Increased Neutrophil Percentage and Neutrophil–T Cell Ratio Precedes Clinical Onset of Experimental Cerebral Malaria
Abstract
:1. Introduction
2. Results
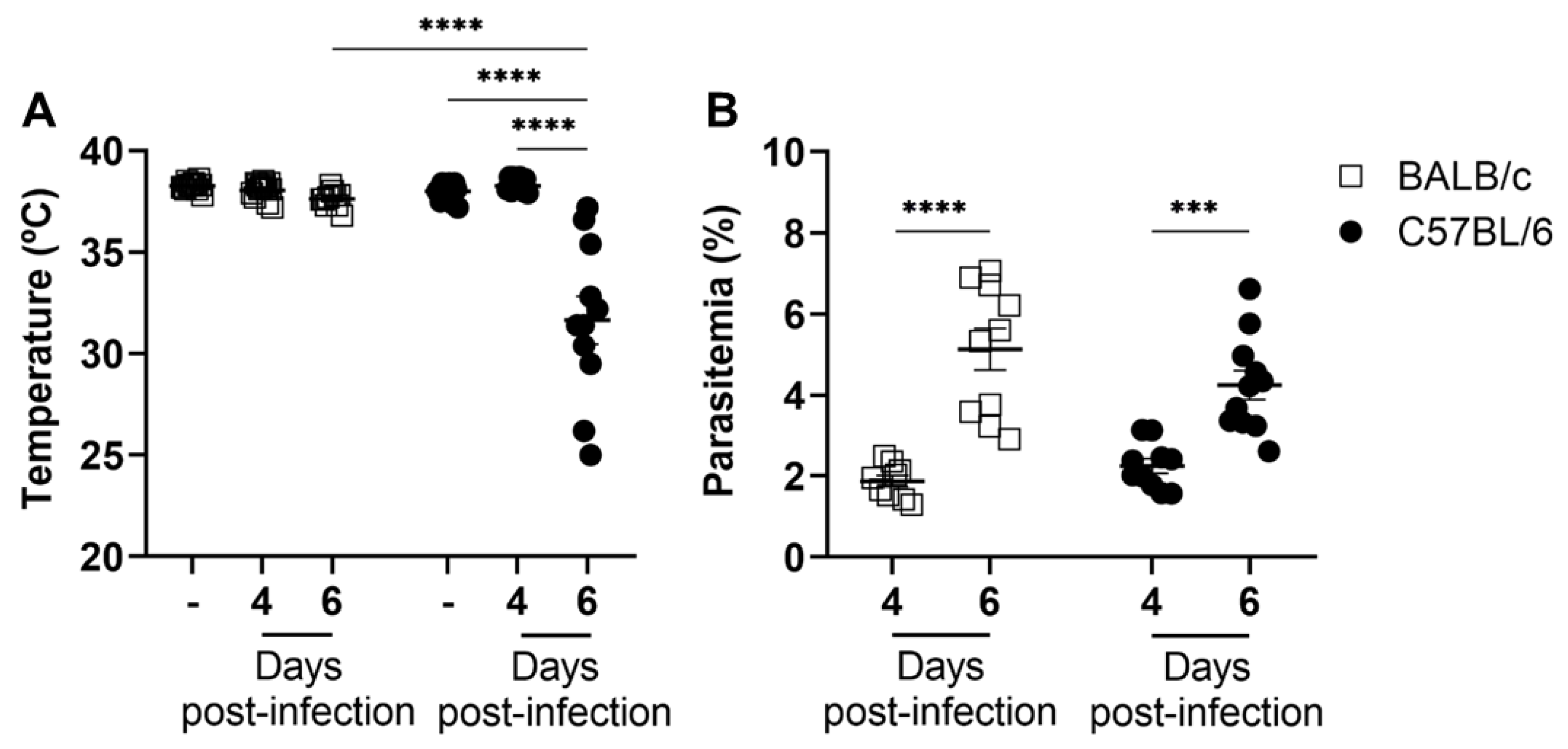
2.1. The Development of Experimental Cerebral Malaria Is Unrelated to Peripheral Parasitemia
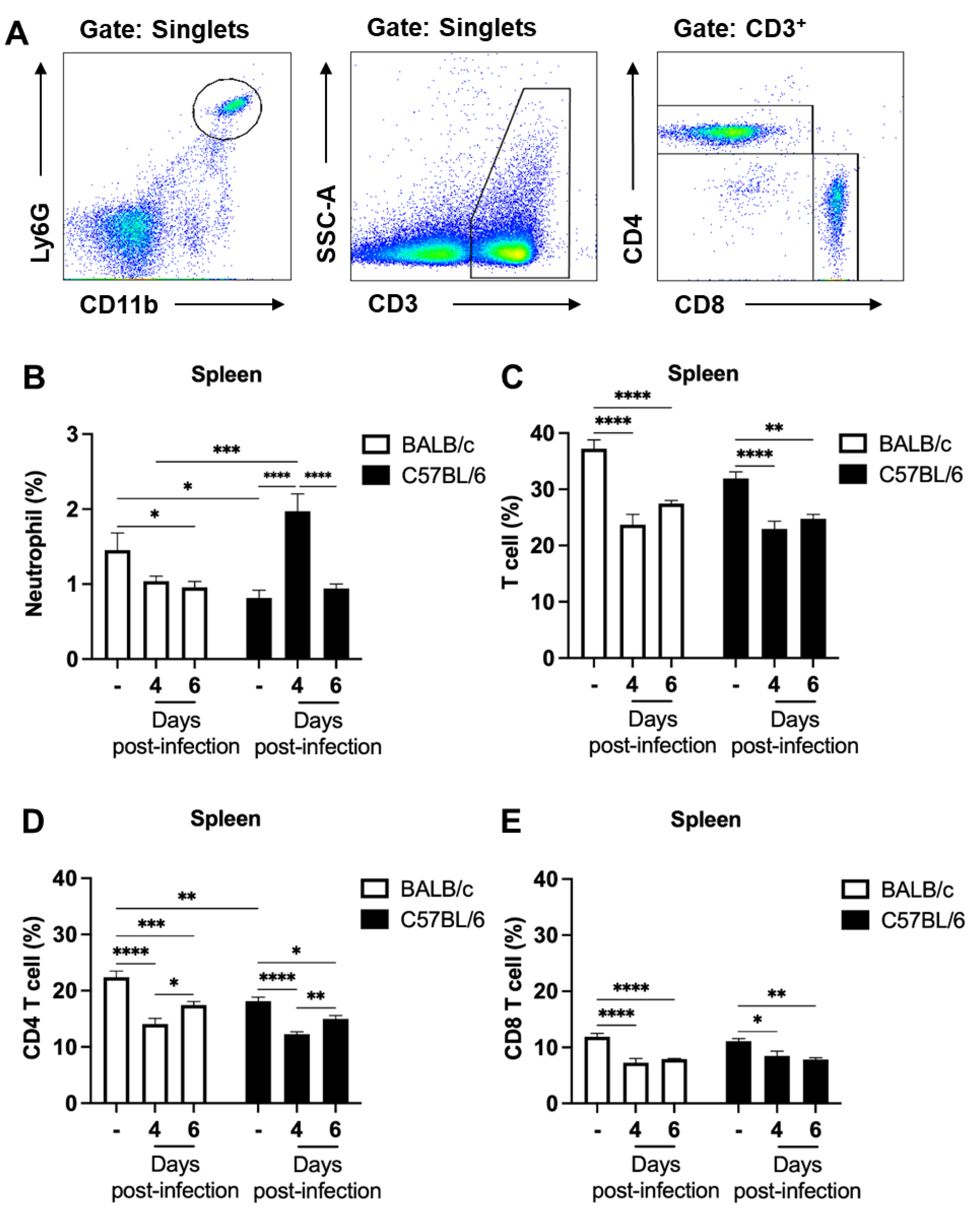
2.2. The Percentage of Neutrophils in the Spleen Significantly Increases Prior to the Development of Clinical Signs of Experimental Cerebral Malaria
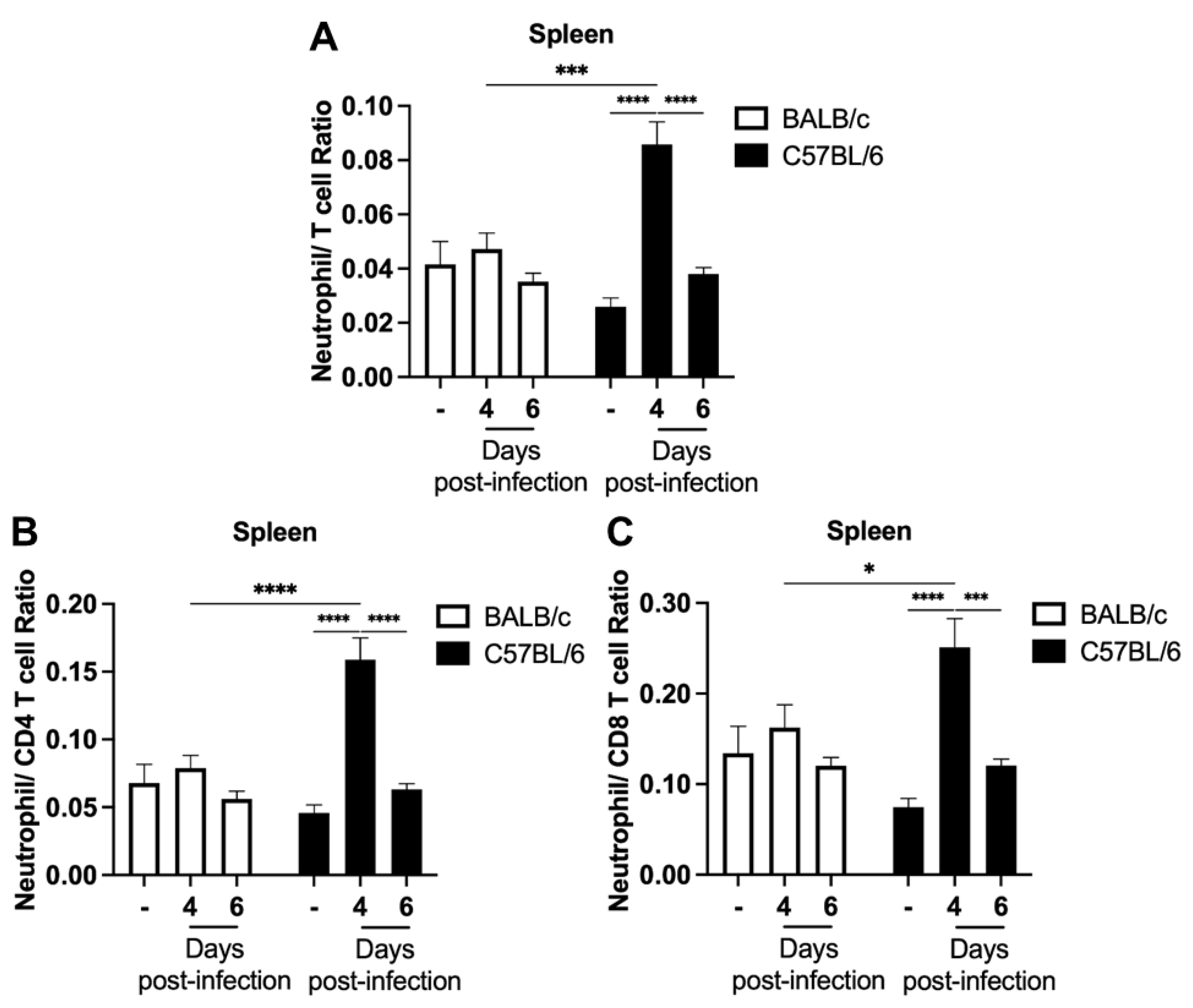
2.3. Increased Neutrophils Percentage and Neutrophil–T Cell Ratios in the Blood of Parasitized C57BL/6 Mice Precede the Development of ECM
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Parasite and Infection
4.3. Clinical Parameters
4.4. Tissue Processing and Immunophenotyping
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). World Malaria Report 2022; WHO: Geneva, Switzerland, 2022; ISBN 9789240064898. [Google Scholar]
- Stevenson, M.M.; Riley, E.M. Innate Immunity to Malaria. Nat. Rev. Immunol. 2004, 4, 169–180. [Google Scholar] [CrossRef]
- Trampuz, A.; Jereb, M.; Muzlovic, I.; Prabhu, R.M. Clinical Review: Severe Malaria. Crit. Care 2003, 7, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Viriyavejakul, P.; Khachonsaksumet, V.; Punsawad, C. Liver Changes in Severe Plasmodium falciparum Malaria: Histopathology, Apoptosis and Nuclear Factor Kappa B Expression. Malar. J. 2014, 13, 106. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfari, N.; Mueller, S.N.; Heath, W.R. Cerebral Malaria in Mouse and Man. Front. Immunol. 2018, 9, 2016. [Google Scholar] [CrossRef] [Green Version]
- Taylor, T.E.; Fu, W.J.; Carr, R.A.; Whitten, R.O.; Mueller, J.G.; Fosiko, N.G.; Lewallen, S.; Liomba, N.G.; Molyneux, M.E. Differentiating the Pathologies of Cerebral Malaria by postmortem Parasite Counts. Nat. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef]
- Mishra, S.K.; Newton, C.R.J.C. Diagnosis and Management of the Neurological Complications of falciparum Malaria. Nat. Rev. Neurol. 2009, 5, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Idro, R.; Marsh, K.; John, C.C.; Newton, C.R.J. Cerebral Malaria: Mechanisms of Brain Injury and Strategies for Improved Neurocognitive Outcome. Pediatr. Res. 2010, 68, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Sahu, P.K.; Satpathi, S.; Behera, P.K.; Mishra, S.K.; Stumhofer, J.S. Pathogenesis of Cerebral Malaria: New Diagnostic Tools, Biomarkers, and Therapeutic Approaches. Front. Cell. Infect. Microbiol. 2015, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Gazzinelli, R.T.; Kalantari, P.; Fitzgerald, K.A.; Golenbock, D.T. Innate Sensing of Malaria Parasites. Nat. Rev. Immunol. 2014, 14, 744–757. [Google Scholar] [CrossRef]
- Gowda, D.C.; Wu, X. Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria. Front. Immunol. 2018, 9, 3006. [Google Scholar] [CrossRef] [Green Version]
- Riggle, B.A.; Manglani, M.; Maric, D.; Johnson, K.R.; Lee, M.H.; Abath Neto, O.L.; Taylor, T.E.; Seydel, K.B.; Nath, A.; Miller, L.H.; et al. CD8+ T Cells Target Cerebrovasculature in Children with Cerebral Malaria. J. Clin. Investig. 2020, 130, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Howland, S.W.; Poh, C.M.; Gun, S.Y.; Claser, C.; Malleret, B.; Shastri, N.; Ginhoux, F.; Grotenbreg, G.M.; Rénia, L. Brain Microvessel Cross-Presentation Is a Hallmark of Experimental Cerebral Malaria. EMBO Mol. Med. 2013, 5, 984–999. [Google Scholar] [CrossRef]
- Knackstedt, S.L.; Georgiadou, A.; Apel, F.; Abu-abed, U.; Moxon, C.A.; Cunnington, A.J.; Raupach, B.; Cunningham, D.; Langhorne, J.; Krüger, R.; et al. Neutrophil Extracellular Traps Drive Inflammatory Pathogenesis in Malaria. Sci. Immunol. 2019, 4, eaaw0336. [Google Scholar] [CrossRef] [Green Version]
- Amulic, B.; Moxon, C.A.; Cunnington, A.J. A More Granular View of Neutrophils in Malaria. Trends Parasitol. 2020, 36, 501–503. [Google Scholar] [CrossRef]
- Feintuch, C.M.; Saidi, A.; Seydel, K.; Chen, G.; Goldman-Yassen, A.; Mita-Mendoza, N.K.; Kim, R.S.; Frenette, P.S.; Taylor, T.; Daily, J.P. Activated Neutrophils Are Associated with Pediatric Cerebral Malaria Vasculopathy in Malawian Children. MBio 2016, 7, e01300-15. [Google Scholar] [CrossRef] [Green Version]
- Schumak, B.; Klocke, K.; Kuepper, J.M.; Biswas, A.; Djie-Maletz, A.; Limmer, A.; Van Rooijen, N.; Mack, M.; Hoerauf, A.; Dunay, I.R. Specific Depletion of Ly6Chi Inflammatory Monocytes Prevents Immunopathology in Experimental Cerebral Malaria. PLoS ONE 2015, 10, e0124080. [Google Scholar] [CrossRef]
- Rocha, B.C.; Marques, P.E.; Leoratti, F.M.d.S.; Junqueira, C.; Pereira, D.B.; Antonelli, L.R.d.V.; Menezes, G.B.; Golenbock, D.T.; Gazzinelli, R.T. Type I Interferon Transcriptional Signature in Neutrophils and Low-Density Granulocytes Are Associated with Tissue Damage in Malaria. Cell Rep. 2015, 13, 2829–2841. [Google Scholar] [CrossRef] [Green Version]
- Lyke, K.E.; Diallo, D.A.; Dicko, A.; Kone, A.; Coulibaly, D.; Guindo, A.; Cissoko, Y.; Sangare, L.; Coulibaly, S.; Dakouo, B.; et al. Association of Intraleukocytic Plasmodium falciparum Malaria Pigment with Disease Severity, Clinical Manifestations, and Prognosis in Severe Malaria. Am. J. Trop. Med. Hyg. 2003, 69, 253–259. [Google Scholar] [CrossRef]
- Van Wolfswinkel, M.E.; Vliegenthart-Jongbloed, K.; De Mendonça Melo, M.; Wever, P.C.; McCall, M.B.; Koelewijn, R.; Van Hellemond, J.J.; Van Genderen, P.J. Predictive Value of Lymphocytopenia and the Neutrophil-Lymphocyte Count Ratio for Severe Imported Malaria. Malar. J. 2013, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Berens-Riha, N.; Kroidl, I.; Schunk, M.; Alberer, M.; Beissner, M.; Pritsch, M.; Kroidl, A.; Fröschl, G.; Hanus, I.; Bretzel, G.; et al. Evidence for Significant Influence of Host Immunity on Changes in Differential Blood Count during Malaria. Malar. J. 2014, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Hunt, N.H.; Grau, G.E. Cytokines: Accelerators and Brakes in the Pathogenesis of Cerebral Malaria. Trends Immunol. 2003, 24, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Rest, J.R. Cerebral Malaria in Inbred Mice. I. A New Model and Its Pathology. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Grau, G.E.; Piguet, P.; Engers, H.D.; Louis, J.A.; Vassalli, P.; Lambert, P. L3T4+ T Lymphocytes Play a Major Role in the Pathogenesis of Murine Cerebral Malaria. J. Immunol. 1986, 137, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, L.P.; Almeida, R.F.; Ribeiro-Gomes, F.L.; Moura Carvalho, L.J.; E Souza, T.M.; De Souza, D.O.G.; Daniel-Ribeiro, C.T. Long-Term Effect of Uncomplicated Plasmodium berghei ANKA Malaria on Memory and Anxiety-like Behaviour in C57BL/6 Mice. Parasites Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Cimperman, C.K.; Pena, M.; Gokcek, S.M.; Theall, B.P.; Patel, M.V.; Sharma, A.; Qi, C.; Sturdevant, D.; Miller, L.H.; Collins, P.L.; et al. Cerebral Malaria Is Regulated by Host-Mediated Changes in Plasmodium Gene Expression. MBio 2023, 14, e0339122. [Google Scholar] [CrossRef]
- Schmidt, K.E.; Kuepper, J.M.; Schumak, B.; Alferink, J.; Hofmann, A.; Howland, S.W.; Rénia, L.; Limmer, A.; Specht, S.; Hoerauf, A. Doxycycline Inhibits Experimental Cerebral Malaria by Reducing Inflammatory Immune Reactions and Tissue-Degrading Mediators. PLoS ONE 2018, 13, e0192717. [Google Scholar] [CrossRef] [Green Version]
- de Kossodo, S.; Grau, G.E. Profiles of Cytokine Production in Relation with Susceptibility to Cerebral Malaria. J. Immunol. 1993, 151, 4811–4820. [Google Scholar] [CrossRef]
- Kotepui, M.; Phunphuech, B.; Phiwklam, N.; Chupeerach, C.; Duangmano, S. Effect of Malarial Infection on Haematological Parameters in Population near Thailand-Myanmar Border. Malar. J. 2014, 13, 218. [Google Scholar] [CrossRef] [Green Version]
- Rosa-Gonçalves, P.; Ribeiro-Gomes, F.L.; Daniel-Ribeiro, C.T. Malaria Related Neurocognitive Deficits and Behavioral Alterations. Front. Cell. Infect. Microbiol. 2022, 12, 829413. [Google Scholar] [CrossRef]
- Hanum, S.P.; Hayano, M.; Kojima, S. Cytokine and Chemokine Responses in a Cerebral Malaria-Susceptible or -Resistant Strain of Mice to Plasmodium berghei ANKA Infection: Early Chemokine Expression in the Brain. Int. Immunol. 2003, 15, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Shibui, A.; Hozumi, N.; Shiraishi, C.; Sato, Y.; Iida, H.; Sugano, S.; Watanabe, J. CD4+ T Cell Response in Early Erythrocytic Stage Malaria: Plasmodium berghei Infection in BALB/c and C57BL/6 Mice. Parasitol. Res. 2009, 105, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Hafalla, J.C.R.; Claser, C.; Couper, K.N.; Grau, G.E.; Renia, L.; de Souza, J.B.; Riley, E.M. The CTLA-4 and PD-1/PD-L1 Inhibitory Pathways Independently Regulate Host Resistance to Plasmodium-Induced Acute Immune Pathology. PLoS Pathog. 2012, 8, e1002504. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Miyauchi, E.; Nakamura, S.; Hirai, M.; Suzue, K.; Imai, T.; Nomura, T.; Handa, T.; Okada, H.; Shimokawa, C.; et al. Plasmodium berghei ANKA Causes Intestinal Malaria Associated with Dysbiosis. Sci. Rep. 2015, 5, 15699. [Google Scholar] [CrossRef] [Green Version]
- Borges, T.K.S.; Alves, É.A.R.; Vasconcelos, H.A.R.; Carneiro, F.P.; Nicola, A.M.; Magalhães, K.G.; Muniz-Junqueira, M.I. Differences in the Modulation of Reactive Species, Lipid Bodies, Cyclooxygenase-2, 5-Lipoxygenase and PPAR-γ in Cerebral Malaria-Susceptible and Resistant Mice. Immunobiology 2017, 222, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, A.J.; Riley, E.M.; Walther, M. Stuck in a Rut? Reconsidering the Role of Parasite Sequestration in Severe Malaria Syndromes. Trends Parasitol. 2013, 29, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The Systemic Inflammation-Based Neutrophil-Lymphocyte Ratio: Experience in Patients with Cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- McNamara, M.G.; Templeton, A.J.; Maganti, M.; Walter, T.; Horgan, A.M.; McKeever, L.; Min, T.; Amir, E.; Knox, J.J. Neutrophil/Lymphocyte Ratio as a Prognostic Factor in Biliary Tract Cancer. Eur. J. Cancer 2014, 50, 1581–1589. [Google Scholar] [CrossRef]
- Isaac, V.; Wu, C.Y.; Huang, C.T.; Baune, B.T.; Tseng, C.L.; McLachlan, C.S. Elevated Neutrophil to Lymphocyte Ratio Predicts Mortality in Medical Inpatients with Multiple Chronic Conditions. Medicine 2016, 95, e3832. [Google Scholar] [CrossRef]
- Ozyurek, B.A.; Ozdemirel, T.S.; Ozden, S.B.; Erdogan, Y.; Kaplan, B.; Kaplan, T. Prognostic Value of the Neutrophil to Lymphocyte Ratio (NLR) in Lung Cancer Cases. Asian Pac. J. Cancer Prev. 2017, 18, 1417–1421. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Luo, X.; Hong, J.; Pan, K.; Lin, X.; Liu, X.; Zhou, L.; Wang, H.; Xu, Y.; et al. Neutrophil-to-Lymphocyte Ratio Positively Correlates to Age in Healthy Population. J. Clin. Lab. Anal. 2015, 29, 437–443. [Google Scholar] [CrossRef]
- Hickman, D.L. Evaluation of the Neutrophil:Lymphocyte Ratio as an Indicator of Chronic Distress in the Laboratory Mouse. Lab Anim. 2017, 46, 303–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamedahmed, K.A.; Abakar, A.D.; Yousif, B.; Nour, M.; Medani, W.; Medani, W.; Medani, W. Evaluation of Neutrophil Lymphocyte Ratio (NLR) in Sudanese Children with Falciparum Malaria. Int. J. Acad. Health Med. Res. 2019, 3, 1–6. [Google Scholar]
- Van Wolfswinkel, M.E.; Langenberg, M.C.C.; Wammes, L.J.; Sauerwein, R.W.; Koelewijn, R.; Hermsen, C.C.; Van Hellemond, J.J.; Van Genderen, P.J. Changes in Total and Differential Leukocyte Counts during the Clinically Silent Liver Phase in a Controlled Human Malaria Infection in Malaria-Naïve Dutch Volunteers. Malar. J. 2017, 16, 457. [Google Scholar] [CrossRef] [Green Version]
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Setyowati, E.Y.; Berbudi, A. Oral Administration of Piperine as Curative and Prophylaxis Reduces Parasitaemia in Plasmodium Berghei ANKA-Infected Mice. J. Trop. Med. 2022, 2022, 5721449. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Castro, J.; Cervantes-Candelas, L.A.; Buendía-González, F.O.; Fernández-Rivera, O.; Nolasco-Pérez, T.D.J.; López-Padilla, M.S.; Chavira-Ramírez, D.R.; Cervantes-Sandoval, A.; Legorreta-Herrera, M. Testosterone Induces Sexual Dimorphism during Infection with Plasmodium Berghei ANKA. Front. Cell. Infect. Microbiol. 2022, 12, 968325. [Google Scholar] [CrossRef]
- Chukwuocha, U.M.; Fernández-Rivera, O.; Legorreta-Herrera, M. Exploring the Antimalarial Potential of Whole Cymbopogon Citratus Plant Therapy. J. Ethnopharmacol. 2016, 193, 517–523. [Google Scholar] [CrossRef]
- Martins, Y.C.; Smith, M.J.; Pelajo-Machado, M.; Werneck, G.L.; Lenzi, H.L.; Daniel-Ribeiro, C.T.; Carvalho, L.J.D.M. Characterization of Cerebral Malaria in the Outbred Swiss Webster Mouse Infected by Plasmodium Berghei ANKA. Int. J. Exp. Pathol. 2009, 90, 119–130. [Google Scholar] [CrossRef]
- Hutchens, M.; Luker, K.E.; Sottile, P.; Sonstein, J.; Lukacs, N.W.; Núñesz, G.; Curtis, J.L.; Luker, G.D. TLR3 Increases Disease Morbidity and Mortality from Vaccinia Infection. J. Immunol. 2008, 180, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Larsen, I.V.; Clausius, H.; Kolb, A.W.; Brandt, C.R. Both CD8 + and CD4 + T Cells Contribute to Corneal Clouding and Viral Clearance Following Vaccinia Virus Infection in C57BL/6 Mice. J. Virol. 2016, 90, 6557–6572. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.P.; Saravolac, E.G.; Clement, J.G.; Nagata, L.P. Development of a Murine Hypothermia Model for Study of Respiratory Tract Influenza Virus Infection. Lab. Anim. Sci. 1997, 47, 143–147. [Google Scholar]
- Lv, J.; Hua, Y.; Wang, D.; Liu, A.; An, J.; Li, A.; Wang, Y.; Wang, X.; Jia, N.; Jiang, Q. Kinetics of Pulmonary Immune Cells, Antibody Responses and Their Correlations with the Viral Clearance of Influenza A Fatal Infection in Mice. Virol. J. 2014, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankenson, F.C.; Ruskoski, N.; Van Saun, M.; Ying, G.S.; Oh, J.; Fraser, N.W. Weight Loss and Reduced Body Temperature Determine Humane Endpoints in a Mouse Model of Ocular Herpesvirus Infection. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 277–285. [Google Scholar] [PubMed]
- Menasria, R.; Canivet, C.; Piret, J.; Boivin, G. Infiltration Pattern of Blood Monocytes into the Central Nervous System during Experimental Herpes Simplex Virus Encephalitis. PLoS ONE 2015, 10, e0145773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, S.H.C.; Sharma, N.; Kwong, A.C.; Dwivedi, D.J.; Khan, M.; Grin, P.M.; Fox-Robichaud, A.E.; Liaw, P.C. Body Temperature and Mouse Scoring Systems as Surrogate Markers of Death in Cecal Ligation and Puncture Sepsis. Intensive Care Med. Exp. 2018, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Du, F.; Averitt V, R.G.; Jakobsson, G.; Rönnow, C.F.; Rahman, M.; Schiopu, A.; Thorlacius, H. Targeting S100a9 Reduces Neutrophil Recruitment, Inflammation and Lung Damage in Abdominal Sepsis. Int. J. Mol. Sci. 2021, 22, 12923. [Google Scholar] [CrossRef]
- Stiles, B.G.; Campbell, Y.G.; Castle, R.M.; Grove, S.A. Correlation of Temperature and Toxicity in Murine Studies of Staphylococcal Enterotoxins and Toxic Shock Syndrome Toxin 1. Infect. Immun. 1999, 67, 1521–1525. [Google Scholar] [CrossRef] [Green Version]
- Schramm, R.; Thorlacius, H. Staphylococcal Enterotoxin B-Induced Acute Inflammation Is Inhibited by Dexamethasone: Important Role of CXC Chemokines KC and Macrophage Inflammatory Protein 2. Infect. Immun. 2003, 71, 2542–2547. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire-Antunes, L.; Ornellas-Garcia, U.; Rangel-Ferreira, M.V.; Ribeiro-Almeida, M.L.; de Sousa, C.H.G.; Carvalho, L.J.d.M.; Daniel-Ribeiro, C.T.; Ribeiro-Gomes, F.L. Increased Neutrophil Percentage and Neutrophil–T Cell Ratio Precedes Clinical Onset of Experimental Cerebral Malaria. Int. J. Mol. Sci. 2023, 24, 11332. https://doi.org/10.3390/ijms241411332
Freire-Antunes L, Ornellas-Garcia U, Rangel-Ferreira MV, Ribeiro-Almeida ML, de Sousa CHG, Carvalho LJdM, Daniel-Ribeiro CT, Ribeiro-Gomes FL. Increased Neutrophil Percentage and Neutrophil–T Cell Ratio Precedes Clinical Onset of Experimental Cerebral Malaria. International Journal of Molecular Sciences. 2023; 24(14):11332. https://doi.org/10.3390/ijms241411332
Chicago/Turabian StyleFreire-Antunes, Lucas, Uyla Ornellas-Garcia, Marcos Vinicius Rangel-Ferreira, Mônica Lucas Ribeiro-Almeida, Carina Heusner Gonçalves de Sousa, Leonardo José de Moura Carvalho, Cláudio Tadeu Daniel-Ribeiro, and Flávia Lima Ribeiro-Gomes. 2023. "Increased Neutrophil Percentage and Neutrophil–T Cell Ratio Precedes Clinical Onset of Experimental Cerebral Malaria" International Journal of Molecular Sciences 24, no. 14: 11332. https://doi.org/10.3390/ijms241411332




