Role of TRP Channels in Liver-Related Diseases
Abstract
1. Introduction
2. Advances in the Treatment of Liver Diseases
3. Expression and Function of TRP Channels in Different Types of Cells in the Liver Tissue
3.1. TRP Channels in Hepatocytes
3.2. TRP Channels in HSCs
3.3. TRP Channels in KCs
3.4. TRP Channels in Endothelial Cells
4. Effects of TRP Channels on the Occurrence and Progression of Liver Diseases
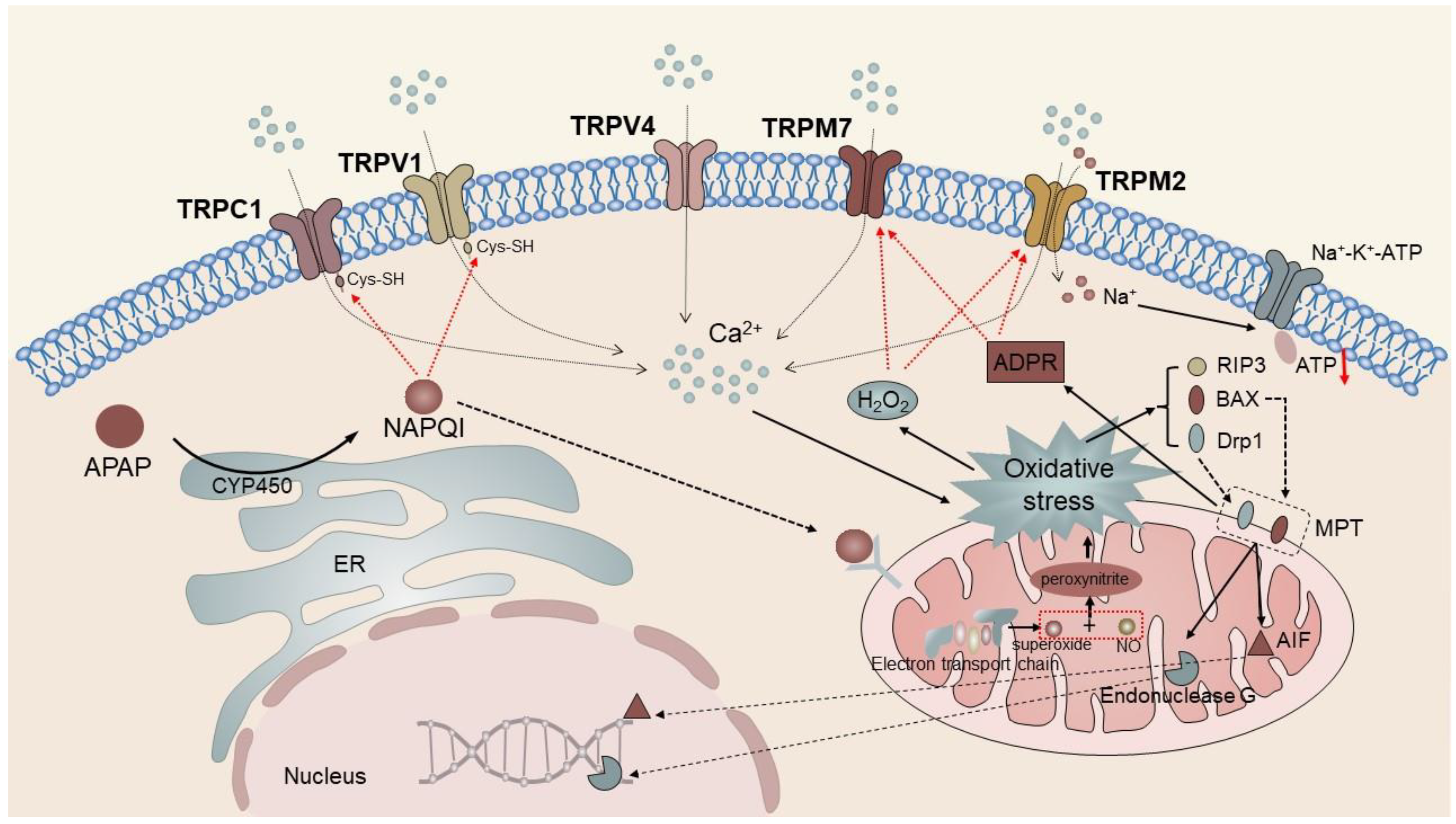
4.1. TRP Channels in Liver Injury
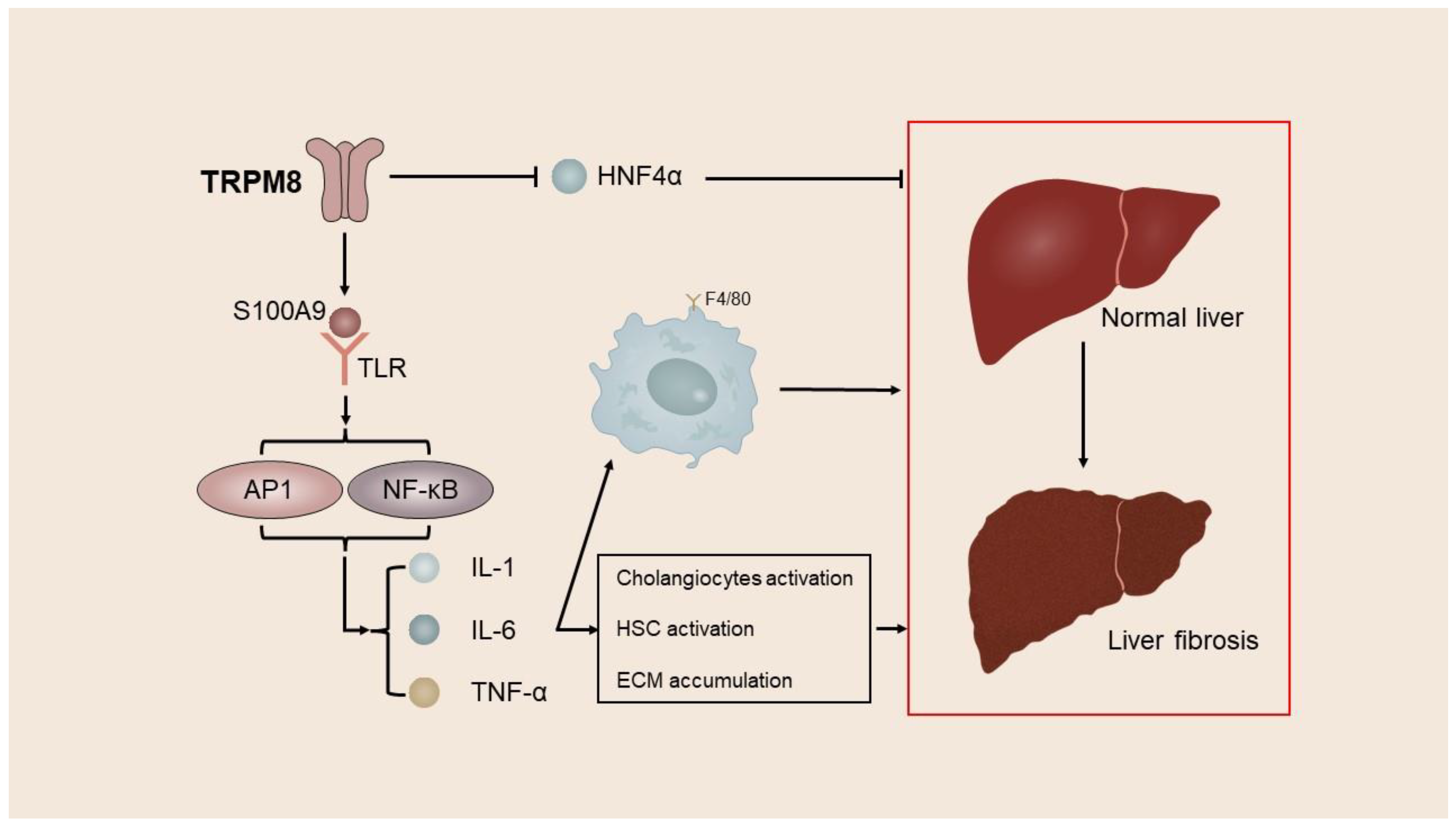
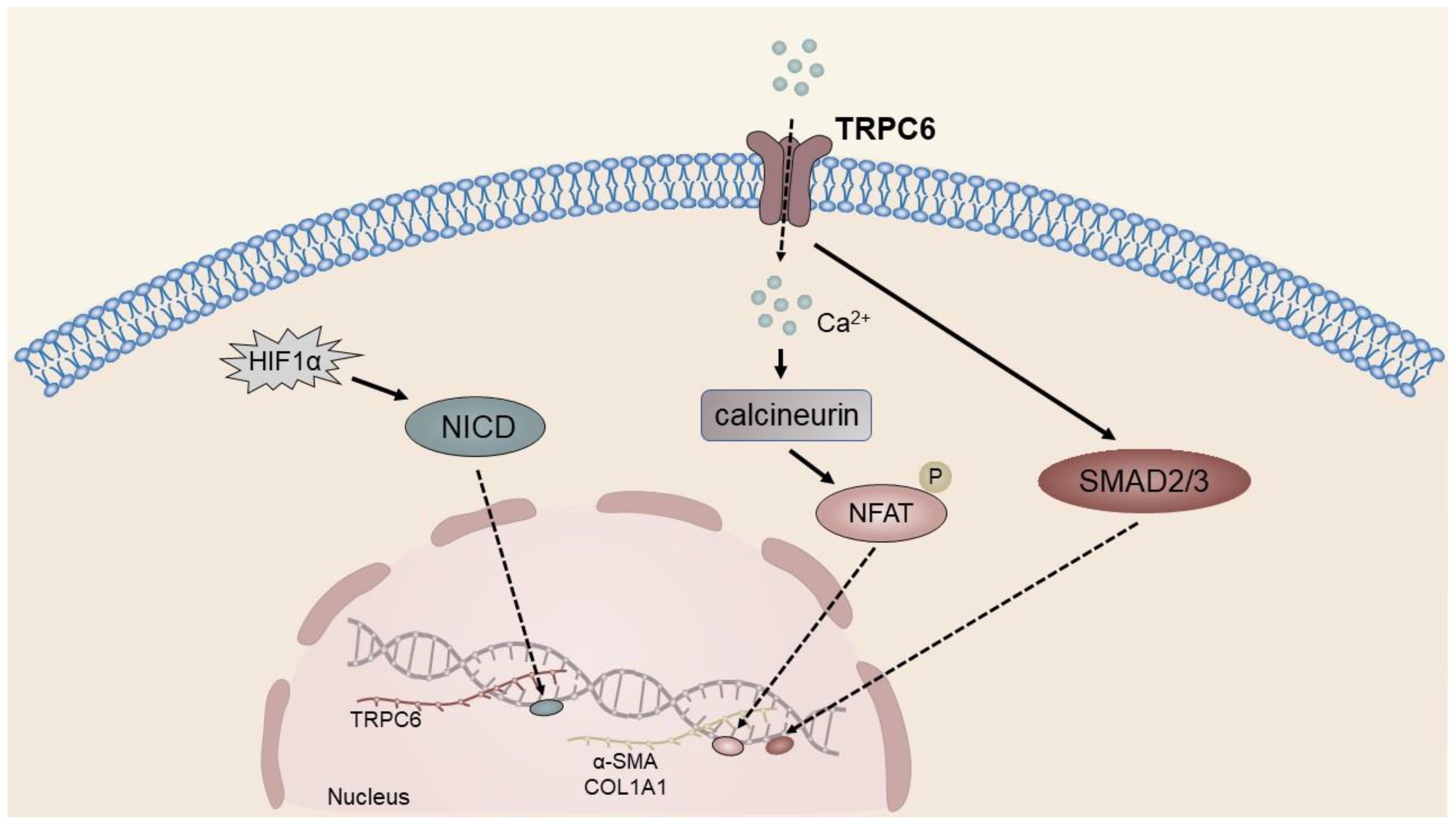
4.2. TRP Channels in Liver Fibrosis
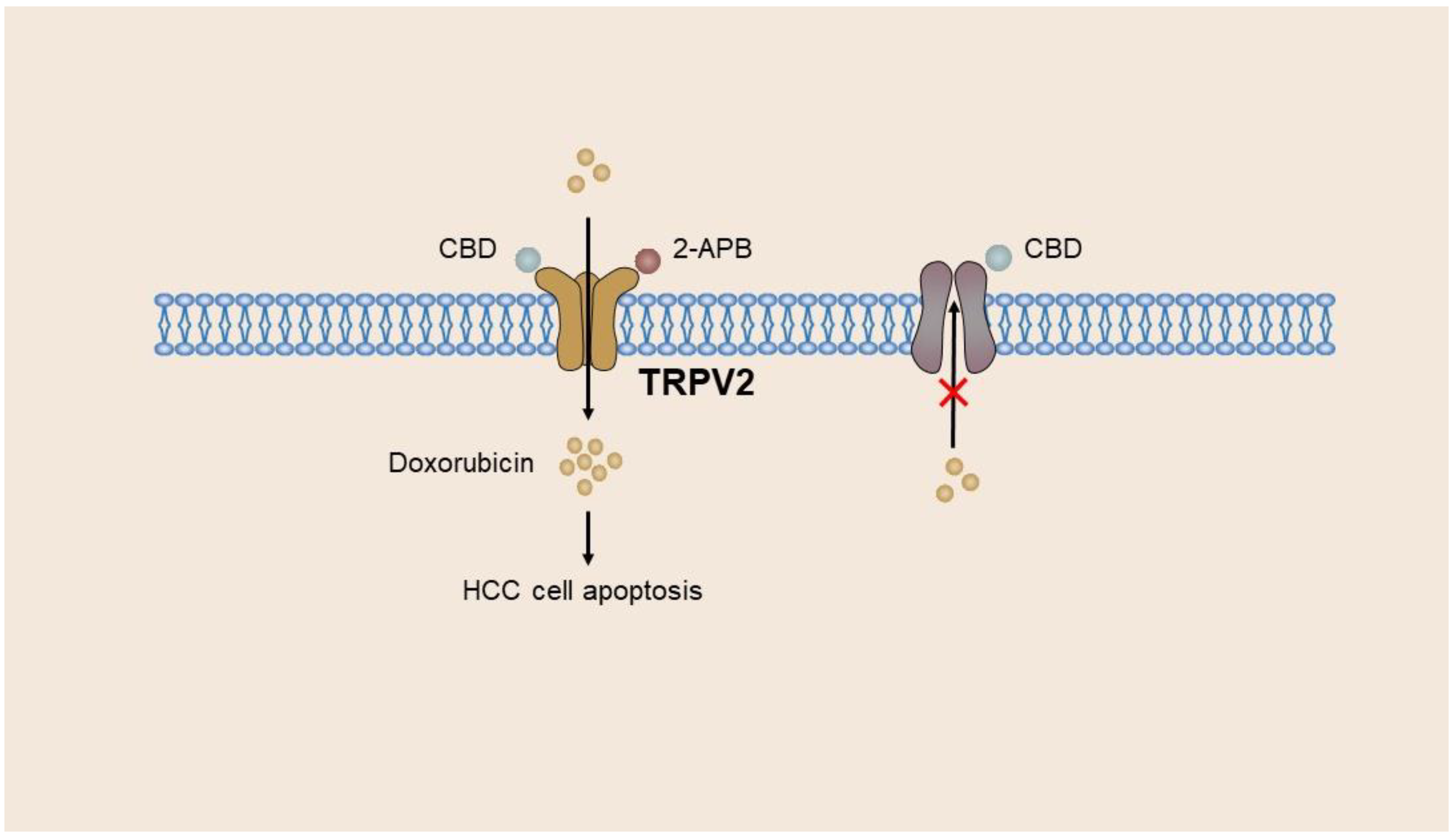
4.3. TRP Channels in Liver Cancer
4.3.1. TRPC
4.3.2. TRPV
4.3.3. TRPM
| Liver Diseases | TRP Channels | Function | Ref. |
|---|---|---|---|
| Hepatic injury | TRPV1, TRPV4, TRPC1, TRPM2, TRPM7 | Enhance the inflammatory response after hepatocyte injury | [44,45,89,91] |
| Hepatic fibrosis | TRPM7, TRPM8, TRPV3, TRPV4, TRPC6 | Promote the activation and proliferation of HSCs and aggravate liver fibrosis | [46,57,59,76,104,140,168] |
| HCC | TRPC1, TRPC6, TRPV6, TRPV4, TRPM7 | Promote the progression of HCC | [40,47,148,153,159,167,169] |
| TRPC5, TRPV1, TRPV2 | Reduce the aggressiveness of tumor cells | [52,55,66,67,68] |
4.4. Drugs for TRP Channels
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Mandato, C.; Di Nuzzi, A.; Vajro, P. Nutrition and Liver Disease. Nutrients 2018, 10, 9. [Google Scholar] [CrossRef]
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- El-Kassas, M.; Cabezas, J.; Coz, P.I.; Zheng, M.; Arab, J.P.; Awad, A. Nonalcoholic Fatty Liver Disease: Current Global Burden. Semin. Liver Dis. 2022, 42, 401–412. [Google Scholar] [CrossRef]
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333. [Google Scholar] [CrossRef]
- Perz, J.F.; Armstrong, G.L.; Farrington, L.A.; Hutin, Y.J.; Bell, B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006, 45, 529–538. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Li, H. TRP Channel Classification. Adv. Exp. Med. Biol. 2017, 976, 1–8. [Google Scholar] [CrossRef]
- Samanta, A.; Hughes, T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef]
- Hardie, R.C. A brief history of trp: Commentary and personal perspective. Pflug. Arch. 2011, 461, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Gees, M.; Owsianik, G.; Nilius, B.; Voets, T. TRP channels. Compr. Physiol. 2012, 2, 563–608. [Google Scholar] [CrossRef]
- Alaimo, A.; Lorenzoni, M.; Ambrosino, P.; Bertossi, A.; Bisio, A.; Macchia, A.; Zoni, E.; Genovesi, S.; Cambuli, F.; Foletto, V.; et al. Calcium cytotoxicity sensitizes prostate cancer cells to standard-of-care treatments for locally advanced tumors. Cell Death Dis. 2020, 11, 1039. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, F.; Du, S.; Li, M.; Wu, T.; Tan, X.; Cheng, W. Mechanism for Regulation of Melanoma Cell Death via Activation of Thermo-TRPV4 and TRPV2. J. Oncol. 2019, 2019, 7362875. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zeng, Y.; Zhu, J.; Chen, H.; Wei, X.; Liu, L. TRPC1 inhibits the proliferation and migration of estrogen receptor-positive Breast cancer and gives a better prognosis by inhibiting the PI3K/AKT pathway. Breast Cancer Res. Treat. 2020, 182, 21–33. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Moran, M.M.; Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. Br. J. Pharmacol. 2018, 175, 2185–2203. [Google Scholar] [CrossRef]
- Rychkov, G.Y.; Barritt, G.J. Expression and function of TRP channels in liver cells. Adv. Exp. Med. Biol. 2011, 704, 667–686. [Google Scholar] [CrossRef]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef]
- D’Amico, G.; Morabito, A.; D’Amico, M.; Pasta, L.; Malizia, G.; Rebora, P.; Valsecchi, M.G. Clinical states of cirrhosis and competing risks. J. Hepatol. 2018, 68, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Resolution of liver fibrosis: Basic mechanisms and clinical relevance. Semin. Liver Dis. 2015, 35, 119–131. [Google Scholar] [CrossRef]
- Jung, Y.K.; Yim, H.J. Reversal of liver cirrhosis: Current evidence and expectations. Korean J. Intern. Med. 2017, 32, 213–228. [Google Scholar] [CrossRef]
- Kim, Y.O.; Popov, Y.; Schuppan, D. Optimized Mouse Models for Liver Fibrosis. Methods Mol. Biol. 2017, 1559, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Van de Bovenkamp, M.; Groothuis, G.M.; Meijer, D.K.; Olinga, P. Liver fibrosis in vitro: Cell culture models and precision-cut liver slices. Toxicol. Vitro 2007, 21, 545–557. [Google Scholar] [CrossRef]
- van Grunsven, L.A. 3D in vitro models of liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 133–146. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Levrero, M.; Subic, M.; Villeret, F.; Zoulim, F. Perspectives and limitations for nucleo(t)side analogs in future HBV therapies. Curr. Opin. Virol. 2018, 30, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Aguilar, S.R.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Duval, F.; Moreno-Cuevas, J.E.; Gonzalez-Garza, M.T.; Maldonado-Bernal, C.; Cruz-Vega, D.E. Liver fibrosis and mechanisms of the protective action of medicinal plants targeting inflammation and the immune response. Int. J. Inflamm. 2015, 2015, 943497. [Google Scholar] [CrossRef] [PubMed]
- Latief, U.; Ahmad, R. Herbal remedies for liver fibrosis: A review on the mode of action of fifty herbs. J. Tradit. Complement. Med. 2018, 8, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Neong, S.F.; Adebayo, D.; Wong, F. An update on the pathogenesis and clinical management of cirrhosis with refractory ascites. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Zhu, Q.; Durham, J.; Popovic, A.; Xavier, S.; Leatherman, J.; Mohan, A.; Mo, G.; Zhang, S.; Gross, N.; et al. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat. Cancer 2021, 2, 891–903. [Google Scholar] [CrossRef]
- Jin, H.; Shi, Y.; Lv, Y.; Yuan, S.; Ramirez, C.F.A.; Lieftink, C.; Wang, L.; Wang, S.; Wang, C.; Dias, M.H.; et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021, 595, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef]
- Kim, R.D.; Sarker, D.; Meyer, T.; Yau, T.; Macarulla, T.; Park, J.; Choo, S.P.; Hollebecque, A.; Sung, M.W.; Lim, H.; et al. First-in-Human Phase I Study of Fisogatinib (BLU-554) Validates Aberrant FGF19 Signaling as a Driver Event in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1696–1707. [Google Scholar] [CrossRef]
- Wang, C.; Vegna, S.; Jin, H.; Benedict, B.; Lieftink, C.; Ramirez, C.; de Oliveira, R.L.; Morris, B.; Gadiot, J.; Wang, W.; et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 2019, 574, 268–272. [Google Scholar] [CrossRef]
- Rudalska, R.; Harbig, J.; Snaebjornsson, M.T.; Klotz, S.; Zwirner, S.; Taranets, L.; Heinzmann, F.; Kronenberger, T.; Forster, M.; Cui, W.; et al. LXRalpha activation and Raf inhibition trigger lethal lipotoxicity in liver cancer. Nat. Cancer 2021, 2, 201–217. [Google Scholar] [CrossRef]
- Anand, P.; Filipenko, P.; Huaman, J.; Lyudmer, M.; Hossain, M.; Santamaria, C.; Huang, K.; Ogunwobi, O.O.; Holford, M. Selective Inhibition of Liver Cancer Cells Using Venom Peptide. Mar. Drugs 2019, 17, 587. [Google Scholar] [CrossRef]
- Lang, F.; Foller, M. Regulation of ion channels and transporters by AMP-activated kinase (AMPK). Channels 2014, 8, 20–28. [Google Scholar] [CrossRef]
- Fiorio, P.A.; Gkika, D. Emerging role of TRP channels in cell migration: From tumor vascularization to metastasis. Front. Physiol. 2013, 4, 311. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, Y.; Xie, J.; Jiang, J.; Yue, L. Transient receptor potential (TRP) channels and cardiac fibrosis. Curr. Top. Med. Chem. 2013, 13, 270–282. [Google Scholar] [CrossRef]
- Wang, W.; Liu, P.; Zhang, Y.; Yan, L.; Zhu, M.X.; Wang, J.; Yu, Y. Expression and functions of transient receptor potential channels in liver diseases. Acta Pharm. Sin. B 2023, 13, 445–459. [Google Scholar] [CrossRef]
- Badr, H.; Kozai, D.; Sakaguchi, R.; Numata, T.; Mori, Y. Different Contribution of Redox-Sensitive Transient Receptor Potential Channels to Acetaminophen-Induced Death of Human Hepatoma Cell Line. Front. Pharmacol. 2016, 7, 26903865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lei, X.; Cao, Z.; Zhang, J.; Yan, L.; Fu, J.; Tong, Q.; Qin, W.; Shao, Y.; Liu, C.; et al. TRPM8 deficiency attenuates liver fibrosis through S100A9-HNF4α signaling. Cell Biosci. 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, Y.; Xie, R.; Liu, J.; Nie, X.; An, J.; Wen, G.; Liu, X.; Jin, H.; Tuo, B. The NCX1/TRPC6 Complex Mediates TGFbeta-Driven Migration and Invasion of Human Hepatocellular Carcinoma Cells. Cancer Res. 2018, 78, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Waning, J.; Vriens, J.; Owsianik, G.; Stüwe, L.; Mally, S.; Fabian, A.; Frippiat, C.; Nilius, B.; Schwab, A. A novel function of capsaicin-sensitive TRPV1 channels: Involvement in cell migration. Cell Calcium 2007, 42, 17–25. [Google Scholar] [CrossRef]
- Liu, H.; Beier, J.I.; Arteel, G.E.; Ramsden, C.E.; Feldstein, A.E.; McClain, C.J.; Kirpich, I.A. Transient receptor potential vanilloid 1 gene deficiency ameliorates hepatic injury in a mouse model of chronic binge alcohol-induced alcoholic liver disease. Am. J. Pathol. 2015, 185, 43–54. [Google Scholar] [CrossRef]
- Stokłosa, P.; Borgström, A.; Kappel, S.; Peinelt, C. TRP Channels in Digestive Tract Cancers. Int. J. Mol. Sci. 2020, 21, 1877. [Google Scholar] [CrossRef]
- Su, K.H.; Lin, S.J.; Wei, J.; Lee, K.I.; Zhao, J.F.; Shyue, S.K.; Lee, T.S. The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis. Acta Physiol. 2014, 212, 191–204. [Google Scholar] [CrossRef]
- Liu, G.; Xie, C.; Sun, F.; Xu, X.; Yang, Y.; Zhang, T.; Deng, Y.; Wang, D.; Huang, Z.; Yang, L.; et al. Clinical significance of transient receptor potential vanilloid 2 expression in human hepatocellular carcinoma. Cancer Genet. Cytogenet. 2010, 197, 54–59. [Google Scholar] [CrossRef]
- Ma, W.; Li, C.; Yin, S.; Liu, J.; Gao, C.; Lin, Z.; Huang, R.; Huang, J.; Li, Z. Novel role of TRPV2 in promoting the cytotoxicity of H2O2-mediated oxidative stress in human hepatoma cells. Free Radic. Biol. Med. 2015, 89, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cao, X.; Fang, Y.; Liu, G.; Xie, C.; Qian, K.; Lei, X.; Cao, Z.; Du, H.; Cheng, X.; et al. Transient receptor potential vanilloid-type 2 targeting on stemness in liver cancer. Biomed. Pharmacother. 2018, 105, 697–706. [Google Scholar] [CrossRef]
- Neumann-Raizel, H.; Shilo, A.; Lev, S.; Mogilevsky, M.; Katz, B.; Shneor, D.; Shaul, Y.D.; Leffler, A.; Gabizon, A.; Karni, R.; et al. 2-APB and CBD-Mediated Targeting of Charged Cytotoxic Compounds Into Tumor Cells Suggests the Involvement of TRPV2 Channels. Front. Pharmacol. 2019, 10, 1198. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Nagase, M.; Fujita, T.; Narumiya, S.; Masaki, T.; Sawamura, T. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: Possible role of LOX-1 ligand and AGE. Biochem. Biophys. Res. Commun. 2001, 287, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, X.; Fu, J.; Liu, Q.; Lei, X.; Cao, Z.; Zhang, J.; Shao, Y.; Tong, Q.; Qin, W.; et al. Inhibition of the transient receptor potential vanilloid 3 channel attenuates carbon tetrachloride-induced hepatic fibrosis. Biochem. Biophys. Res. Commun. 2021, 558, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Janssens, A.; Prenen, J.; Nilius, B.; Wondergem, R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 2004, 36, 19–28. [Google Scholar] [CrossRef]
- Zhan, L.; Yang, Y.; Ma, T.; Huang, C.; Meng, X.; Zhang, L.; Li, J. Transient receptor potential vanilloid 4 inhibits rat HSC-T6 apoptosis through induction of autophagy. Mol. Cell. Biochem. 2015, 402, 9–22. [Google Scholar] [CrossRef]
- Moccia, F. Endothelial Ca(2+) Signaling and the Resistance to Anticancer Treatments: Partners in Crime. Int. J. Mol. Sci. 2018, 19, 217. [Google Scholar] [CrossRef]
- Brereton, H.M.; Chen, J.; Rychkov, G.; Harland, M.L.; Barritt, G.J. Maitotoxin activates an endogenous non-selective cation channel and is an effective initiator of the activation of the heterologously expressed hTRPC-1 (transient receptor potential) non-selective cation channel in H4-IIE liver cells. Biochim. Biophys. Acta 2001, 1540, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Du, L.L.; Shen, Z.; Li, Z.; Ye, X.; Wu, M.; Hong, L.; Zhao, Y. TRPC1 Deficiency Impairs the Endothelial Progenitor Cell Function via Inhibition of Calmodulin/eNOS Pathway. J. Cardiovasc. Transl. Res. 2018, 11, 339–345. [Google Scholar] [CrossRef]
- Liu, H.; Nan, B.; Yang, C.; Li, X.; Yan, H.; Yuan, Y. Elaidic acid induced NLRP3 inflammasome activation via ERS-MAPK signaling pathways in Kupffer cells. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2022, 1867, 159061. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, P.; Eccles, S.A.; Yaqoob, M.M. Coupling between the TRPC3 ion channel and the NCX1 transporter contributed to VEGF-induced ERK1/2 activation and angiogenesis in human primary endothelial cells. Cell. Signal. 2017, 37, 12–30. [Google Scholar] [CrossRef]
- Song, H.B.; Jun, H.O.; Kim, J.H.; Fruttiger, M.; Kim, J.H. Suppression of transient receptor potential canonical channel 4 inhibits vascular endothelial growth factor-induced retinal neovascularization. Cell Calcium 2015, 57, 101–108. [Google Scholar] [CrossRef]
- Yin, Z.; Ma, T.; Lin, Y.; Lu, X.; Zhang, C.; Chen, S.; Jian, Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell. Biochem. 2018, 119, 9419–9432. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, Y.; Lai, Y.; He, W.; Li, Y.; Wang, R.; Luo, X.; Chen, R.; Chen, T. Long non-coding RNA cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J. Cell. Biochem. 2018, 119, 2951–2963. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Kuwahara, K.; Wang, Y.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Hill, J.A.; Olson, E.N. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Investig. 2006, 116, 3114–3126. [Google Scholar] [CrossRef]
- Ge, R.; Tai, Y.; Sun, Y.; Zhou, K.; Yang, S.; Cheng, T.; Zou, Q.; Shen, F.; Wang, Y. Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett. 2009, 283, 43–51. [Google Scholar] [CrossRef]
- Kheradpezhouh, E.; Zhou, F.H.; Barritt, G.J.; Rychkov, G.Y. Oxidative stress promotes redistribution of TRPM2 channels to the plasma membrane in hepatocytes. Biochem. Biophys. Res. Commun. 2018, 503, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Smani, T.; Gómez, L.J.; Regodon, S.; Woodard, G.E.; Siegfried, G.; Khatib, A.; Rosado, J.A. TRP Channels in Angiogenesis and Other Endothelial Functions. Front. Physiol. 2018, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Barritt, G.J.; Chen, J.; Rychkov, G.Y. Ca(2+) -permeable channels in the hepatocyte plasma membrane and their roles in hepatocyte physiology. Biochim. Biophys. Acta 2008, 1783, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, B.; Yao, H.; Meng, X.; Huang, C.; Ni, M.; Li, J. Novel Insights Into TRPM7 Function in Fibrotic Diseases: A Potential Therapeutic Target. J. Cell. Physiol. 2015, 230, 1163–1169. [Google Scholar] [CrossRef]
- Fang, L.; Zhan, S.; Huang, C.; Cheng, X.; Lv, X.; Si, H.; Li, J. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol. Appl. Pharmacol. 2013, 272, 713–725. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Huang, Y.; Huang, C. Inhibition of transient receptor potential melastain 7 channel increases HSCs apoptosis induced by TRAIL. Life Sci. 2012, 90, 612–618. [Google Scholar] [CrossRef]
- Zhang, F.; Li, P.L. Reconstitution and characterization of a nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive Ca2+ release channel from liver lysosomes of rats. J. Biol. Chem. 2007, 282, 25259–25269. [Google Scholar] [CrossRef]
- Mohammed, F.F.; Khokha, R. Thinking outside the cell: Proteases regulate hepatocyte division. Trends Cell Biol. 2005, 15, 555–563. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Gomes, D.A.; Sehgal, S.; Nathanson, M.H. Hormonal regulation of nuclear permeability. J. Biol. Chem. 2007, 282, 4210–4217. [Google Scholar] [CrossRef]
- Nieuwenhuijs, V.B.; De Bruijn, M.T.; Padbury, R.T.; Barritt, G.J. Hepatic ischemia-reperfusion injury: Roles of Ca2+ and other intracellular mediators of impaired bile flow and hepatocyte damage. Dig. Dis. Sci. 2006, 51, 1087–1102. [Google Scholar] [CrossRef]
- Kaplowitz, N. Liver biology and pathobiology. Hepatology 2006, 43, S235–S238. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.J.; White, P.J.; Hall, J.F.; Kingston, S.; Boarder, M.R. Regulation of human hepatocytes by P2Y receptors: Control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J. Pharmacol. Exp. Ther. 2005, 313, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Robb-Gaspers, L.D.; Burnett, P.; Rutter, G.A.; Denton, R.M.; Rizzuto, R.; Thomas, A.P. Integrating cytosolic calcium signals into mitochondrial metabolic responses. Embo J. 1998, 17, 4987–5000. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.; Gomes, D.A.; Leite, M.F.; Grant, W.; Zhang, L.; Lam, W.; Cheng, Y.C.; Bennett, A.M.; Nathanson, M.H. Nucleoplasmic calcium is required for cell proliferation. J. Biol. Chem. 2007, 282, 17061–17068. [Google Scholar] [CrossRef]
- El, B.C.; Bidaux, G.; Enfissi, A.; Delcourt, P.; Prevarskaya, N.; Capiod, T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology 2008, 47, 2068–2077. [Google Scholar] [CrossRef]
- Goswami, C.; Dreger, M.; Otto, H.; Schwappach, B.; Hucho, F. Rapid disassembly of dynamic microtubules upon activation of the capsaicin receptor TRPV1. J. Neurochem. 2006, 96, 254–266. [Google Scholar] [CrossRef]
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef]
- Bubolz, A.H.; Mendoza, S.A.; Zheng, X.; Zinkevich, N.S.; Li, R.; Gutterman, D.D.; Zhang, D.X. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: Role of Ca2+ entry and mitochondrial ROS signaling. Am. J. Physiol.-Heart Circul. Physiol. 2012, 302, H634–H642. [Google Scholar] [CrossRef]
- Toth, B.; Csanady, L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J. Biol. Chem. 2010, 285, 30091–30102. [Google Scholar] [CrossRef]
- Nagamine, K.; Kudoh, J.; Minoshima, S.; Kawasaki, K.; Asakawa, S.; Ito, F.; Shimizu, N. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 1998, 54, 124–131. [Google Scholar] [CrossRef]
- Iordanov, I.; Mihalyi, C.; Toth, B.; Csanady, L. The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity. eLife 2016, 5, e17600. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Rao, V.; Ta, R.; Shobeiri, N.; Hill, C.E. Mg2+- and MgATP-inhibited and Ca2+/calmodulin-sensitive TRPM7-like current in hepatoma and hepatocytes. Am. J. Physiol.-Gastroint. Liver Physiol. 2009, 297, G687–G694. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Barritt, G.J. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca(2+)-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem. J. 2003, 373, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Xu, J.; Liu, X.; Brenner, D.A. New Developments on the Treatment of Liver Fibrosis. Dig. Dis. 2016, 34, 589–596. [Google Scholar] [CrossRef]
- Trivedi, P.; Wang, S.; Friedman, S.L. The Power of Plasticity-Metabolic Regulation of Hepatic Stellate Cells. Cell Metab. 2021, 33, 242–257. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, X.; Liang, J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp. Cell Res. 2017, 352, 420–426. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.R.; Tian, Z. Roles of hepatic stellate cells in acute liver failure: From the perspective of inflammation and fibrosis. World J. Hepatol. 2019, 11, 412–420. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Zhang, Y.; Li, W.; Zheng, W.; Yu, J.; Wang, B.; Chen, L.; Zhuo, Q.; Chen, L.; et al. MicroRNA-212 activates hepatic stellate cells and promotes liver fibrosis via targeting SMAD7. Biochem. Biophys. Res. Commun. 2018, 496, 176–183. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Preziosi, M.E.; Monga, S.P. Update on the Mechanisms of Liver Regeneration. Semin. Liver Dis. 2017, 37, 141–151. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.H.; Grant, C.E.; Hill, C.E. Differential expression of TRPM7 in rat hepatoma and embryonic and adult hepatocytes. Can. J. Physiol. Pharmacol. 2012, 90, 435–444. [Google Scholar] [CrossRef]
- Zhu, Y.; Men, R.; Wen, M.; Hu, X.; Liu, X.; Yang, L. Blockage of TRPM7 channel induces hepatic stellate cell death through endoplasmic reticulum stress-mediated apoptosis. Life Sci. 2014, 94, 37–44. [Google Scholar] [CrossRef]
- Sahni, J.; Scharenberg, A.M. TRPM7 ion channels are required for sustained phosphoinositide 3-kinase signaling in lymphocytes. Cell Metab. 2008, 8, 84–93. [Google Scholar] [CrossRef]
- Baldoli, E.; Maier, J.A. Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis 2012, 15, 47–57. [Google Scholar] [CrossRef]
- Song, Y.; Zhan, L.; Yu, M.; Huang, C.; Meng, X.; Ma, T.; Zhang, L.; Li, J. TRPV4 channel inhibits TGF-beta1-induced proliferation of hepatic stellate cells. PLoS ONE 2014, 9, e101179. [Google Scholar] [CrossRef]
- Breitkopf, K.; Godoy, P.; Ciuclan, L.; Singer, M.V.; Dooley, S. TGF-beta/Smad signaling in the injured liver. Z. Gastroent. 2006, 44, 57–66. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okazaki, I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 2007, 56, 284–292. [Google Scholar] [CrossRef]
- Um, J.Y.; Kang, S.Y.; Kim, H.J.; Chung, B.Y.; Park, C.W.; Kim, H.O. Transient receptor potential vanilloid-3 (TRPV3) channel induces dermal fibrosis via the TRPV3/TSLP/Smad2/3 pathways in dermal fibroblasts. J. Dermatol. Sci. 2020, 97, 117–124. [Google Scholar] [CrossRef]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harbor Perspect. Biol. 2010, 2, a3962. [Google Scholar] [CrossRef]
- Taye, A.; El-Sheikh, A.A. Lectin-like oxidized low-density lipoprotein receptor 1 pathways. Eur. J. Clin. Investig. 2013, 43, 740–745. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, H.; E, M.; Shi, P.; Zhang, Q.; Li, S.; Wang, Y.; Cao, Y.; Chen, Y.; Ba, L.; et al. Transient receptor potential vanilloid-3 (TRPV3) activation plays a central role in cardiac fibrosis induced by pressure overload in rats via TGF-beta(1) pathway. Naunyn-Schmiedebergs Arch. Pharmacol. 2018, 391, 131–143. [Google Scholar] [CrossRef]
- Liu, C.; Tao, Q.; Sun, M.; Wu, J.Z.; Yang, W.; Jian, P.; Peng, J.; Hu, Y.; Liu, C.; Liu, P. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab. Investig. 2010, 90, 1805–1816. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer cells in the pathogenesis of liver disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Z.M.; Liu, L.; Zhang, C.; Zhang, Y.L.; Zhang, Z.C. Effects of Ca2+ channel blockers on store-operated Ca2+ channel currents of Kupffer cells after hepatic ischemia/reperfusion injury in rats. World J. Gastroenterol. 2006, 12, 4694–4698. [Google Scholar] [CrossRef]
- Ong, H.L.; de Souza, L.B.; Cheng, K.T.; Ambudkar, I.S. Physiological functions and regulation of TRPC channels. Handb. Exp. Pharmacol. 2014, 223, 1005–1034. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Yamakage, M.; Namiki, A. Calcium channels—basic aspects of their structure, function and gene encoding; anesthetic action on the channels—a review. Can. J. Anesth. 2002, 49, 151–164. [Google Scholar] [CrossRef]
- de la Harpe, A.; Beukes, N.; Frost, C.L. CBD activation of TRPV1 induces oxidative signaling and subsequent ER stress in breast cancer cell lines. Biotechnol. Appl. Biochem. 2022, 69, 420–430. [Google Scholar] [CrossRef]
- Vestuto, V.; Di Sarno, V.; Musella, S.; Di Dona, G.; Moltedo, O.; Gomez-Monterrey, I.M.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Ciaglia, T. New Frontiers on ER Stress Modulation: Are TRP Channels the Leading Actors? Int. J. Mol. Sci. 2023, 24, 185. [Google Scholar] [CrossRef]
- Toki, Y.; Takenouchi, T.; Harada, H.; Tanuma, S.; Kitani, H.; Kojima, S.; Tsukimoto, M. Extracellular ATP induces P2X7 receptor activation in mouse Kupffer cells, leading to release of IL-1β, HMGB1, and PGE2, decreased MHC class I expression and necrotic cell death. Biochem. Biophys. Res. Commun. 2015, 458, 771–776. [Google Scholar] [CrossRef]
- Marrone, G.; Shah, V.H.; Gracia-Sancho, J. Sinusoidal communication in liver fibrosis and regeneration. J. Hepatol. 2016, 65, 608–617. [Google Scholar] [CrossRef]
- Xu, M.; Xu, H.; Lin, Y.; Sun, X.; Wang, L.; Fang, Z.; Su, X.; Liang, X.; Hu, Y.; Liu, Z.; et al. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell 2019, 178, 1478–1492. [Google Scholar] [CrossRef]
- Cao, S.; Anishkin, A.; Zinkevich, N.S.; Nishijima, Y.; Korishettar, A.; Wang, Z.; Fang, J.; Wilcox, D.A.; Zhang, D.X. Transient receptor potential vanilloid 4 (TRPV4) activation by arachidonic acid requires protein kinase A-mediated phosphorylation. J. Biol. Chem. 2018, 293, 5307–5322. [Google Scholar] [CrossRef]
- Wong, C.O.; Yao, X. TRP channels in vascular endothelial cells. Adv. Exp. Med. Biol. 2011, 704, 759–780. [Google Scholar] [CrossRef]
- Loh, K.P.; Ng, G.; Yu, C.Y.; Fhu, C.K.; Yu, D.; Vennekens, R.; Nilius, B.; Soong, T.W.; Liao, P. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflug. Arch. 2014, 466, 563–576. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. Mechanisms of acetaminophen hepatotoxicity and their translation to the human pathophysiology. J. Clin. Transl. Res. 2017, 3, 157–169. [Google Scholar] [CrossRef]
- Perraud, A.L.; Fleig, A.; Dunn, C.A.; Bagley, L.A.; Launay, P.; Schmitz, C.; Stokes, A.J.; Zhu, Q.; Bessman, M.J.; Penner, R.; et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001, 411, 595–599. [Google Scholar] [CrossRef]
- Echtermeyer, F.; Eberhardt, M.; Risser, L.; Herzog, C.; Gueler, F.; Khalil, M.; Engel, M.; Vondran, F.; Leffler, A. Acetaminophen-induced liver injury is mediated by the ion channel TRPV4. FASEB J. 2019, 33, 10257–10268. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol.-Mech. Dis. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Moreira, R.K. Hepatic stellate cells and liver fibrosis. Arch. Pathol. Lab. Med. 2007, 131, 1728–1734. [Google Scholar] [CrossRef]
- Parviz, F.; Matullo, C.; Garrison, W.D.; Savatski, L.; Adamson, J.W.; Ning, G.; Kaestner, K.H.; Rossi, J.M.; Zaret, K.S.; Duncan, S.A. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 2003, 34, 292–296. [Google Scholar] [CrossRef]
- Park, S.M. The crucial role of cholangiocytes in cholangiopathies. Gut Liver 2012, 6, 295–304. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; Strazzabosco, M.; Larusso, N.F. The cholangiopathies: Disorders of biliary epithelia. Gastroenterology 2004, 127, 1565–1577. [Google Scholar] [CrossRef]
- Sawamura, T.; Kume, N.; Aoyama, T.; Moriwaki, H.; Hoshikawa, H.; Aiba, Y.; Tanaka, T.; Miwa, S.; Katsura, Y.; Kita, T.; et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77. [Google Scholar] [CrossRef]
- Habib, A.; Chokr, D.; Wan, J.; Hegde, P.; Mabire, M.; Siebert, M.; Ribeiro-Parenti, L.; Le Gall, M.; Letteron, P.; Pilard, N.; et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut 2019, 68, 522–532. [Google Scholar] [CrossRef]
- Son, M.K.; Ryu, Y.L.; Jung, K.H.; Lee, H.; Lee, H.S.; Yan, H.H.; Park, H.J.; Ryu, J.K.; Suh, J.K.; Hong, S.; et al. HS-173, a novel PI3K inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci. Rep. 2013, 3, 3470. [Google Scholar] [CrossRef]
- Iyer, S.C.; Kannan, A.; Gopal, A.; Devaraj, N.; Halagowder, D. Receptor channel TRPC6 orchestrate the activation of human hepatic stellate cell under hypoxia condition. Exp. Cell Res. 2015, 336, 66–75. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Wang, W.; Smits, R.; Hao, H.; He, C. Wnt/beta-Catenin Signaling in Liver Cancers. Cancers 2019, 11, 926. [Google Scholar] [CrossRef]
- Nakagawa, H.; Fujita, M.; Fujimoto, A. Genome sequencing analysis of liver cancer for precision medicine. Semin. Cancer Biol. 2019, 55, 120–127. [Google Scholar] [CrossRef]
- Montagner, A.; Le Cam, L.; Guillou, H. beta-catenin oncogenic activation rewires fatty acid catabolism to fuel hepatocellular carcinoma. Gut 2019, 68, 183–185. [Google Scholar] [CrossRef]
- Gill, D.L.; Waldron, R.T.; Rys-Sikora, K.E.; Ufret-Vincenty, C.A.; Graber, M.N.; Favre, C.J.; Alfonso, A. Calcium pools, calcium entry, and cell growth. Biosci. Rep. 1996, 16, 139–157. [Google Scholar] [CrossRef]
- Qi, H.; Wu, F.; Wang, H. Function of TRPC1 in modulating hepatocellular carcinoma progression. Med. Oncol. 2023, 40, 97. [Google Scholar] [CrossRef] [PubMed]
- Selli, C.; Erac, Y.; Kosova, B.; Erdal, E.S.; Tosun, M. Silencing of TRPC1 regulates store-operated calcium entry and proliferation in Huh7 hepatocellular carcinoma cells. Biomed. Pharmacother. 2015, 71, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Selli, C.; Pearce, D.A.; Sims, A.H.; Tosun, M. Differential expression of store-operated calcium- and proliferation-related genes in hepatocellular carcinoma cells following TRPC1 ion channel silencing. Mol. Cell. Biochem. 2016, 420, 129–140. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm. Sin. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Liang, C.; Chen, E.; Chen, W.; Liang, F.; Zhi, X.; Wei, T.; Xue, F.; Li, G.; Yang, Q.; et al. Regulation of Multi-drug Resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci. Rep. 2016, 6, 23269. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Lin, G.B.; Lin, S.H.; Lu, C.H.; Hsieh, C.H.; Ma, B.L.; Chao, C.Y. Static magnetic field enhances the anticancer efficacy of capsaicin on HepG2 cells via capsaicin receptor TRPV1. PLoS ONE 2018, 13, e191078. [Google Scholar] [CrossRef]
- Dai, L.; Mugaanyi, J.; Cai, X.; Dong, M.; Lu, C.; Lu, C. Comprehensive bioinformatic analysis of MMP1 in hepatocellular carcinoma and establishment of relevant prognostic model. Sci. Rep. 2022, 12, 13639. [Google Scholar] [CrossRef]
- Li, W.H.; Lee, Y.M.; Kim, J.Y.; Kang, S.; Kim, S.; Kim, K.H.; Park, C.H.; Chung, J.H. Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J. Investig. Dermatol. 2007, 127, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Wang, J.S.; Markowitz, J.S.; Donovan, J.L.; Gibson, B.B.; Gefroh, H.A.; Devane, C.L. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J. Pharmacol. Exp. Ther. 2006, 317, 850–857. [Google Scholar] [CrossRef]
- DeHaven, W.I.; Smyth, J.T.; Boyles, R.R.; Bird, G.S.; Putney, J.J. Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J. Biol. Chem. 2008, 283, 19265–19273. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, G.; Xie, C.; Qian, K.; Lei, X.; Liu, Q.; Liu, G.; Cao, Z.; Fu, J.; Du, H.; et al. Pharmacological inhibition of TRPV4 channel suppresses malignant biological behavior of hepatocellular carcinoma via modulation of ERK signaling pathway. Biomed. Pharmacother. 2018, 101, 910–919. [Google Scholar] [CrossRef]
- Lee, W.H.; Choong, L.Y.; Mon, N.N.; Lu, S.; Lin, Q.; Pang, B.; Yan, B.; Krishna, V.S.; Singh, H.; Tan, T.Z.; et al. TRPV4 Regulates Breast Cancer Cell Extravasation, Stiffness and Actin Cortex. Sci. Rep. 2016, 6, 27903. [Google Scholar] [CrossRef]
- Qu, L.; Liu, B. Cyclooxygeanse-2 promotes metastasis in osteosarcoma. Cancer Cell Int. 2015, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; Kawarabayashi, Y.; Imai, Y.; Honda, A.; Inoue, R. Counteracting effect of TRPC1-associated Ca2+ influx on TNF-alpha-induced COX-2-dependent prostaglandin E2 production in human colonic myofibroblasts. Am. J. Physiol.-Gastroint. Liver Physiol. 2011, 301, G356–G367. [Google Scholar] [CrossRef]
- Nzeako, U.C.; Guicciardi, M.E.; Yoon, J.H.; Bronk, S.F.; Gores, G.J. COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology 2002, 35, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhong, J.; Zhou, M.; Zhou, Y.; Xiu, P.; Liu, F.; Wang, F.; Li, Z.; Tang, Y.; Chen, Y.; et al. Targeting of the COX-2/PGE2 axis enhances the antitumor activity of T7 peptide in vitro and in vivo. Drug Deliv. 2021, 28, 844–855. [Google Scholar] [CrossRef]
- Sharma, J.N.; Jawad, N.M. Adverse effects of COX-2 inhibitors. Sci. World J. 2005, 5, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Nissen, S.E.; Topol, E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA-J. Am. Med. Assoc. 2001, 286, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Y.; Sun, S.; Wang, Z.; Liu, P.; Liu, S.; Jiang, J. Bradykinin promotes migration and invasion of hepatocellular carcinoma cells through TRPM7 and MMP2. Exp. Cell Res. 2016, 349, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, X.; Cheng, X.; Zhang, Y.; Chen, L.; Shi, L.; Liu, Z.; Qian, H.; Wu, M.; Yin, Z. Intratumoral hepatic stellate cells as a poor prognostic marker and a new treatment target for hepatocellular carcinoma. PLoS ONE 2013, 8, e80212. [Google Scholar] [CrossRef]
- Chigurupati, S.; Venkataraman, R.; Barrera, D.; Naganathan, A.; Madan, M.; Paul, L.; Pattisapu, J.V.; Kyriazis, G.A.; Sugaya, K.; Bushnev, S.; et al. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res. 2010, 70, 418–427. [Google Scholar] [CrossRef]
- Ann, J.; Kim, H.S.; Thorat, S.A.; Kim, H.; Ha, H.J.; Choi, K.; Kim, Y.H.; Kim, M.; Hwang, S.W.; Pearce, L.V.; et al. Discovery of Nonpungent Transient Receptor Potential Vanilloid 1 (TRPV1) Agonist as Strong Topical Analgesic. J. Med. Chem. 2020, 63, 418–424. [Google Scholar] [CrossRef]
- Manitpisitkul, P.; Flores, C.M.; Moyer, J.A.; Romano, G.; Shalayda, K.; Tatikola, K.; Hutchison, J.S.; Mayorga, A.J. A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep). Scand. J. Pain 2018, 18, 151–164. [Google Scholar] [CrossRef]
- Phe, V.; Schneider, M.P.; Peyronnet, B.; Abo, Y.N.; Mordasini, L.; Chartier-Kastler, E.; Bachmann, L.M.; Kessler, T.M. Intravesical vanilloids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: A systematic review and meta-analysis. A report from the Neuro-Urology Promotion Committee of the International Continence Society (ICS). Neurourol. Urodyn. 2018, 37, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Li, X.; Chen, J.; Su, B.; Li, X.; Yang, S.; Guan, Z.; Wang, R. Resiniferatoxin for treatment of lifelong premature ejaculation: A preliminary study. Int. J. Urol. 2014, 21, 923–926. [Google Scholar] [CrossRef]
- Deruyver, Y.; Weyne, E.; Dewulf, K.; Rietjens, R.; Pinto, S.; Van Ranst, N.; Franken, J.; Vanneste, M.; Albersen, M.; Gevaert, T.; et al. Intravesical Activation of the Cation Channel TRPV4 Improves Bladder Function in a Rat Model for Detrusor Underactivity. Eur. Urol. 2018, 74, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Shirai, K.; Miyajima, M.; Reinach, P.S.; Yamanaka, O.; Sumioka, T.; Kokado, M.; Tomoyose, K.; Saika, S. Loss of TRPV4 Function Suppresses Inflammatory Fibrosis Induced by Alkali-Burning Mouse Corneas. PLoS ONE 2016, 11, e167200. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Fribo, J.; Nagy, I.; Gotfredsen, C.; Charrua, A.; Hansen, J.B.; Hansen, A.J.; Szallasi, A. TRPV1 Antagonists as Novel Anti-Diabetic Agents: Regulation of Oral Glucose Tolerance and Insulin Secretion Through Reduction of Low-Grade Inflammation? Med. Sci 2019, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; Del, C.D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef]
- Fu, J.; Du, H.; Zhang, X.; Xu, X. Pharmacological Inhibition of Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Alleviates Carbon Tetrachloride-Induced Liver Fibrosis in Mice. J. Nippon Med. Sch. 2019, 86, 258–262. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Stock, K.; Kumar, J.; Synowitz, M.; Petrosino, S.; Imperatore, R.; Smith, E.S.; Wend, P.; Purfurst, B.; Nuber, U.A.; Gurok, U.; et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat. Med. 2012, 18, 1232–1238. [Google Scholar] [CrossRef]
- Larsson, M.H.; Hakansson, P.; Jansen, F.P.; Magnell, K.; Brodin, P. Ablation of TRPM5 in Mice Results in Reduced Body Weight Gain and Improved Glucose Tolerance and Protects from Excessive Consumption of Sweet Palatable Food when Fed High Caloric Diets. PLoS ONE 2015, 10, e138373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clemmensen, C.; Jall, S.; Kleinert, M.; Quarta, C.; Gruber, T.; Reber, J.; Sachs, S.; Fischer, K.; Feuchtinger, A.; Karlas, A.; et al. Coordinated targeting of cold and nicotinic receptors synergistically improves obesity and type 2 diabetes. Nat. Commun. 2018, 9, 4304. [Google Scholar] [CrossRef] [PubMed]
- Monet, M.; Gkika, D.; Lehen’Kyi, V.; Pourtier, A.; Vanden, A.F.; Bidaux, G.; Juvin, V.; Rassendren, F.; Humez, S.; Prevarsakaya, N. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim. Biophys. Acta 2009, 1793, 528–539. [Google Scholar] [CrossRef] [PubMed]
- von Moos, R.; Strasser, F.; Gillessen, S.; Zaugg, K. Metastatic bone pain: Treatment options with an emphasis on bisphosphonates. Support. Care Cancer 2008, 16, 1105–1115. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J. Biol. Chem. 2010, 285, 19362–19371. [Google Scholar] [CrossRef]
- Gavva, N.R.; Treanor, J.J.; Garami, A.; Fang, L.; Surapaneni, S.; Akrami, A.; Alvarez, F.; Bak, A.; Darling, M.; Gore, A.; et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008, 136, 202–210. [Google Scholar] [CrossRef]
- Eid, S.R. Therapeutic targeting of TRP channels--the TR(i)P to pain relief. Curr. Top. Med. Chem. 2011, 11, 2118–2130. [Google Scholar] [CrossRef]
- Miller, F.; Bjornsson, M.; Svensson, O.; Karlsten, R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp. Clin. Trials 2014, 37, 189–199. [Google Scholar] [CrossRef]
- Sun, R.; Fang, L.; Lv, X.; Fang, J.; Wang, Y.; Chen, D.; Wang, L.; Chen, J.; Qi, Y.; Tang, Z.; et al. In vitro and in vivo evaluation of self-assembled chitosan nanoparticles selectively overcoming hepatocellular carcinoma via asialoglycoprotein receptor. Drug Deliv. 2021, 28, 2071–2084. [Google Scholar] [CrossRef]
- Teng, W.; Zhao, L.; Yang, S.; Zhang, C.; Liu, M.; Luo, J.; Jin, J.; Zhang, M.; Bao, C.; Li, D.; et al. The hepatic-targeted, resveratrol loaded nanoparticles for relief of high fat diet-induced nonalcoholic fatty liver disease. J. Controll. Release 2019, 307, 139–149. [Google Scholar] [CrossRef]
| TRP Channel | Expression | Related Diseases | Main Functions | Ref. |
|---|---|---|---|---|
| TRPV1 | Hepatocytes, HepG2 cells, ECs | hepatitis, hepatic injury, HCC | Regulates the stability of microtubule and cell migration; manipulates the biological function of KCs; mediates APAP-induced liver injury; and promotes angiogenesis. | [44,48,49,50,51] |
| TRPV2 | HepG2 cells, Huh-7 cells, BNL1 ME cells | HCC | Mediates HCC cell survival and cell stemness; and mediates doxorubicin entry into BNL1 ME cells. | [52,53,54,55] |
| TRPV3 | HSCs | hepatic fibrosis, cirrhosis | Regulates expression of inflammation-related gene Olr1 and proliferation of HSCs. | [56,57] |
| TRPV4 | Hepatocytes, HepG2 cells, HSC-T6 cells, ECs | hepatic injury, hepatic fibrosis, HCC | Mediates APAP-induced liver injury; mediates activation and proliferation of HSCs; attenuates EMT and induceds inactivation of p-ERK; and promotes angiogenesis. | [45,58,59,60] |
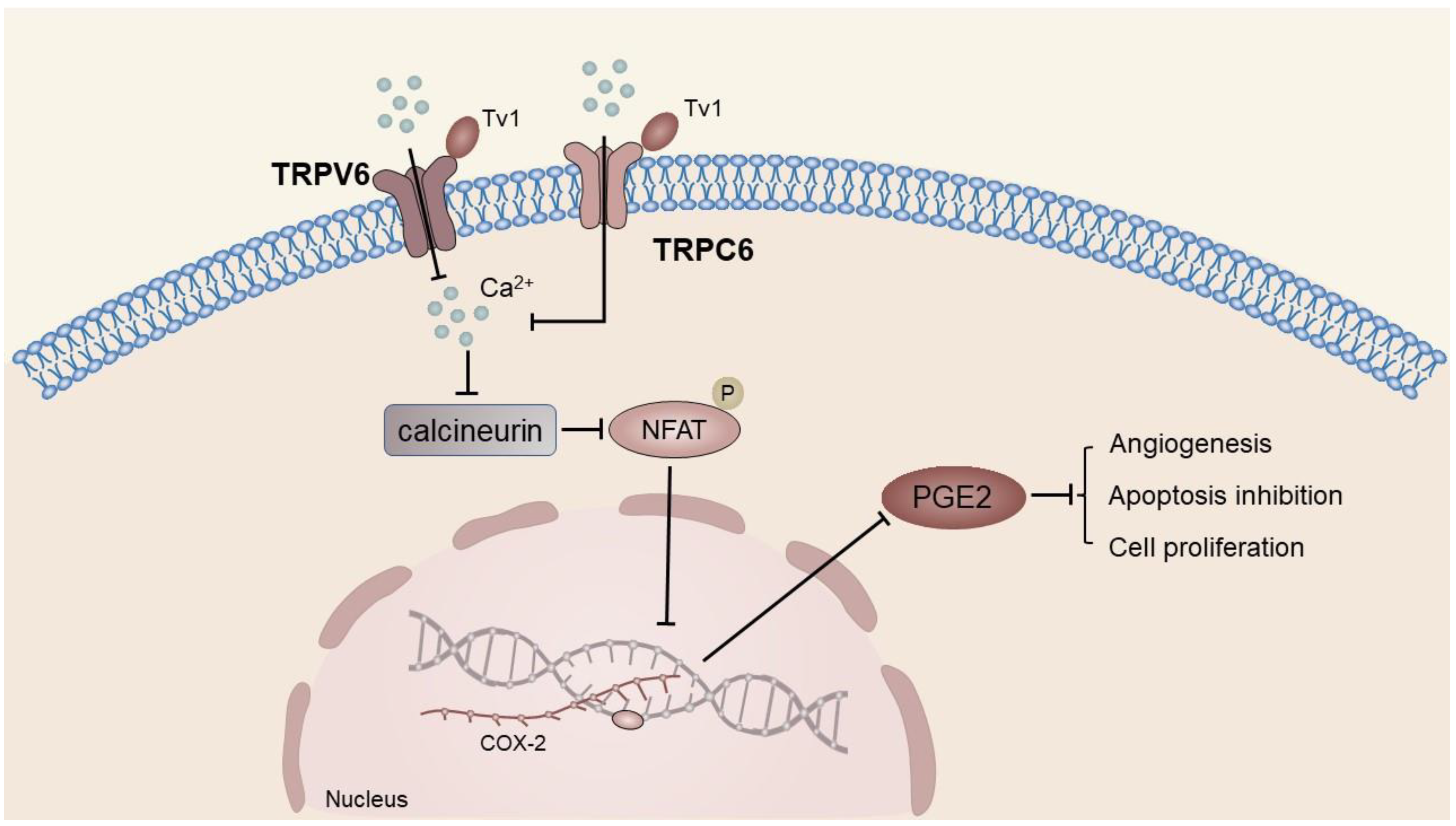
| TRPV6 | 1MEA cells | HCC | Regulates the expression of COX-2. | [40] |
| TRPC1 | H4-IIE cells, Huh-7 cells, ECs | hepatic injury, HCC | Regulates the volume of hepatocytes; mediates APAP-induced liver injury; regulates the SOCE and cellular activity of HCC cells; and promotes angiogenesis. | [45,61,62] |
| TRPC3 | KCs, ECs | IRI | Mediates calcium overload and activation of KCs; and promotes angiogenesis. | [63,64] |
| TRPC4 | ECs | - | Promotes angiogenesis. | [65] |
| TRPC5 | Paracancerous tissues | HCC | Macrophage differentiation is inhibited by regulating the Akt/IκB/NF-κB signaling pathway. | [66,67,68] |
| TRPC6 | lx-2 cells, HepG2 cells, Huh-7 cells, 1MEA cells, ECs | hepatic fibrosis, HCC | Mediates expression of α-SMA and COL1A1; mediates migration and MDR of HCC cells; regulates the expression of COX-2; and promotes angiogenesis. | [69,70] |
| TRPM2 | Hepatocytes, ECs | hepatic injury | Mediates apoptosis and necrosis pathways; mediates APAP-induced liver injury; and mediates EC migration. | [45,71,72] |
| TRPM7 | Hepatocytes, WIF-B cells, HSC-T6 cells | hepatic injury, hepatic fibrosis, HCC | Mediates hepatocyte proliferation and ROS-induced apoptosis; involved in TRAIL-induced HSC apoptosis; and mediates APAP-induced liver injury. | [45,73,74,75,76] |
| TRPM8 | Hepatocytes | hepatic fibrosis | Downregulates the expression of S100A9 and promotes the expression of HNF4α. | [46] |
| TRPML1 | Hepatocytes | - | Mediates lysosome release of Ca2+ to maintain intracellular calcium homeostasis. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lyu, Y.; Zhu, L.; Wang, H. Role of TRP Channels in Liver-Related Diseases. Int. J. Mol. Sci. 2023, 24, 12509. https://doi.org/10.3390/ijms241512509
Liu Y, Lyu Y, Zhu L, Wang H. Role of TRP Channels in Liver-Related Diseases. International Journal of Molecular Sciences. 2023; 24(15):12509. https://doi.org/10.3390/ijms241512509
Chicago/Turabian StyleLiu, Yusheng, Yihan Lyu, Lijuan Zhu, and Hongmei Wang. 2023. "Role of TRP Channels in Liver-Related Diseases" International Journal of Molecular Sciences 24, no. 15: 12509. https://doi.org/10.3390/ijms241512509
APA StyleLiu, Y., Lyu, Y., Zhu, L., & Wang, H. (2023). Role of TRP Channels in Liver-Related Diseases. International Journal of Molecular Sciences, 24(15), 12509. https://doi.org/10.3390/ijms241512509







