Resveratrol May Reduce the Degree of Periodontitis by Regulating ERK Pathway in Gingival-Derived MSCs
Abstract
1. Introduction
2. Results
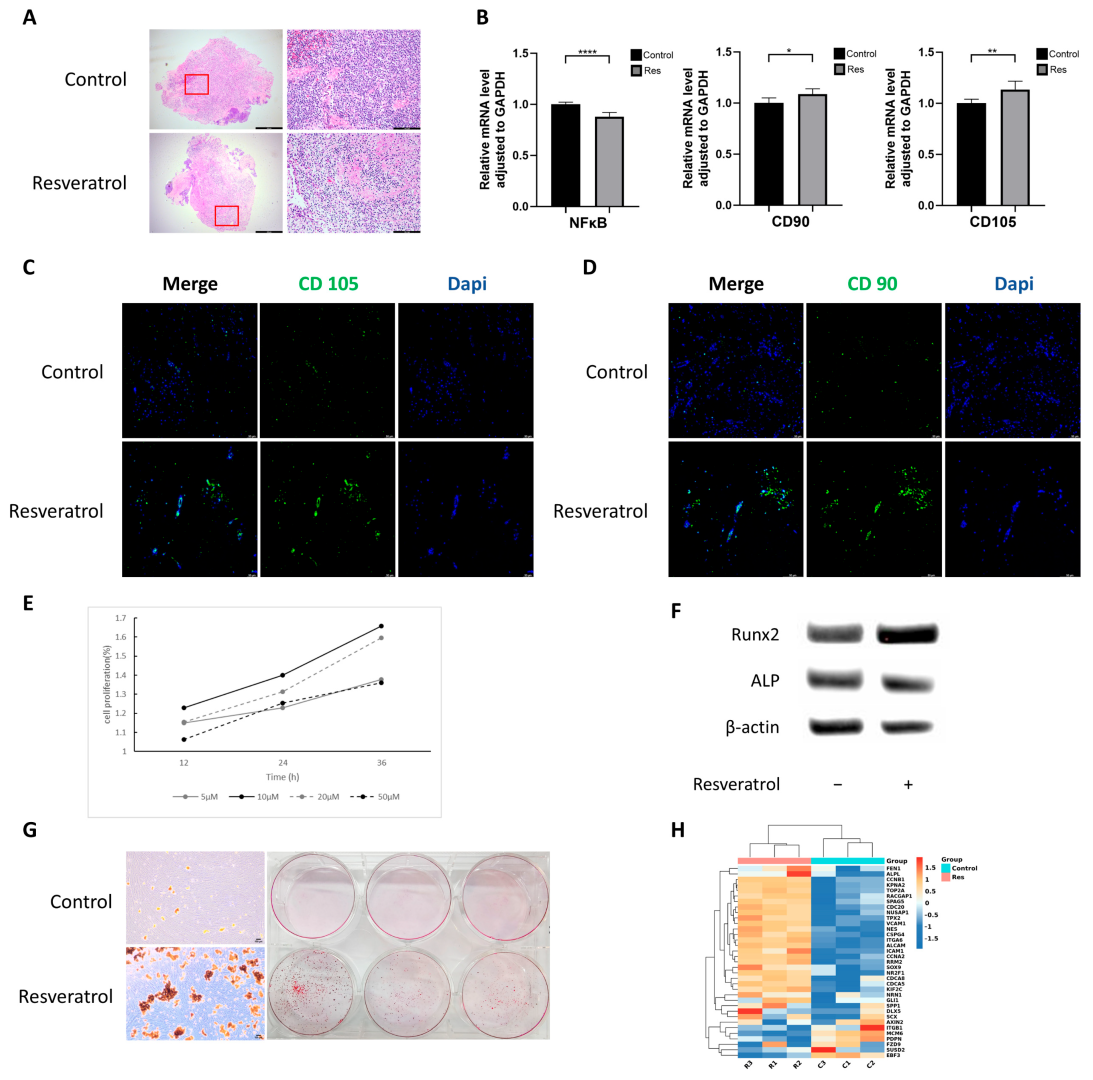
2.1. Resveratrol Reduces Inflammation and Enhances Cell Stemness in Gingival Tissue
2.2. Resveratrol Promotes hGMSCs Proliferation and Osteogenic Differentiation Capacity
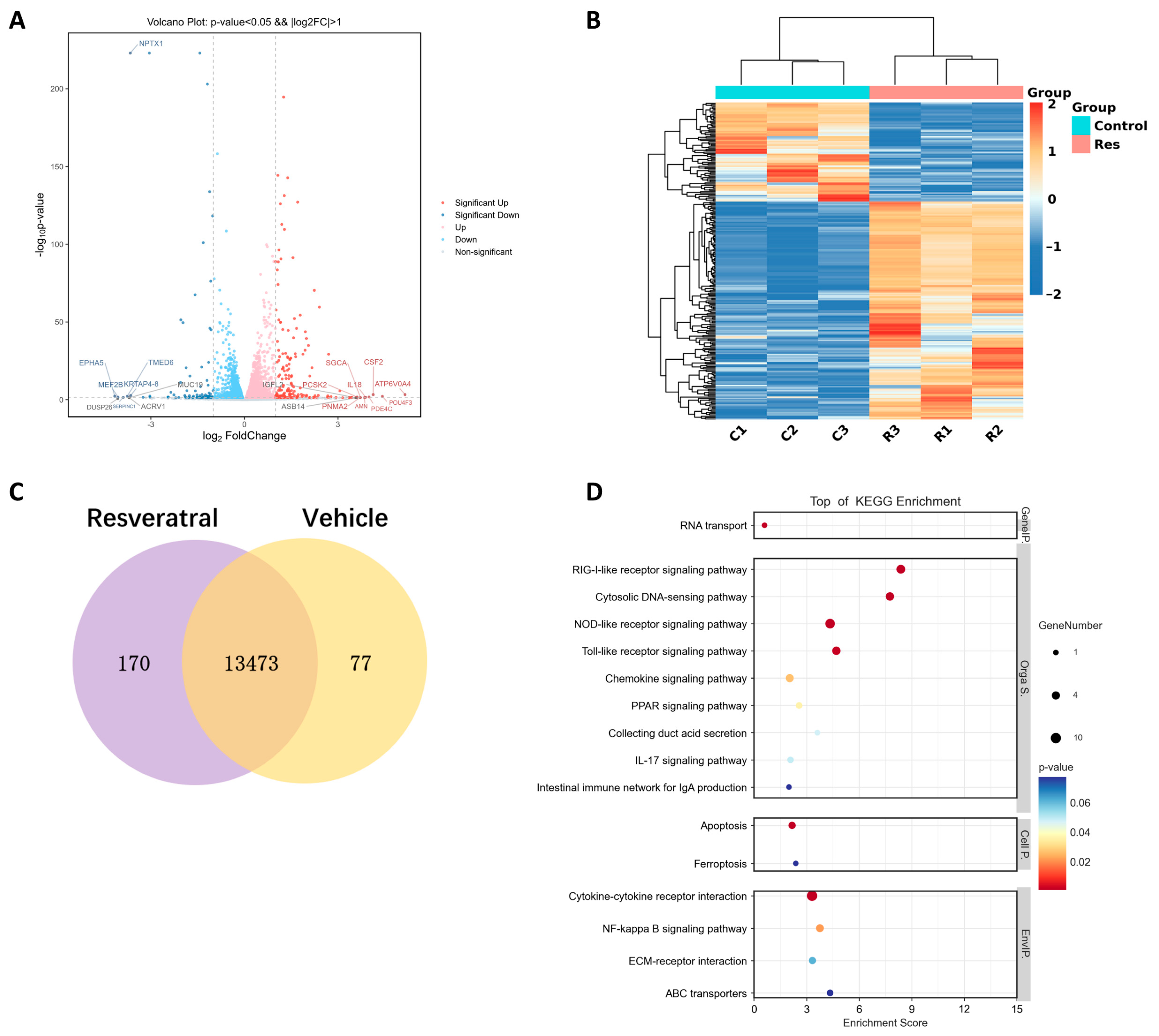
2.3. Resveratrol Enhances Stemness of hGMSCs In Vitro
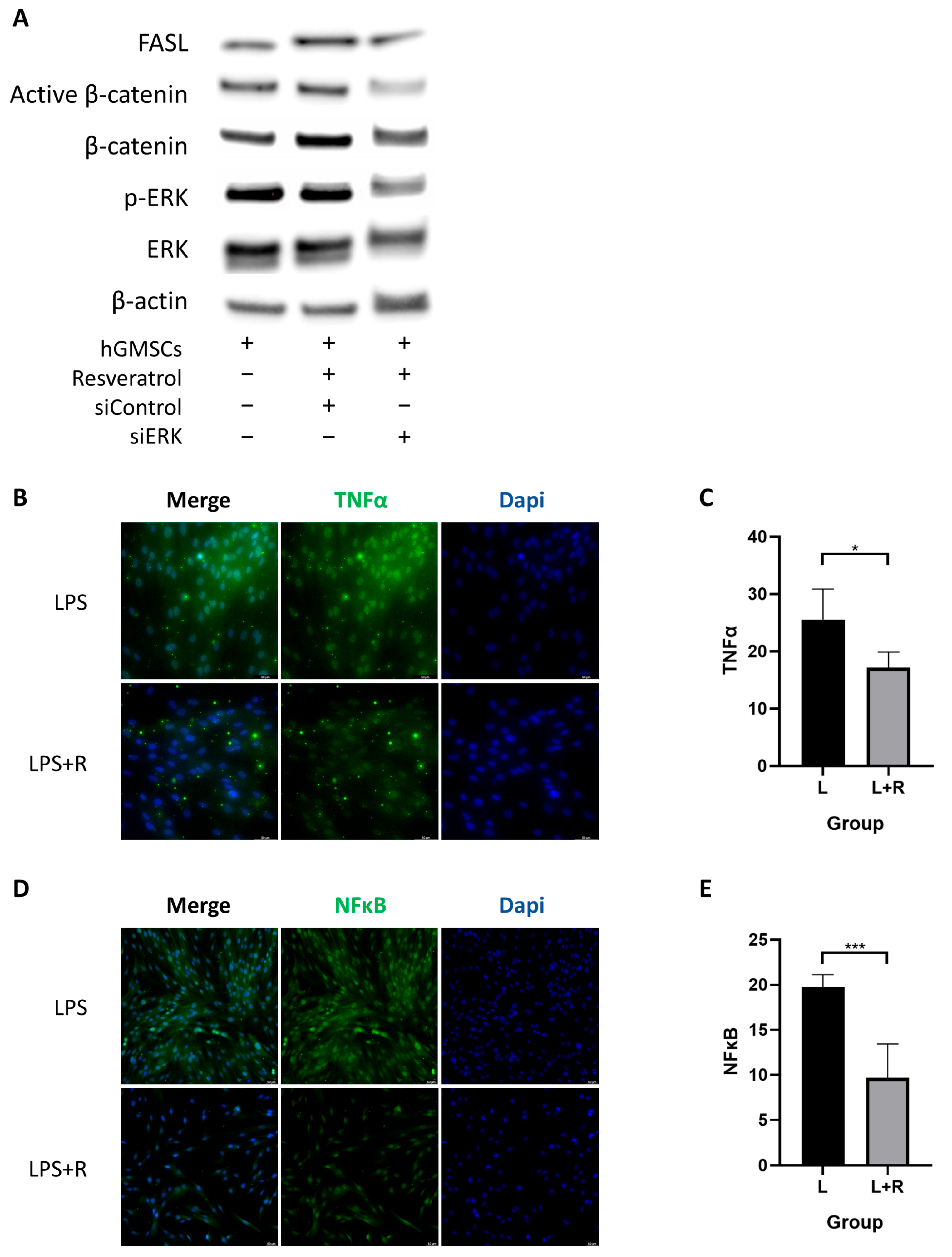
2.4. Resveratrol Activates ERK/Wnt Signaling Pathway in hGMSCs and Enhances FASL-Mediated Immunomodulation
2.5. Resveratrol Inhibits the Inflammatory Pathway in hGMSCs
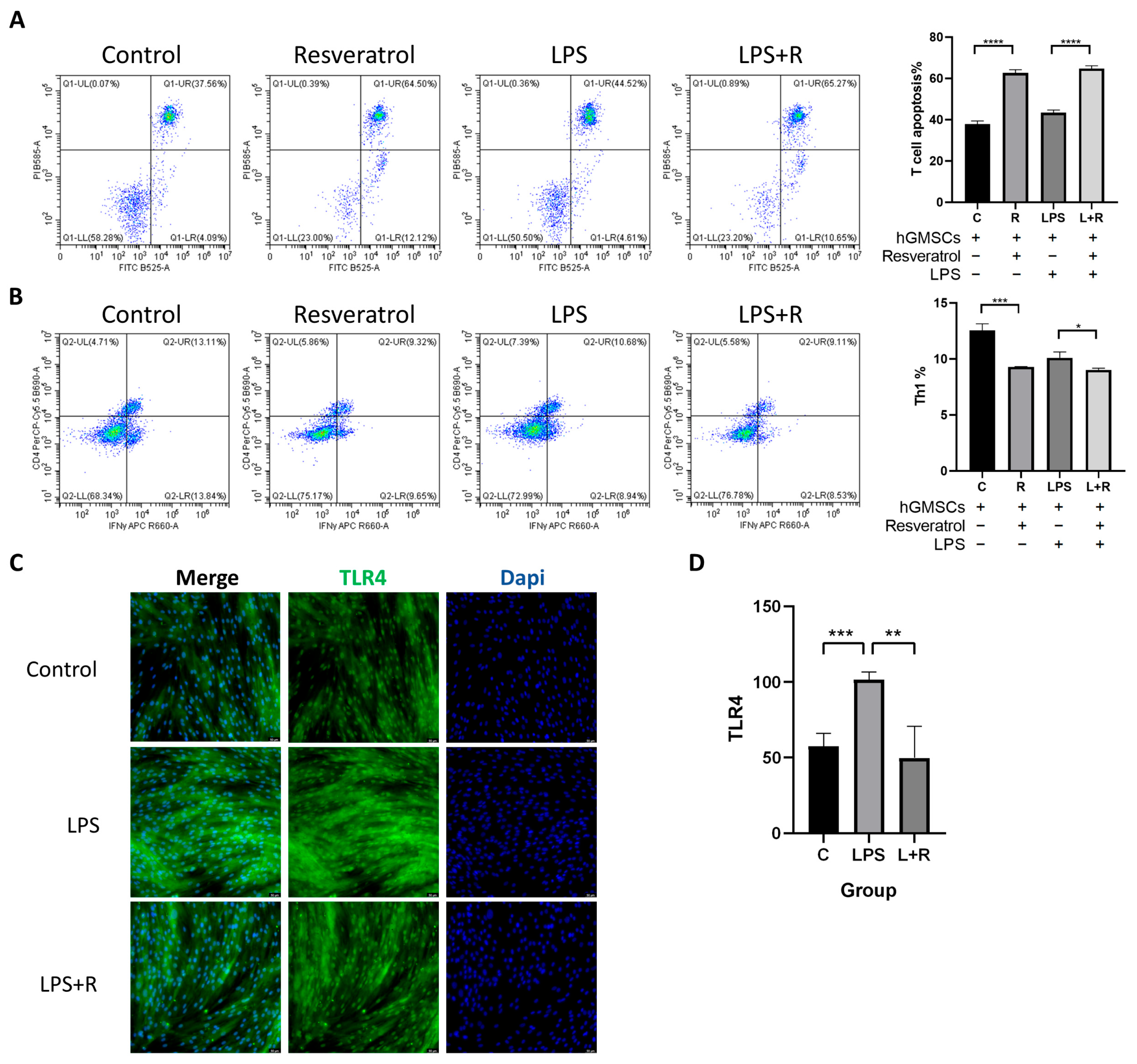
2.6. Resveratrol Is Associated with hGMSCs-Mediated Immunomodulation
2.7. TLR4 in hGMSCs Can Be Activated by LPS, and Resveratrol Can Reverse This Process
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J. Periodontal regeneration. J. Periodontol. 2005, 76, 1601–1622. [Google Scholar] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; Keating. International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef]
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Front. Immunol. 2021, 12, 667221. [Google Scholar] [CrossRef]
- Kasper, G.; Mao, L.; Geissler, S.; Draycheva, A.; Trippens, J.; Kühnisch, J.; Tschirschmann, M.; Kaspar, K.; Perka, C.; Duda, G.N.; et al. Insights into mesenchymal stem cell aging: Involvement of antioxidant defense and actin cytoskeleton. Stem. Cells 2009, 27, 1288–1297. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Li, S.; Zhang, B.; Li, S.; Wang, X.; Wang, Y.; Jia, C.; Chang, Y. sTNFRII-Fc modification protects human UC-MSCs against apoptosis/autophagy induced by TNF-alpha and enhances their efficacy in alleviating inflammatory arthritis. Stem. Cell Res. Ther. 2021, 12, 535. [Google Scholar] [CrossRef]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35 Pt 5, 1156–1160. [Google Scholar] [CrossRef]
- Chudzińska, M.; Rogowicz, D.; Wołowiec, Ł.; Banach, J.; Sielski, S.; Bujak, R.; Sinkiewicz, A.; Grześk, G. Resveratrol and cardiovascular system-the unfulfilled hopes. Ir. J. Med Sci. 2021, 190, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar]
- Dai, Z.; Li, Y.; Quarles, L.D.; Song, T.; Pan, W.; Zhou, H.; Xiao, Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 2007, 14, 806–814. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Napimoga, M.H.; Casati, M.Z.; Casarin, R.C.V. Systemic treatment with resveratrol reduces the progression of experimental periodontitis and arthritis in rats. PLoS ONE 2018, 13, e0204414. [Google Scholar] [CrossRef] [PubMed]
- Javid, A.Z.; Hormoznejad, R.; Yousefimanesh, H.A.; Haghighi-Zadeh, M.H.; Zakerkish, M. Impact of resveratrol supplementation on inflammatory, antioxidant, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Diabetes Metab. Syndr. 2019, 13, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- Berta, G.N.; Romano, F.; Vallone, R.; Abbadessa, G.; Di Scipio, F.; Defabianis, P. An Innovative Strategy for Oral Biofilm Control in Early Childhood Based on a Resveratrol-Cyclodextrin Nanotechnology Approach. Materials 2021, 14, 3801. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, X.; Yu, W.; Xu, Y.; Huang, R.; Park, J.; Moshaverinia, A.; Arora, P.; Chen, C. Activation of Functional Somatic Stem Cells Promotes Endogenous Tissue Regeneration. J. Dent. Res. 2022, 101, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, E.K.; Dorfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem. Cells Int. 2016, 2016, 7154327. [Google Scholar]
- Zhao, Y.; Wang, L.; Jin, Y.; Shi, S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J. Dent. Res. 2012, 91, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, M.; Trusca, V.G.; Savu, L.; Stancu, I.G.; Ratiu, A.C.; Simionescu, M.; Gafencu, A.V. Adenovirus-Mediated FasL Minigene Transfer Endows Transduced Cells with Killer Potential. Int. J. Mol. Sci. 2020, 21, 6011. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Shi, S. Interplay between mesenchymal stem cells and lymphocytes: Implications for immunotherapy and tissue regeneration. J. Dent. Res. 2012, 91, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein. Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [PubMed]
- Zhang, H.; Liu, L.; Jiang, C.; Pan, K.; Deng, J.; Wan, C. MMP9 protects against LPS-induced inflammation in osteoblasts. Innate Immun. 2020, 26, 259–269. [Google Scholar] [CrossRef]
- Liao, H.-T.; Chen, C.-T.; Chen, C.-H.; Chen, J.-P.; Tsai, J.-C. Combination of guided osteogenesis with autologous platelet-rich fibrin glue and mesenchymal stem cell for mandibular reconstruction. J. Trauma 2011, 70, 228–237. [Google Scholar] [CrossRef]
- Peng, J.; Wen, C.; Wang, A.; Wang, Y.; Xu, W.; Zhao, B.; Zhang, L.; Lu, S.; Qin, L.; Guo, Q.; et al. Micro-CT-based bone ceramic scaffolding and its performance after seeding with mesenchymal stem cells for repair of load-bearing bone defect in canine femoral head. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 316–325. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Toraldo, D.; Del Boccio, P.; Vergaro, V.; Leporatti, S.; Pieragostino, D.; Tinelli, A.; De Domenico, S.; Alberti, S.; et al. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol. Biosyst. 2012, 8, 1078–1087. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Jian, Y.; Ma, Y.; Yan, W.; Chen, X.; Xu, H.; Li, L. Resveratrol induces apoptosis in human melanoma cell through negatively regulating Erk/PKM2/Bcl-2 axis. OncoTargets Ther. 2018, 11, 8995–9006. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, K.; Su, H.; Zhang, P.; Zhao, N. Resveratrol ameliorates brain injury via the TGF-beta-mediated ERK signaling pathway in a rat model of cerebral hemorrhage. Exp. Ther. Med. 2019, 18, 3397–3404. [Google Scholar]
- Zhao, X.-E.; Yang, Z.; Zhang, H.; Yao, G.; Liu, J.; Wei, Q.; Ma, B. Resveratrol Promotes Osteogenic Differentiation of Canine Bone Marrow Mesenchymal Stem Cells through Wnt/Beta-Catenin Signaling Pathway. Cell. Reprogram. 2018, 20, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dudley, I.J.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Wu, L.; Du, L.; Ju, Q.; Chen, Z.; Ma, Y.; Bai, T.; Ji, G.; Wu, Y.; Liu, Z.; Shao, Y.; et al. Silencing TLR4/MyD88/NF-kappaB Signaling Pathway Alleviated Inflammation of Corneal Epithelial Cells Infected by ISE. Inflammation 2021, 44, 633–644. [Google Scholar] [CrossRef]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef]
- Xu, H.; Oriss, T.B.; Fei, M.; Henry, A.C.; Melgert, B.N.; Chen, L.; Mellor, A.L.; Munn, D.H.; Irvin, C.G.; Ray, P.; et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 6690–6695. [Google Scholar] [CrossRef] [PubMed]
- Cavalla, F.; Hernandez, M. Polarization Profiles of T Lymphocytes and Macrophages Responses in Periodontitis. Adv. Exp. Med. Biol. 2022, 1373, 195–208. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Ni, J.; Hu, L.; Xiang, Z.; Zeng, J.; Shi, J.; Chen, Q.; Li, W. Resveratrol May Reduce the Degree of Periodontitis by Regulating ERK Pathway in Gingival-Derived MSCs. Int. J. Mol. Sci. 2023, 24, 11294. https://doi.org/10.3390/ijms241411294
Jiang H, Ni J, Hu L, Xiang Z, Zeng J, Shi J, Chen Q, Li W. Resveratrol May Reduce the Degree of Periodontitis by Regulating ERK Pathway in Gingival-Derived MSCs. International Journal of Molecular Sciences. 2023; 24(14):11294. https://doi.org/10.3390/ijms241411294
Chicago/Turabian StyleJiang, Han, Jia Ni, Longshuang Hu, Zichao Xiang, Jincheng Zeng, Jiejun Shi, Qianming Chen, and Wen Li. 2023. "Resveratrol May Reduce the Degree of Periodontitis by Regulating ERK Pathway in Gingival-Derived MSCs" International Journal of Molecular Sciences 24, no. 14: 11294. https://doi.org/10.3390/ijms241411294
APA StyleJiang, H., Ni, J., Hu, L., Xiang, Z., Zeng, J., Shi, J., Chen, Q., & Li, W. (2023). Resveratrol May Reduce the Degree of Periodontitis by Regulating ERK Pathway in Gingival-Derived MSCs. International Journal of Molecular Sciences, 24(14), 11294. https://doi.org/10.3390/ijms241411294




