Role of TRPC3 in Right Ventricular Dilatation under Chronic Intermittent Hypoxia in 129/SvEv Mice
Abstract
1. Introduction
2. Results
2.1. Cardiac Function Measured by MRI
2.2. TRPC3 Deletion and Hypoxia Aggravated RV Function
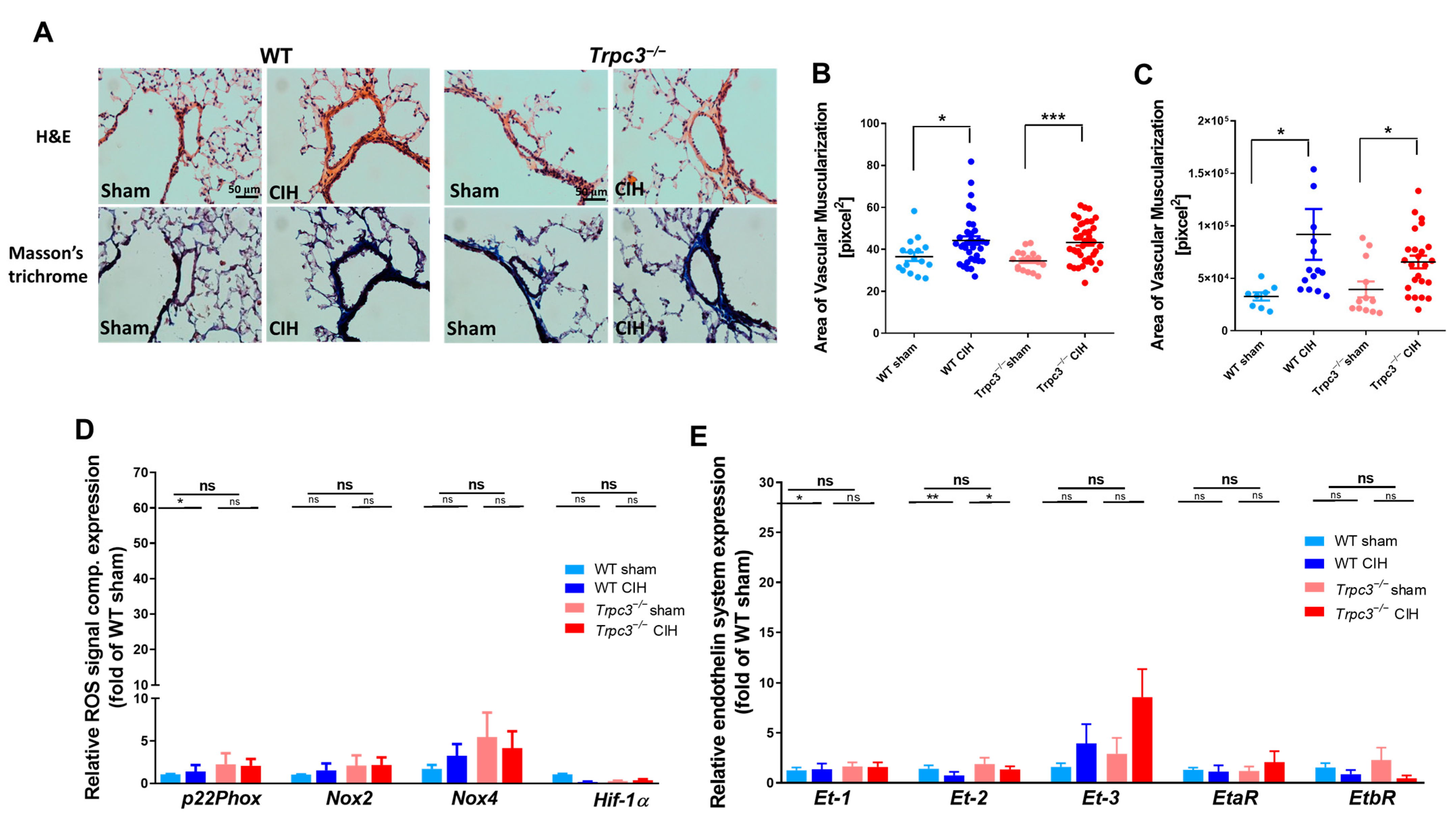
2.3. Changes in RV Function Were Not Influenced by Lung Vascularity
3. Discussion
4. Material and Methods
4.1. Experimental Animals and CIH Exposure
4.2. Measurement of Cardiac Function by MRI
4.3. Specimen Harvesting and Histological Analysis
4.4. RV Cardiacmyocyte Area Analysis
4.5. Pulmonary Vascular Morphology Analysis
4.6. Measuring mRNA Expression in the Heart and Lungs
4.7. Enzyme-Linked Immunosorbent Assay Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Minai, O.A.; Ricaurte, B.; Kaw, R.; Hammel, J.; Mansour, M.; McCarthy, K.; Golish, J.A.; Stoller, J.K. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am. J. Cardiol. 2009, 104, 1300–1306. [Google Scholar] [CrossRef]
- Javaheri, S.; Javaheri, S.; Javaheri, A. Sleep apnea, heart failure, and pulmonary hypertension. Curr. Heart Fail. Rep. 2013, 10, 315–320. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef]
- Bogaard, H.J.; Natarajan, R.; Henderson, S.C.; Long, C.S.; Kraskauskas, D.; Smithson, L.; Ockaili, R.; McCord, J.M.; Voelkel, N.F. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009, 120, 1951–1960. [Google Scholar] [CrossRef]
- Ryan, J.J.; Archer, S.L. The right ventricle in pulmonary arterial hypertension: Disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ. Res. 2014, 115, 176–188. [Google Scholar] [CrossRef]
- Maripov, A.; Mamazhakypov, A.; Sartmyrzaeva, M.; Akunov, A.; Muratali Uulu, K.; Duishobaev, M.; Cholponbaeva, M.; Sydykov, A.; Sarybaev, A. Right Ventricular Remodeling and Dysfunction in Obstructive Sleep Apnea: A Systematic Review of the Literature and Meta-Analysis. Can. Respir. J. 2017, 2017, 1587865. [Google Scholar] [CrossRef]
- Viswanathan, G.; Mamazhakypov, A.; Schermuly, R.T.; Rajagopal, S. The Role of G Protein-Coupled Receptors in the Right Ventricle in Pulmonary Hypertension. Front. Cardiovasc. Med. 2018, 5, 179. [Google Scholar] [CrossRef]
- Nagendran, J.; Sutendra, G.; Paterson, I.; Champion, H.C.; Webster, L.; Chiu, B.; Haromy, A.; Rebeyka, I.M.; Ross, D.B.; Michelakis, E.D. Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ. Res. 2013, 112, 347–354. [Google Scholar] [CrossRef]
- D’Orléans-Juste, P.; Labonté, J.; Bkaily, G.; Choufani, S.; Plante, M.; Honoré, J.C. Function of the endothelin(B) receptor in cardiovascular physiology and pathophysiology. Pharmacol. Ther. 2002, 95, 221–238. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, G.E.; Holewinski, R.J.; Venkatraman, V.; Zhu, G.; Bedja, D.; Kass, D.A.; Van Eyk, J.E. Transient receptor potential channel 6 regulates abnormal cardiac S-nitrosylation in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA 2017, 114, E10763–E10771. [Google Scholar] [CrossRef]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef]
- Wu, X.; Eder, P.; Chang, B.; Molkentin, J.D. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2010, 107, 7000–7005. [Google Scholar] [CrossRef]
- Nakayama, H.; Wilkin, B.J.; Bodi, I.; Molkentin, J.D. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006, 20, 1660–1670. [Google Scholar] [CrossRef]
- Onohara, N.; Nishida, M.; Inoue, R.; Kobayashi, H.; Sumimoto, H.; Sato, Y.; Mori, Y.; Nagao, T.; Kurose, H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006, 25, 5305–5316. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, Y.H.; Yoen, S.I.; Abramowitz, J.; Birnbaumer, L.; Lee, M.G.; Kim, J.Y. Resistance to pathologic cardiac hypertrophy and reduced expression of CaV1.2 in Trpc3-depleted mice. Mol. Cell. Biochem. 2016, 421, 55–65. [Google Scholar] [CrossRef]
- Huang, J.H.; He, G.W.; Xue, H.M.; Yao, X.Q.; Liu, X.C.; Underwood, M.J.; Yang, Q. TRPC3 channel contributes to nitric oxide release: Significance during normoxia and hypoxia-reoxygenation. Cardiovasc. Res. 2011, 91, 472–482. [Google Scholar] [CrossRef]
- Yeon, S.I.; Kim, J.Y.; Yeon, D.S.; Abramowitz, J.; Birnbaumer, L.; Muallem, S.; Lee, Y.H. Transient receptor potential canonical type 3 channels control the vascular contractility of mouse mesenteric arteries. PLoS ONE 2014, 9, e110413. [Google Scholar] [CrossRef]
- Carlson, J.T.; Rångemark, C.; Hedner, J.A. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J. Hypertens. 1996, 14, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Roberts-Thomson, P.; Phillips, B.G.; Haynes, W.G.; Winnicki, M.; Accurso, V.; Somers, V.K. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 2000, 102, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Siques, P.; López de Pablo, A.L.; Brito, J.; Arribas, S.M.; Flores, K.; Arriaza, K.; Naveas, N.; González, M.C.; Hoorntje, A.; León-Velarde, F.; et al. Nitric oxide and superoxide anion balance in rats exposed to chronic and long term intermittent hypoxia. BioMed Res. Int. 2014, 2014, 610474. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, B.; Song, D.; Ye, X.; Liu, S.F. Chronic intermittent hypoxia down-regulates endothelial nitric oxide synthase expression by an NF-κB-dependent mechanism. Sleep Med. 2013, 14, 165–171. [Google Scholar] [CrossRef]
- van Wolferen, S.A.; Marcus, J.T.; Westerhof, N.; Spreeuwenberg, M.D.; Marques, K.M.; Bronzwaer, J.G.; Henkens, I.R.; Gan, C.T.; Boonstra, A.; Postmus, P.E.; et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur. Heart J. 2008, 29, 120–127. [Google Scholar] [CrossRef]
- Lau, E.M.T.; Giannoulatou, E.; Celermajer, D.S.; Humbert, M. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef]
- Seo, K.; Rainer, P.P.; Shalkey Hahn, V.; Lee, D.I.; Jo, S.H.; Andersen, A.; Liu, T.; Xu, X.; Willette, R.N.; Lepore, J.J.; et al. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2014, 111, 1551–1556. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Lu, J.R.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef]
- Stewart, D.J.; Levy, R.D.; Cernacek, P.; Langleben, D. Increased plasma endothelin-1 in pulmonary hypertension: Marker or mediator of disease? Ann. Intern. Med. 1991, 114, 464–469. [Google Scholar] [CrossRef]
- Hirsch, L.J.; Rooney, M.W.; Wat, S.S.; Kleinmann, B.; Mathru, M. Norepinephrine and phenylephrine effects on right ventricular function in experimental canine pulmonary embolism. Chest 1991, 100, 796–801. [Google Scholar] [CrossRef]
- Hussain, M.B.; Marshall, I. Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br. J. Pharmacol. 1997, 122, 849–858. [Google Scholar] [CrossRef]
- Murdoch, C.E.; Zhang, M.; Cave, A.C.; Shah, A.M. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc. Res. 2006, 71, 208–215. [Google Scholar] [CrossRef]
- Kitajima, N.; Numaga-Tomita, T.; Watanabe, M.; Kuroda, T.; Nishimura, A.; Miyano, K.; Yasuda, S.; Kuwahara, K.; Sato, Y.; Ide, T.; et al. TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Sci. Rep. 2016, 6, 37001. [Google Scholar] [CrossRef]
- Shimauchi, T.; Numaga-Tomita, T.; Ito, T.; Nishimura, A.; Matsukane, R.; Oda, S.; Hoka, S.; Ide, T.; Koitabashi, N.; Uchida, K.; et al. TRPC3-Nox2 complex mediates doxorubicin-induced myocardial atrophy. JCI Insight 2017, 2, 93358. [Google Scholar] [CrossRef]
- He, X.; Du, T.; Long, T.; Liao, X.; Dong, Y.; Huang, Z.-P. Signaling cascades in the failing heart and emerging therapeutic strategies. Signal Transduct. Target. Ther. 2022, 7, 134. [Google Scholar] [CrossRef]
- Steppan, J.; Jandu, S.; Wang, H.; Kang, S.; Savage, W.; Narayanan, R.; Nandakumar, K.; Santhanam, L. Commonly used mouse strains have distinct vascular properties. Hypertens. Res. 2020, 43, 1175–1181. [Google Scholar] [CrossRef]
- Nootens, M.; Kaufmann, E.; Rector, T.; Toher, C.; Judd, D.; Francis, G.S.; Rich, S. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: Relation to hemodynamic variables and endothelin levels. J. Am. Coll. Cardiol. 1995, 26, 1581–1585. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef]
- Hölscher, M.; Schäfer, K.; Krull, S.; Farhat, K.; Hesse, A.; Silter, M.; Lin, Y.; Pichler, B.J.; Thistlethwaite, P.; El-Armouche, A.; et al. Unfavourable consequences of chronic cardiac HIF-1α stabilization. Cardiovasc. Res. 2012, 94, 77–86. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Trpc3 | 5′-GCCATTGCCAGTGTGATCTA-3′ | 5′-AGGTTGGAGGCACCATCAA-3′ |
| Trpc1 | 5′-GAATCGCGTAACCAGCTCAG-3′ | 5′-AGTGGGCCCAAAATAGAGCT-3′ |
| Trpc6 | 5′-TCTCGAGTTGGGGATGCTTT-3′ | 5′-GCGAGAATGATTGGGGTCAC-3′ |
| p22Phox | 5′- GCCATTGCCAGTGTGATCTA -3′ | 5′-AGGTTGGAGGCACCATCAA-3′ |
| Nox2 | 5′-TTCCAGTGCGTGTTGCTCGAC-3′ | 5′-GATGGCGGTGTGCAGTGCTAT-3′ |
| Nox4 | 5′-GGA TCA CAG AAG GTC CCT AGC AG-3′ | 5′-GCG GCT ACA TGC ACA CCT GAG AA-3′ |
| Hif-1α | 5′-CAGTACAGGATGCTTGCCAAAA-3′ | 5′-ATACCACTTACAACATAATTCACACACACA-3′ |
| Et-1 | 5′-CTGCTGTTC GTGACTTTCCA-3′ | 5′-AGCTCCGGTGCTGAGTTC-3′ |
| Et-2 | 5′-TGCGTTTT CGTCGATGCTC-3′ | 5′-CTGTCTGTCCCGCAGTGTTCA-3′ |
| Et-3 | 5′-TGGAC ACGCTTGCGTTGTACT-3′ | 5′-CGGAATAACTGGTGACATCTCTGG-3′ |
| EtγA | 5′-GAGGCGTAATGGCTGACAAT-3′ | 5′-GTGGTGCCCAGAAAGTTGAT-3′ |
| EtγB | 5′-CTCTGTTGGCTTCCCCTTC-3′ | 5′-CGATTGGATTGATGCAGGA-3′ |
| Anp | 5′-CCTCGTCTTGGCCTTTTGGCT-3′ | 5′-CCTCCAGGTGGTCTAGCAGGTTC-3′ |
| Bnp | 5′-AAGTCCTAGCCAGTCTCCAGA-3′ | 5′-CTGCCTTGAGACCGAAGG-3′ |
| β-Mhc | 5′-AGATGTTTTTGTGCCCGATGACA-3′ | 5′-CACCGTCTTGCCATTCTCCGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.-Y.; Heo, W.; Kang, M.; Ahn, T.; Kim, D.; Choi, A.; Birnbaumer, L.; Cho, H.-J.; Kim, J.Y. Role of TRPC3 in Right Ventricular Dilatation under Chronic Intermittent Hypoxia in 129/SvEv Mice. Int. J. Mol. Sci. 2023, 24, 11284. https://doi.org/10.3390/ijms241411284
Park D-Y, Heo W, Kang M, Ahn T, Kim D, Choi A, Birnbaumer L, Cho H-J, Kim JY. Role of TRPC3 in Right Ventricular Dilatation under Chronic Intermittent Hypoxia in 129/SvEv Mice. International Journal of Molecular Sciences. 2023; 24(14):11284. https://doi.org/10.3390/ijms241411284
Chicago/Turabian StylePark, Do-Yang, Woon Heo, Miran Kang, Taeyoung Ahn, DoHyeon Kim, Ayeon Choi, Lutz Birnbaumer, Hyung-Ju Cho, and Joo Young Kim. 2023. "Role of TRPC3 in Right Ventricular Dilatation under Chronic Intermittent Hypoxia in 129/SvEv Mice" International Journal of Molecular Sciences 24, no. 14: 11284. https://doi.org/10.3390/ijms241411284
APA StylePark, D.-Y., Heo, W., Kang, M., Ahn, T., Kim, D., Choi, A., Birnbaumer, L., Cho, H.-J., & Kim, J. Y. (2023). Role of TRPC3 in Right Ventricular Dilatation under Chronic Intermittent Hypoxia in 129/SvEv Mice. International Journal of Molecular Sciences, 24(14), 11284. https://doi.org/10.3390/ijms241411284






