Experimental Liver Cirrhosis Inhibits Restenosis after Balloon Angioplasty
Abstract
1. Introduction
2. Results
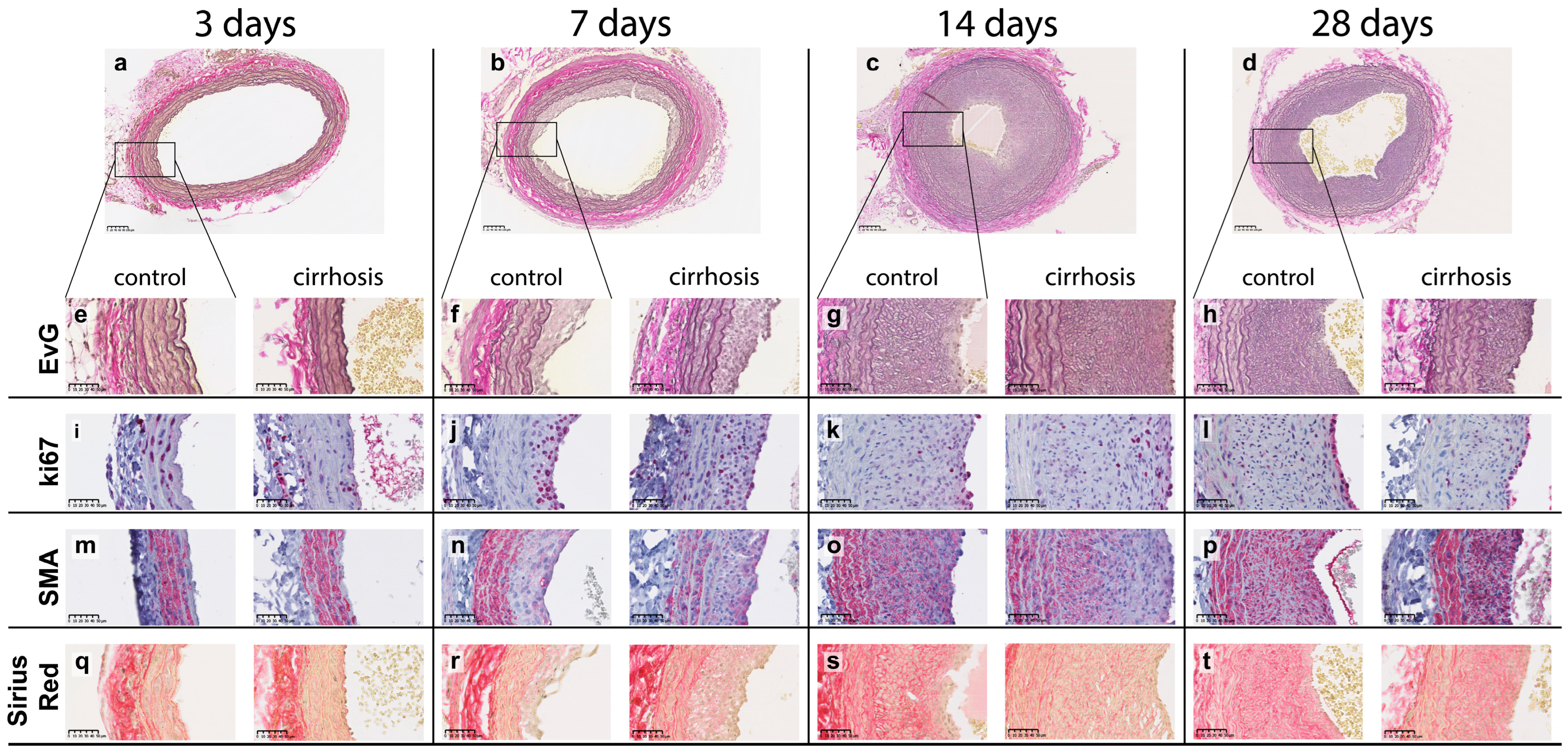
2.1. Pathology and Morphometry
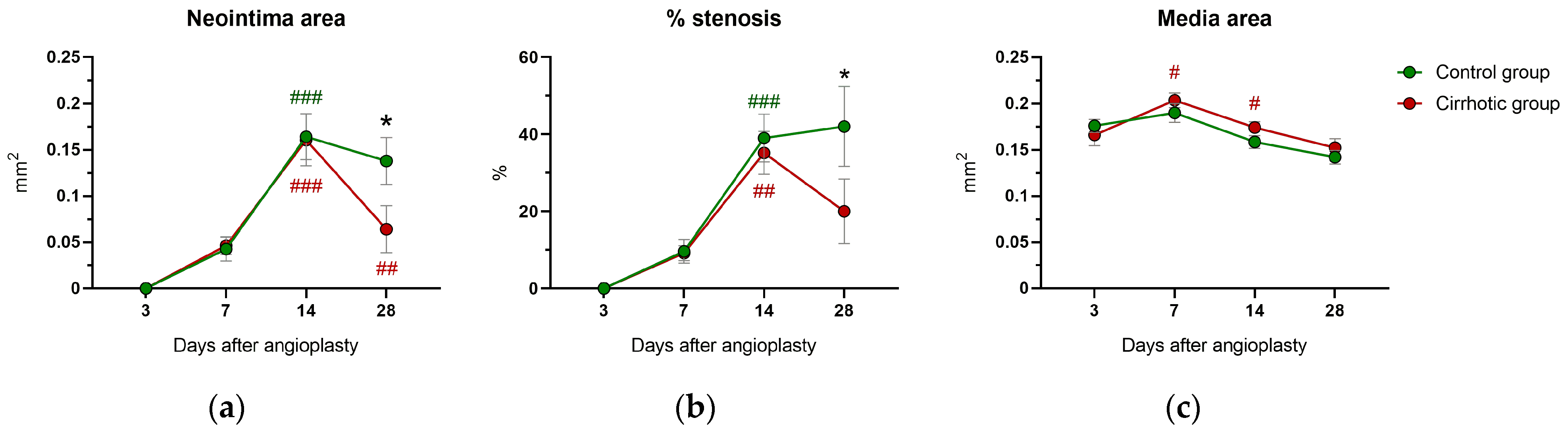
2.2. Neointima Area and Vascular Stenosis
2.3. Media Area and Circumferences
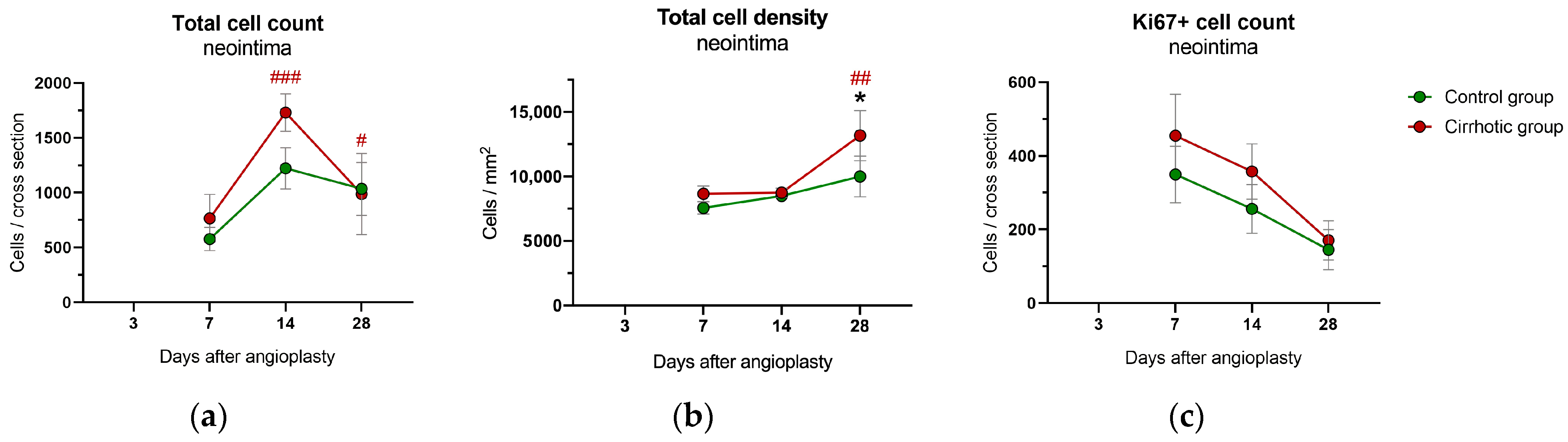
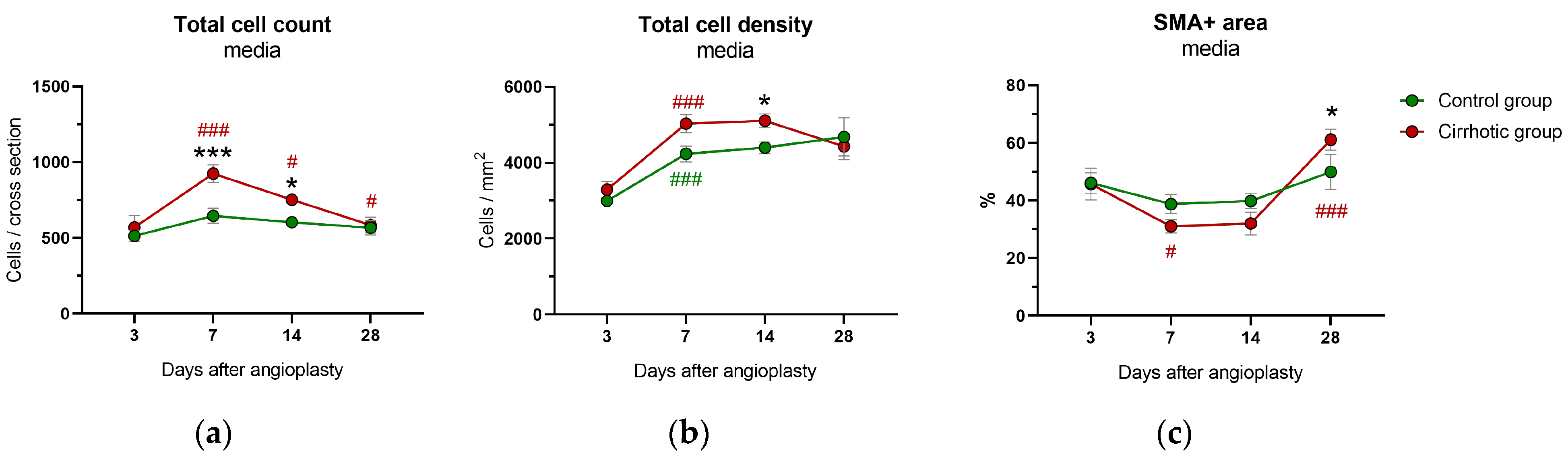
2.4. Immunohistology and Immunohistochemistry
2.4.1. Neointima
2.4.2. Media
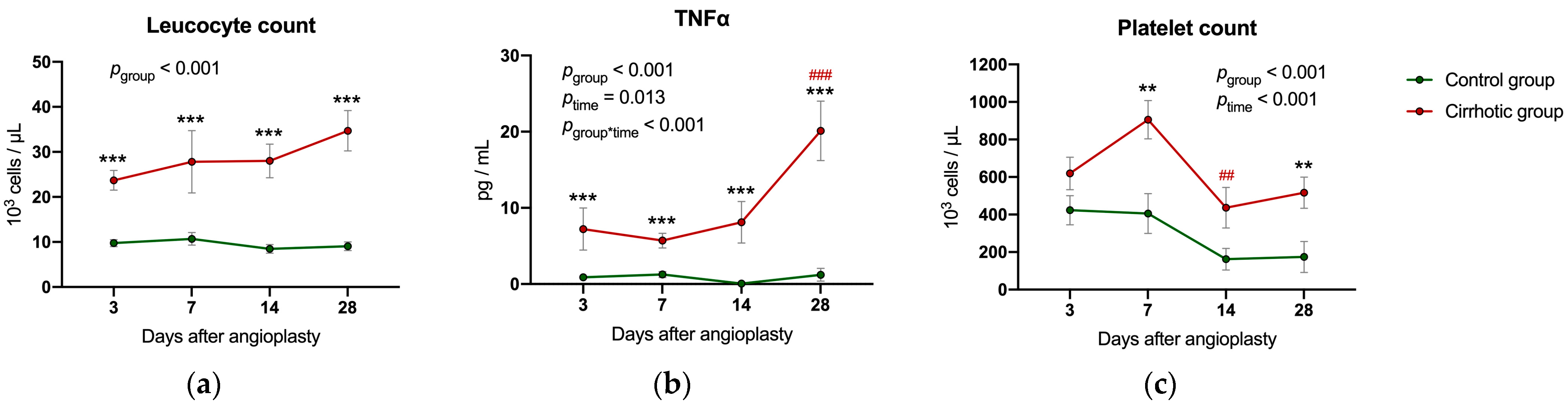
2.5. Inflammatory Parameters and Liver Values Following Balloon Dilatation
3. Discussion
3.1. Proliferative and Migratory Phase until Day 14
3.2. Neointimal Condensation 28 Days after Balloon Dilatation
3.3. Limitations
3.4. Clinical Relevance
4. Materials and Methods
4.1. Animals and Housing
4.2. Experimental Protocol
4.3. Anesthesia
4.4. Cirrhosis Induction
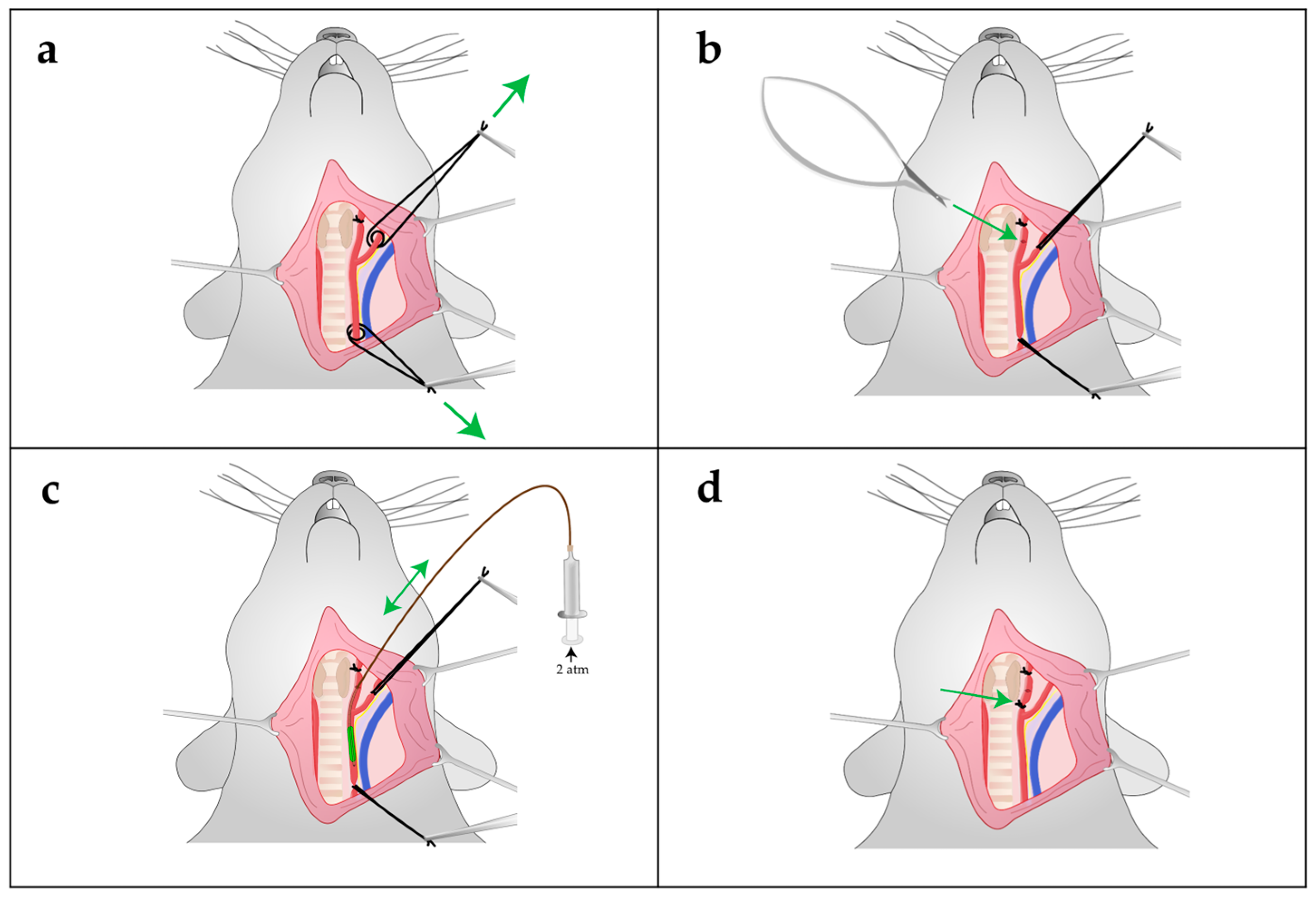
4.5. Carotid Artery Balloon Angioplasty
4.6. Follow-Up Care
4.7. Final Surgery: Sample Collection, Organ Harvesting and Sample Processing
4.8. Pathology and Morphometry
4.9. Immunohistology and Immunohistochemistry
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keeffe, B.G.; Valantine, H.; Keeffe, E.B. Detection and treatment of coronary artery disease in liver transplant candidates. Liver. Transpl. 2001, 7, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Munk, K.; Ovrehus, K.A.; Jensen, J.M.; Siggaard, C.B.; Gronbaek, H.; Norgaard, B.L.; Vilstrup, H. High burden of coronary atherosclerosis in patients with cirrhosis. Eur. J. Clin. Investig. 2017, 47, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Steitieh, D.; Feldman, D.N.; Cheung, J.W.; Wong, S.C.; Halazun, H.; Halazun, K.J.; Amin, N.; Wang, J.; Chae, J.; et al. Impact Of Cirrhosis On 90-Day Outcomes After Percutaneous Coronary Intervention (from A Nationwide Database). Am. J. Cardiol. 2020, 125, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.; Scovotti, J.C.; Tobis, J.; Niemann, C.U.; Planinsic, R.; Walia, A.; Findlay, J.; Wagener, G.; Cywinski, J.B.; Markovic, D.; et al. Liver transplantation outcome in patients with angiographically proven coronary artery disease: A multi-institutional study. Am. J. Transplant. 2013, 13, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Saybolt, M.D.; Kiss, D.H.; Matthai, W.H.; Forde, K.A.; Giri, J.; Wilensky, R.L. One-Year Outcomes of Percutaneous Coronary Intervention in Patients with End-Stage Liver Disease. Clin. Med. Insights Cardiol. 2020, 14, 1179546820901491. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Li, D.; Chen, X.; Liu, Z.; Hu, G.; Wang, J.; Ling, W. Association between liver fibrosis scores and the risk of mortality among patients with coronary artery disease. Atherosclerosis 2020, 299, 45–52. [Google Scholar] [CrossRef]
- Liu, H.H.; Cao, Y.X.; Jin, J.L.; Hua, Q.; Li, Y.F.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Gao, R.L.; Li, J.J. Liver Fibrosis Scoring Systems as Novel Tools for Predicting Cardiovascular Outcomes in Patients Following Elective Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2021, 10, e018869. [Google Scholar] [CrossRef]
- Chaabane, C.; Otsuka, F.; Virmani, R.; Bochaton-Piallat, M.L. Biological responses in stented arteries. Cardiovasc. Res. 2013, 99, 353–363. [Google Scholar] [CrossRef]
- Goel, S.A.; Guo, L.W.; Liu, B.; Kent, K.C. Mechanisms of post-intervention arterial remodelling. Cardiovasc. Res. 2012, 96, 363–371. [Google Scholar] [CrossRef]
- Zargham, R.; Pepin, J.; Thibault, G. α8β1 Integrin is up-regulated in the neointima concomitant with late luminal loss after balloon injury. Cardiovasc. Pathol. 2007, 16, 212–220. [Google Scholar] [CrossRef]
- Haarer, S.L.; Emig, L.L.; Keiser, J.A. Vascular remodeling in balloon injured rabbit iliac arteries. Basic. Res. Cardiol. 1998, 93, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Alvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; McDaid, J.; Tyagi, S.; Csizmadia, E.; Graca-Souza, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Chong Nguyen, C.; Duboc, D.; Rainteau, D.; Sokol, H.; Humbert, L.; Seksik, P.; Bellino, A.; Abdoul, H.; Bouazza, N.; Treluyer, J.M.; et al. Circulating bile acids concentration is predictive of coronary artery disease in human. Sci. Rep. 2021, 11, 22661. [Google Scholar] [CrossRef] [PubMed]
- Mechelinck, M.; Peschel, M.; Habigt, M.A.; Kroy, D.; Lehrke, M.; Helmedag, M.J.; Rossaint, R.; Barton, M.; Hein, M. Serum from Patients with Severe Alcoholic Liver Cirrhosis Inhibits Proliferation and Migration of Human Coronary Artery Smooth Muscle Cells. J. Clin. Med. 2021, 10, 5471. [Google Scholar] [CrossRef]
- Nevzorova, Y.A.; Boyer-Diaz, Z.; Cubero, F.J.; Gracia-Sancho, J. Animal models for liver disease—A practical approach for translational research. J. Hepatol. 2020, 73, 423–440. [Google Scholar] [CrossRef]
- Appanna, G.; Kallis, Y. An update on the management of cholestatic liver diseases. Clin. Med. 2020, 20, 513–516. [Google Scholar] [CrossRef]
- Mitra, A.K.; Agrawal, D.K. In stent restenosis: Bane of the stent era. J. Clin. Pathol. 2006, 59, 232–239. [Google Scholar] [CrossRef]
- Grewe, P.H.; Deneke, T.; Machraoui, A.; Barmeyer, J.; Muller, K.M. Acute and chronic tissue response to coronary stent implantation: Pathologic findings in human specimen. J. Am. Coll. Cardiol. 2000, 35, 157–163. [Google Scholar] [CrossRef]
- Riessen, R.; Wight, T.N.; Pastore, C.; Henley, C.; Isner, J.M. Distribution of Hyaluronan During Extracellular Matrix Remodeling in Human Restenotic Arteries and Balloon-Injured Rat Carotid Arteries. Circulation 1996, 93, 1141–1147. [Google Scholar] [CrossRef]
- Ebert, M.L.A.; Schmidt, V.F.; Pfaff, L.; von Thaden, A.; Kimm, M.A.; Wildgruber, M. Animal Models of Neointimal Hyperplasia and Restenosis. JACC Basic Transl. Sci. 2021, 6, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Torella, D.; Coppola, C.; Stabile, E.; Esposito, G.; Curcio, A.; Pisani, A.; Cavuto, L.; Arcucci, O.; Cireddu, M.; et al. Rat carotid artery dilation by PTCA balloon catheter induces neointima formation in presence of IEL rupture. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H760–H767. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.P.; Kenagy, R.D.; Hasenstab, D.; Bowen-Pope, D.F.; Seifert, R.A.; Coats, S.; Hawkins, S.M.; Clowes, A.W. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ. Res. 1999, 85, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; Chen, M.; Hong, T.; Zhu, L.Y.; He, D.; Feng, J.G.; Jiang, L.P. Lentivirus-mediated RNAi knockdown of the gap junction protein, Cx43, attenuates the development of vascular restenosis following balloon injury. Int. J. Mol. Med. 2015, 35, 885–892. [Google Scholar] [CrossRef]
- Creighton, W.M.; Taylor, A.J.; Dichek, D.A.; Dong, G.; Roberts, A.B.; Schulick, A.H.; Mannam, P.; Virmani, R. Regional Variability in the Time Course of TGF-β1 Expression, Cellular Proliferation and Extracellular Matrix Expansion following Arterial Injury. Growth Factors 1997, 14, 297–306. [Google Scholar] [CrossRef]
- Han, Y.; Deng, J.; Guo, L.; Yan, C.; Liang, M.; Kang, J.; Liu, H.; Graham, A.M.; Li, S. CREG promotes a mature smooth muscle cell phenotype and reduces neointimal formation in balloon-injured rat carotid artery. Cardiovasc. Res. 2008, 78, 597–604. [Google Scholar] [CrossRef]
- Park, S.H.; Marso, S.P.; Zhou, Z.; Foroudi, F.; Topol, E.J.; Lincoff, A.M. Neointimal hyperplasia after arterial injury is increased in a rat model of non-insulin-dependent diabetes mellitus. Circulation 2001, 104, 815–819. [Google Scholar] [CrossRef]
- Mills, C.J.; Northrup, J.L.; Hullinger, T.G.; Simmons, C.A.; Shebuski, R.J.; Jones, D.A. Temporal expression of c-fos mRNA following balloon injury in the rat common carotid artery. Cardiovasc. Res. 1996, 32, 954–961. [Google Scholar] [CrossRef]
- Schoenhagen, P.; Ziada, K.M.; Vince, D.G.; Nissen, S.E.; Tuzcu, E.M. Arterial remodeling and coronary artery disease: The concept of “dilated” versus “obstructive” coronary atherosclerosis. J. Am. Coll. Cardiol. 2001, 38, 297–306. [Google Scholar] [CrossRef]
- Ferns, G.A.A.; Avades, T.Y. The mechanisms of coronary restenosis: Insights from experimental models. Int. J. Exp. Pathol. 2000, 81, 63–88. [Google Scholar] [CrossRef]
- Zargham, R.; Thibault, G. α8β1 Integrin expression in the rat carotid artery: Involvement in smooth muscle cell migration and neointima formation. Cardiovasc. Res. 2005, 65, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Gold, H.K.; Tang, A.; Weber, D.K.; Wight, T.N.; Clermont, A.; Virmani, R.; Kolodgie, F.D. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J. Vasc. Res. 2002, 39, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L. Animal models of restenosis. Trends Cardiovasc. Med. 1994, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Larena-Avellaneda, A.; Wipper, S. Experimental Atherosclerosis Research on Large and Small Animal Models in Vascular Surgery. J. Vasc. Res. 2022, 59, 221–228. [Google Scholar] [CrossRef]
- Chen, C.H.; Chang, L.T.; Tung, W.C.; Chen, Y.L.; Chang, C.L.; Leu, S.; Sun, C.K.; Tsai, T.H.; Tsai, I.T.; Chang, H.W.; et al. Levels and values of circulating endothelial progenitor cells, soluble angiogenic factors, and mononuclear cell apoptosis in liver cirrhosis patients. J. Biomed. Sci. 2012, 19, 66. [Google Scholar] [CrossRef]
- Li, C.P.; Lee, F.Y.; Hwang, S.J.; Lu, R.H.; Lee, W.P.; Chao, Y.; Wang, S.S.; Chang, F.Y.; Whang-Peng, J.; Lee, S.D. Spider angiomas in patients with liver cirrhosis: Role of vascular endothelial growth factor and basic fibroblast growth factor. World J. Gastroenterol. WJG 2003, 9, 2832–2835. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Hepatitis C virus core protein augments androgen receptor-mediated signaling. J. Virol. 2008, 82, 11066–11072. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Zhuang, Z.; Bhardwaj, S.; Murakami, M.; De Muinck, E.; Yla-Herttuala, S.; Ferrara, N.; Martin, J.F.; Zachary, I.; Simons, M. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation 2004, 110, 2436–2443. [Google Scholar] [CrossRef]
- Singh, V.; Patel, N.J.; Rodriguez, A.P.; Shantha, G.; Arora, S.; Deshmukh, A.; Cohen, M.G.; Grines, C.; De Marchena, E.; Badheka, A.; et al. Percutaneous Coronary Intervention in Patients With End-Stage Liver Disease. Am. J. Cardiol. 2016, 117, 1729–1734. [Google Scholar] [CrossRef]
- Alabboodi, Y.; Al-Azzawi, Y.; Fasullo, M.; Ridha, A.; Naguib, T. The Morbidity and Mortality Risks Following Percutaneous Interventions in Cirrhosis. Am. J. Gastroenterol. 2017, 112, S496–S498. [Google Scholar] [CrossRef]
- Kalaitzakis, E.; Bjornsson, E. Coronary artery disease in liver cirrhosis: Does the aetiology of liver disease matter? J. Hepatol. 2009, 51, 962–963; Author Reply 963–964. [Google Scholar] [CrossRef]
- Patel, S.; Kiefer, T.L.; Ahmed, A.; Ali, Z.A.; Tremmel, J.A.; Lee, D.P.; Yeung, A.C.; Fearon, W.F. Comparison of the frequency of coronary artery disease in alcohol-related versus non-alcohol-related endstage liver disease. Am. J. Cardiol. 2011, 108, 1552–1555. [Google Scholar] [CrossRef]
- Nicolau-Raducu, R.; Gitman, M.; Ganier, D.; Loss, G.E.; Cohen, A.J.; Patel, H.; Girgrah, N.; Sekar, K.; Nossaman, B. Adverse cardiac events after orthotopic liver transplantation: A cross-sectional study in 389 consecutive patients. Liver. Transpl. 2015, 21, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Albeldawi, M.; Aggarwal, A.; Madhwal, S.; Cywinski, J.; Lopez, R.; Eghtesad, B.; Zein, N.N. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012, 18, 370–375. [Google Scholar] [CrossRef]
- Carey, W.D.; Dumot, J.A.; Pimentel, R.R.; Barnes, D.S.; Hobbs, R.E.; Henderson, J.M.; Vogt, D.P.; Mayes, J.T.; Westveer, M.K.; Easley, K.A. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation 1995, 59, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, E.; Rosengren, A.; Skommevik, T.; Bjornsson, E. Coronary artery disease in patients with liver cirrhosis. Dig. Dis. Sci. 2010, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Gologorsky, E.; Pretto, E.A., Jr.; Fukazawa, K. Coronary artery disease and its risk factors in patients presenting for liver transplantation. J. Clin. Anesth. 2013, 25, 618–623. [Google Scholar] [CrossRef]
- Patel, S.S.; Nabi, E.; Guzman, L.; Abbate, A.; Bhati, C.; Stravitz, R.T.; Reichman, T.; Matherly, S.C.; Driscoll, C.; Lee, H.; et al. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transplant. 2018, 24, 333–342. [Google Scholar] [CrossRef]
- Kadayifci, A.; Tan, V.; Ursell, P.C.; Merriman, R.B.; Bass, N.M. Clinical and pathologic risk factors for atherosclerosis in cirrhosis: A comparison between NASH-related cirrhosis and cirrhosis due to other aetiologies. J. Hepatol. 2008, 49, 595–599. [Google Scholar] [CrossRef]
- Krueger, J.C.; Habigt, M.A.; Helmedag, M.J.; Uhlig, M.; Moss, M.; Bleich, A.; Tolba, R.H.; Rossaint, R.; Hein, M.; Mechelinck, M. Evaluation of score parameters for severity assessment of surgery and liver cirrhosis in rats. Anim. Welf. 2023, 32, e29. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Uhlig, M.; Hein, M.; Habigt, M.A.; Tolba, R.H.; Braunschweig, T.; Helmedag, M.J.; Arici, M.; Theissen, A.; Klinkenberg, A.; Klinge, U.; et al. Cirrhotic Cardiomyopathy Following Bile Duct Ligation in Rats-A Matter of Time? Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Sci. 2020, 4, e100115. [Google Scholar] [CrossRef] [PubMed]
- Roehl, A.B.; Teubner, A.; Funcke, S.; Goetzenich, A.; Rossaint, R.; Tolba, R.; Hein, M. Accidental renal injury by an external heating device during surgery in rats. Lab. Anim. 2011, 45, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Morales, A.S.; Del Toro-Arreola, S.; Garcia-Benavides, L.; Bastidas-Ramirez, B.E.; Fafutis-Morris, M.; Pereira-Suarez, A.L.; Bueno-Topete, M.R. Liver fibrosis in bile duct-ligated rats correlates with increased hepatic IL-17 and TGF-beta2 expression. Ann. Hepatol. 2016, 15, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Beal, S.L. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 2001, 28, 481–504. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Smith, J.D.; Bryant, S.R.; Couper, L.L.; Vary, C.P.; Gotwals, P.J.; Koteliansky, V.E.; Lindner, V. Soluble transforming growth factor-beta type II receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ. Res. 1999, 84, 1212–1222. [Google Scholar] [CrossRef]
- Tao, M.; Mauro, C.R.; Yu, P.; Favreau, J.T.; Nguyen, B.; Gaudette, G.R.; Ozaki, C.K. A simplified murine intimal hyperplasia model founded on a focal carotid stenosis. Am. J. Pathol. 2013, 182, 277–287. [Google Scholar] [CrossRef]
- Ryan, S.T.; Koteliansky, V.E.; Gotwals, P.J.; Lindner, V. Transforming growth factor-beta-dependent events in vascular remodeling following arterial injury. J. Vasc. Res. 2003, 40, 37–46. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Ai, T.J.; Sun, J.Y.; Du, L.J.; Shi, C.; Li, C.; Sun, X.N.; Liu, Y.; Li, L.; Xia, Z.; Jia, L.; et al. Inhibition of neddylation by MLN4924 improves neointimal hyperplasia and promotes apoptosis of vascular smooth muscle cells through p53 and p62. Cell Death Differ. 2018, 25, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.W.; Cha, M.J.; Yoon, N.; Lee, C.Y.; Lee, J.; Seo, H.H.; Shin, S.; Choi, J.W.; Lee, S.; et al. TAK-733 inhibits inflammatory neointimal formation by suppressing proliferation, migration, and inflammation in vitro and in vivo. Exp. Mol. Med. 2018, 50, 37. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Breen, D.M.; Pereira, T.J.; Dalvi, P.S.; Zhang, H.; Mori, Y.; Ghanim, H.; Tumiati, L.; Fantus, I.G.; Bendeck, M.P.; et al. The effect of insulin to decrease neointimal growth after arterial injury is endothelial nitric oxide synthase-dependent. Atherosclerosis 2015, 241, 111–120. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechelinck, M.; Hein, M.; Kupp, C.; Braunschweig, T.; Helmedag, M.J.; Klinkenberg, A.; Habigt, M.A.; Klinge, U.; Tolba, R.H.; Uhlig, M. Experimental Liver Cirrhosis Inhibits Restenosis after Balloon Angioplasty. Int. J. Mol. Sci. 2023, 24, 11351. https://doi.org/10.3390/ijms241411351
Mechelinck M, Hein M, Kupp C, Braunschweig T, Helmedag MJ, Klinkenberg A, Habigt MA, Klinge U, Tolba RH, Uhlig M. Experimental Liver Cirrhosis Inhibits Restenosis after Balloon Angioplasty. International Journal of Molecular Sciences. 2023; 24(14):11351. https://doi.org/10.3390/ijms241411351
Chicago/Turabian StyleMechelinck, Mare, Marc Hein, Carolin Kupp, Till Braunschweig, Marius J. Helmedag, Axel Klinkenberg, Moriz A. Habigt, Uwe Klinge, René H. Tolba, and Moritz Uhlig. 2023. "Experimental Liver Cirrhosis Inhibits Restenosis after Balloon Angioplasty" International Journal of Molecular Sciences 24, no. 14: 11351. https://doi.org/10.3390/ijms241411351
APA StyleMechelinck, M., Hein, M., Kupp, C., Braunschweig, T., Helmedag, M. J., Klinkenberg, A., Habigt, M. A., Klinge, U., Tolba, R. H., & Uhlig, M. (2023). Experimental Liver Cirrhosis Inhibits Restenosis after Balloon Angioplasty. International Journal of Molecular Sciences, 24(14), 11351. https://doi.org/10.3390/ijms241411351




