur, A.; Thomassen, M.J. Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis. Int. J. Mol. Sci. 2021, 22, 3705. [Google Scholar] [CrossRef]
- Malur, A.; Barna, B.P.; Patel, J.; McPeek, M.; Wingard, C.J.; Dobbs, L.; Thomassen, M.J. Exposure to a Mycobacterial Antigen, ESAT-6, Exacerbates Granulomatous and Fibrotic Changes in a Multiwall Carbon Nanotube Model of Chronic Pulmonary Disease. J. Nanomed. Nanotechnol. 2015, 6, 340. [Google Scholar] [CrossRef]
- Malur, A.; Mohan, A.; Barrington, R.A.; Leffler, N.; Malur, A.; Muller-Borer, B.; Murray, G.; Kew, K.; Zhou, C.; Russell, J.; et al. Peroxisome Proliferator–Activated Receptor-γ Deficiency Exacerbates Fibrotic Response to Mycobacteria Peptide in Murine Sarcoidosis Model. Am. J. Respir. Cell Mol. Biol. 2019, 61, 198–208. [Google Scholar] [CrossRef]
- Linke, M.; Pham, H.T.T.; Katholnig, K.; Schnöller, T.; Miller, A.; Demel, F.; Schütz, B.; Rosner, M.; Kovacic, B.; Sukhbaatar, N.; et al. Chronic Signaling via the Metabolic Checkpoint Kinase MTORC1 Induces Macrophage Granuloma Formation and Marks Sarcoidosis Progression. Nat. Immunol. 2017, 18, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.; Lim, C.X.; Weichhart, T.; Valeyre, D.; Bentaher, A.; Calender, A. Sarcoidosis and the MTOR, Rac1, and Autophagy Triad. Trends Immunol. 2020, 41, 286–299. [Google Scholar] [CrossRef]
- Kishore, A.; Petersen, B.-S.; Nutsua, M.; Müller-Quernheim, J.; Franke, A.; Fischer, A.; Schreiber, S.; Petrek, M. Whole-Exome Sequencing Identifies Rare Genetic Variations in German Families with Pulmonary Sarcoidosis. Hum. Genet. 2018, 137, 705–716. [Google Scholar] [CrossRef]
- Calender, A.; Weichhart, T.; Valeyre, D.; Pacheco, Y. Current Insights in Genetics of Sarcoidosis: Functional and Clinical Impacts. J. Clin. Med. 2020, 9, 2633. [Google Scholar] [CrossRef]
- Pizzini, A.; Bacher, H.; Aichner, M.; Franchi, A.; Watzinger, K.; Tancevski, I.; Sonnweber, T.; Mosheimer-Feistritzer, B.; Duftner, C.; Zelger, B.; et al. High Expression of MTOR Signaling in Granulomatous Lesions Is Not Predictive for the Clinical Course of Sarcoidosis. Respir. Med. 2021, 177, 106294. [Google Scholar] [CrossRef] [PubMed]
- Manzia, T.M.; Bellini, M.I.; Corona, L.; Toti, L.; Fratoni, S.; Cillis, A.; Orlando, G.; Tisone, G. Successful Treatment of Systemic de Novo Sarcoidosis with Cyclosporine Discontinuation and Provision of Rapamune after Liver Transplantation. Transpl. Int. 2011, 24, e69–e70. [Google Scholar] [CrossRef]
- Albergante, L.; Timmis, J.; Beattie, L.; Kaye, P.M. A Petri Net Model of Granulomatous Inflammation: Implications for IL-10 Mediated Control of Leishmania Donovani Infection. PLoS Comput. Biol. 2013, 9, e1003334. [Google Scholar] [CrossRef] [PubMed]
- Sellares, J.; Strambu, I.; Crouser, E.D.; Freudenberg, M.A.; Gulati, M.; Hart, S.; Herzog, E.; Kolb, M.; Weichhart, T.; Drake, W.P.; et al. New Advances in the Development of Sarcoidosis Models: A Synopsis of a Symposium Sponsored by the Foundation for Sarcoidosis Research. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 2–4. [Google Scholar] [CrossRef]
- Hao, W.; Crouser, E.D.; Friedman, A. Mathematical Model of Sarcoidosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16065–16070. [Google Scholar] [CrossRef] [PubMed]
- Sieroń, A.; Sieroń-Stołtny, K.; Kawczyk-Krupka, A.; Latos, W.; Kwiatek, S.; Straszak, D.; Bugaj, A.M. The Role of Fluorescence Diagnosis in Clinical Practice. Onco Targets Ther. 2013, 6, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H.; Younis, M.A.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int. J. Nanomed. 2022, 17, 1951–1970. [Google Scholar] [CrossRef] [PubMed]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.S.; Shanavas, M.S. Revisiting the Cytotoxicity of Quantum Dots: An in-Depth Overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum Dots-Characterization, Preparation and Usage in Biological Systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef]
- Novikoff, A.B.; Novikoff, P.M.; Quintana, N.; Davis, C. Diffusion artifacts in 3,3’-diaminobenzidine cytochemistry. J. Histochem. Cytochem. 1972, 20, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Lemmer, B. HistoGreen: A New Alternative to 3,3’-Diaminobenzidine-Tetrahydrochloride-Dihydrate (DAB) as a Peroxidase Substrate in Immunohistochemistry? Brain Res. Brain Res. Protoc. 2005, 14, 107–118. [Google Scholar] [CrossRef]
- Vu, T.Q.; Lam, W.Y.; Hatch, E.W.; Lidke, D.S. Quantum Dots for Quantitative Imaging: From Single Molecules to Tissue. Cell Tissue Res. 2015, 360, 71–86. [Google Scholar] [CrossRef]
- Goldman, E.R.; Uyeda, H.T.; Hayhurst, A.; Mattoussi, H. Luminescent Biocompatible Quantum Dots: A Tool for Immunosorbent Assay Design. Methods Mol. Biol. 2007, 374, 207–227. [Google Scholar] [CrossRef]
- Xing, Y.; Chaudry, Q.; Shen, C.; Kong, K.Y.; Zhau, H.E.; Chung, L.W.; Petros, J.A.; O’Regan, R.M.; Yezhelyev, M.V.; Simons, J.W.; et al. Bioconjugated Quantum Dots for Multiplexed and Quantitative Immunohistochemistry. Nat. Protoc. 2007, 2, 1152–1165. [Google Scholar] [CrossRef]
- Akhtar, R.S.; Latham, C.B.; Siniscalco, D.; Fuccio, C.; Roth, K.A. Immunohistochemical Detection with Quantum Dots. Methods Mol. Biol. 2007, 374, 11–28. [Google Scholar] [CrossRef]
- Zheng, H.; Li, X.; Chen, C.; Chen, J.; Sun, J.; Sun, S.; Jin, L.; Li, J.; Sun, S.; Wu, X. Quantum Dot-Based Immunofluorescent Imaging and Quantitative Detection of TOP2A and Prognostic Value in Triple-Negative Breast Cancer. IJN 2016, 11, 5519–5529. [Google Scholar] [CrossRef]
- Kairdolf, B.A.; Smith, A.M.; Stokes, T.H.; Wang, M.D.; Young, A.N.; Nie, S. Semiconductor Quantum Dots for Bioimaging and Biodiagnostic Applications. Annu. Rev. Anal. Chem. 2013, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lau, S.K.; Varma, V.A.; Moffitt, R.A.; Caldwell, M.; Liu, T.; Young, A.N.; Petros, J.A.; Osunkoya, A.O.; Krogstad, T.; et al. Molecular Mapping of Tumor Heterogeneity on Clinical Tissue Specimens with Multiplexed Quantum Dots. ACS Nano 2010, 4, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-W.; Liu, X.-L.; Chen, C.; Liu, X.; Yang, X.-Q.; Pang, D.-W.; Zhu, X.-B.; Li, Y. Patterns of Cancer Invasion Revealed by QDs-Based Quantitative Multiplexed Imaging of Tumor Microenvironment. Biomaterials 2011, 32, 2907–2917. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, S.; Jiang, Q.; Lan, T.; Cheng, Z.; Liu, H. Small-Protein-Stabilized Semiconductor Nanoprobe for Targeted Imaging of Cancer Cells. Chembiochem 2016, 17, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, A.; Mizutani, A.; Takekoshi, S.; Itoh, J.; Okinaga, H.; Nishina, Y.; Takano, K.; Nagashima, T.; Osamura, R.Y.; Teramoto, A. Analyses of the Mechanism of Intracellular Transport and Secretion of Pituitary Hormone, with an Insight of the Subcellular Localization of Pituitary Hormone and Its MRNA. Brain Tumor Pathol. 2006, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, A.; Itoh, J.; Takekoshi, S.; Nagashima, T.; Osamura, R.Y. Three-Dimensional Imaging of the Intracellular Localization of Growth Hormone and Prolactin and Their MRNA Using Nanocrystal (Quantum Dot) and Confocal Laser Scanning Microscopy Techniques. J. Histochem. Cytochem. 2005, 53, 833–838. [Google Scholar] [CrossRef]
- Chen, H.; Xue, J.; Zhang, Y.; Zhu, X.; Gao, J.; Yu, B. Comparison of Quantum Dots Immunofluorescence Histochemistry and Conventional Immunohistochemistry for the Detection of Caveolin-1 and PCNA in the Lung Cancer Tissue Microarray. J. Mol. Histol. 2009, 40, 261–268. [Google Scholar] [CrossRef]
- Zacheo, A.; Quarta, A.; Mangoni, A.; Pompa, P.P.; Mastria, R.; Capogrossi, M.C.; Rinaldi, R.; Pellegrino, T. CdSe/CdS Semiconductor Quantum Rods as Robust Fluorescent Probes for Paraffin-Embedded Tissue Imaging. IEEE Trans. Nanobiosci. 2011, 10, 209–215. [Google Scholar] [CrossRef]
- Ornberg, R.L.; Liu, H. Immunofluorescent Labeling of Proteins in Cultured Cells with Quantum Dot Secondary Antibody Conjugates. Methods Mol. Biol. 2007, 374, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, L.; Rehberg, M.; Stoeger, T.; Zhang, J.; Chen, S. Applications and Immunological Effects of Quantum Dots on Respiratory System. Front. Immunol. 2021, 12, 795232. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Terada, N.; Saitoh, S.; Ohno, N.; Jin, T.; Ohno, S. Histochemical Analyses and Quantum Dot Imaging of Microvascular Blood Flow with Pulmonary Edema in Living Mouse Lungs by “in Vivo Cryotechnique”. Histochem. Cell Biol. 2012, 137, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, P.; Gao, D.; Zhang, Y.; Li, P.; Liu, L.; Wang, C.; Wang, H.; Ma, Y.; Cai, L. Noninvasive Visualization of Respiratory Viral Infection Using Bioorthogonal Conjugated Near-Infrared-Emitting Quantum Dots. ACS Nano 2014, 8, 5468–5477. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow Cytometry: Basic Principles and Applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef]
- Cabral Filho, P.E.; Pereira, M.I.A.; Fernandes, H.P.; de Thomaz, A.A.; Cesar, C.L.; Santos, B.S.; Barjas-Castro, M.L.; Fontes, A. Blood Group Antigen Studies Using CdTe Quantum Dots and Flow Cytometry. Int. J. Nanomed. 2015, 10, 4393–4404. [Google Scholar] [CrossRef]
- Chattopadhyay, P.K. Quantum Dot Technology in Flow Cytometry. Methods Cell Biol. 2011, 102, 463–477. [Google Scholar] [CrossRef]
- Jin, S.; Hu, Y.; Gu, Z.; Liu, L.; Wu, H.-C. Application of Quantum Dots in Biological Imaging. J. Nanomater. 2011, 2011, e834139. [Google Scholar] [CrossRef]
- Buranda, T.; Wu, Y.; Sklar, L.A. Quantum Dots for Quantitative Flow Cytometry. Methods Mol. Biol. 2011, 699, 67–84. [Google Scholar] [CrossRef]
- Drobintseva, A.O.; Matyushkin, L.B.; Aleksandrova, O.A.; Drobintsev, P.D.; Kvetnoi, I.M.; Mazing, D.S.; Moshnikov, V.A.; Polyakova, V.O.; Musikhin, S.F. Colloidal CdSe and ZnSe/Mn Quantum Dots: Their Cytotoxicity and Effects on Cell Morphology. St. Petersburg Polytech. Univ. J. Phys. Math. 2015, 1, 272–277. [Google Scholar] [CrossRef]
- Ho, C.-C.; Chang, H.; Tsai, H.-T.; Tsai, M.-H.; Yang, C.-S.; Ling, Y.-C.; Lin, P. Quantum Dot 705, a Cadmium-Based Nanoparticle, Induces Persistent Inflammation and Granuloma Formation in the Mouse Lung. Nanotoxicology 2013, 7, 105–115. [Google Scholar] [CrossRef] [PubMed]

| Experimental Model(s) | Marker | Function | Effects (Increased/Decreased) |
|---|---|---|---|
| BAL cells obtained from patients with sarcoidosis | TWIST1 | Transcription factor associated with macrophage polarisation | increased [18] |
| TNF-α | Pro-inflammatory cytokine | increased [21] | |
| CCL18 | Chemokine | ||
| CD163 | Scavenger–receptor, bacterial sensor | ||
| BM-MSCs co-cultivated with BAL cells obtained from patients with sarcoidosis (anti-inflammatory “reprogramming” BAL cells) | TNF-α | Pro-inflammatory cytokine | decreased [19] |
| IL-10 | Anti-inflammatory cytokine | increased [19] | |
| Serum obtained from patients with sarcoidosis | Lysozyme | Enzyme, serum marker of macrophage activation | increased [21] |
| sACE | Enzyme, sarcoidosis marker | ||
| SAA | Acute phase protein | ||
| sIL-2R | Soluble cytokine receptor | ||
| BAL cells stimulated by LPS or TNFa | TWIST1 | Transcription factor associated with macrophage polarisation | increased [18] |
| PBMCs stimulated by MWCNTs | CD68 | Monocyte-macrophages marker | increased [27] |
| CD3 | TCR co-receptor | ||
| PBMC obtained from patients with sarcoidosis | IFN–γ | Pro-inflammatory cytokine | increased [4] |
| PBMCs stimulated by MAB microparticles | IL-7 | Pro-inflammatory cytokine | increased, but decreased during α-MSH stimulation [24] |
| IL-7R | Pro-inflammatory cytokine receptor | ||
| IFN-γ | Pro-inflammatory cytokine | ||
| BAL obtained from C57BL/6 mice stimulated by P. acnes lysate | IFN-γ | Pro-inflammatory cytokine | increased [32] |
| BAL obtained from Mmp12-knocked out mice stimulated MWCNTs | IFN-γ | Pro-inflammatory cytokine | increased [41] |
| PPARγ | Nuclear receptor protein that functions as transcription factor | increased [41] | |
| BAL obtained from PPARγ-knocked out mice stimulated by P. acnes lysate | CCL2 (MCP-1) | Chemokine that regulates migration and infiltration of monocytes/macrophages | increased [36] |
| Osteopontin | Extracellular matrix protein expressed by osteoblastic and mesenchymal stem cells | increased [36] | |
| WT and PPARγ-knocked out mice stimulated by MWCNTs | MMP12 | Matrix metalloprotease | increased [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linkova, N.; Diatlova, A.; Zinchenko, Y.; Kornilova, A.; Snetkov, P.; Morozkina, S.; Medvedev, D.; Krasichkov, A.; Polyakova, V.; Yablonskiy, P. Pulmonary Sarcoidosis: Experimental Models and Perspectives of Molecular Diagnostics Using Quantum Dots. Int. J. Mol. Sci. 2023, 24, 11267. https://doi.org/10.3390/ijms241411267
Linkova N, Diatlova A, Zinchenko Y, Kornilova A, Snetkov P, Morozkina S, Medvedev D, Krasichkov A, Polyakova V, Yablonskiy P. Pulmonary Sarcoidosis: Experimental Models and Perspectives of Molecular Diagnostics Using Quantum Dots. International Journal of Molecular Sciences. 2023; 24(14):11267. https://doi.org/10.3390/ijms241411267
Chicago/Turabian StyleLinkova, Natalia, Anastasiia Diatlova, Yulia Zinchenko, Anastasiia Kornilova, Petr Snetkov, Svetlana Morozkina, Dmitrii Medvedev, Alexandr Krasichkov, Victoria Polyakova, and Piotr Yablonskiy. 2023. "Pulmonary Sarcoidosis: Experimental Models and Perspectives of Molecular Diagnostics Using Quantum Dots" International Journal of Molecular Sciences 24, no. 14: 11267. https://doi.org/10.3390/ijms241411267
APA StyleLinkova, N., Diatlova, A., Zinchenko, Y., Kornilova, A., Snetkov, P., Morozkina, S., Medvedev, D., Krasichkov, A., Polyakova, V., & Yablonskiy, P. (2023). Pulmonary Sarcoidosis: Experimental Models and Perspectives of Molecular Diagnostics Using Quantum Dots. International Journal of Molecular Sciences, 24(14), 11267. https://doi.org/10.3390/ijms241411267








