Spatially Resolved Proteomic and Transcriptomic Profiling of Anaplastic Lymphoma Kinase-Rearranged Pulmonary Adenocarcinomas Reveals Key Players in Inter- and Intratumoral Heterogeneity
Abstract
:1. Introduction
2. Results
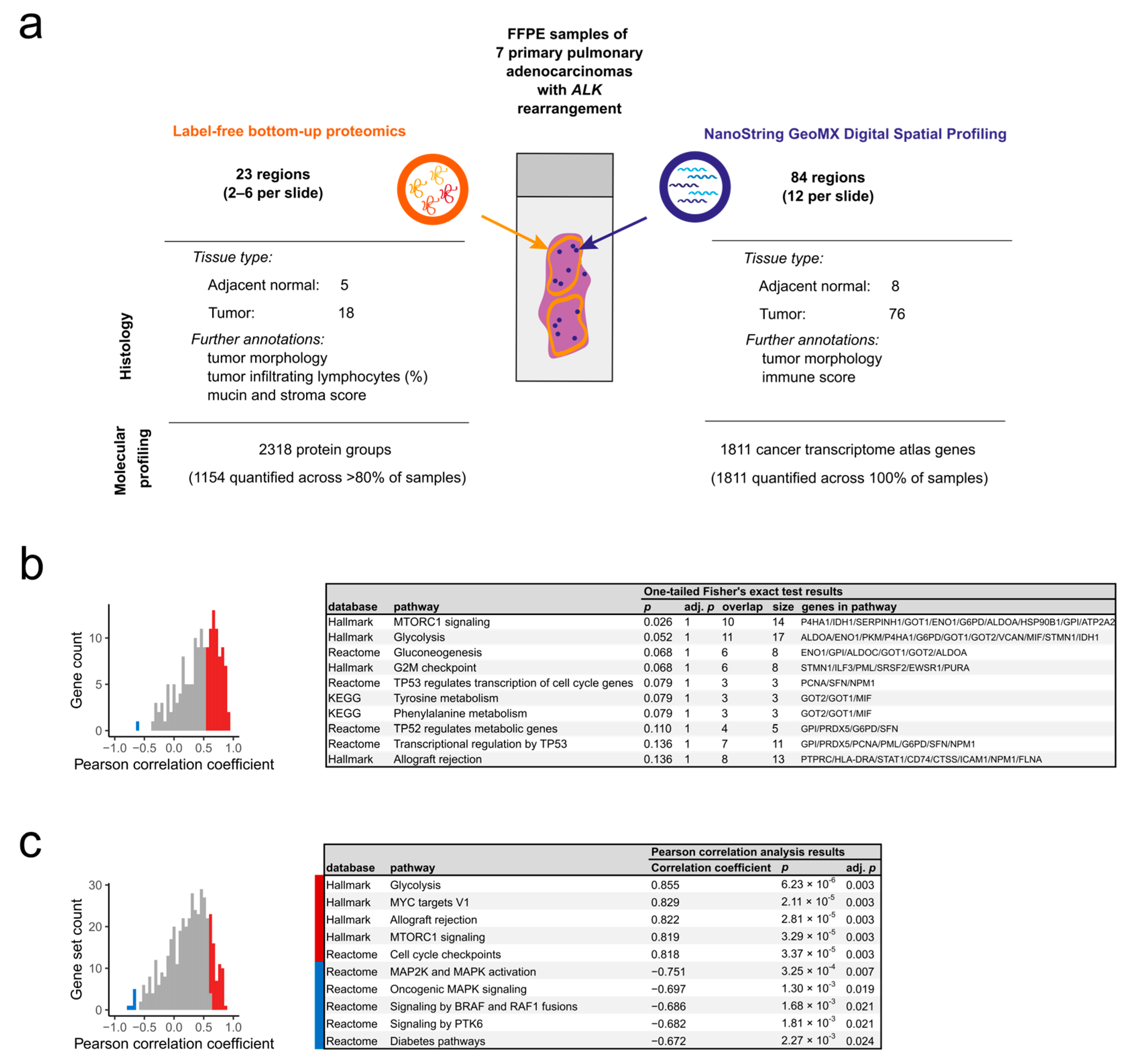
2.1. Spatially Resolved Characterization of the Proteome and Transcriptome in ALK-Rearranged pADCs
2.1.1. Histopathological Description of the Sample Cohort
2.1.2. Overview of the Collected Proteomic and Transcriptomic Data
2.2. Multi-Omic Signatures of Histopathological Features
2.2.1. Molecular Characteristics Associated with pADCs Compared to Normal Adjacent Tissues
2.2.2. Multi-Omic Signatures Related to Varying Levels of Immune Infiltration
2.2.3. Proteomic Changes Associated with Mucin and Stroma Scores
2.3. Assessment of Intratumoral Heterogeneity in Seven ALK-Rearranged pADC Cases
2.3.1. Homogeneously Expressed Proteins and Genes within the Tumors and Associated Pathways
2.3.2. Key Players in Intratumoral Heterogeneity
2.3.3. Co-Localization Patterns of Tumor Microenvironment Elements
3. Discussion
4. Materials and Methods
4.1. Patients and Collected Histopathological Data
4.2. NanoString GeoMx Profiling
4.3. Mass-Spectrometry-Based Proteomic Measurements
4.3.1. On-Tissue Proteolytic Digestion and Solid-Phase Extraction Purification
4.3.2. nanoUHPLC–MS/MS Measurements
4.4. Data Analysis
4.4.1. Quality Control and Data Normalization
4.4.2. Retrieval of External Proteomic and Transcriptomic Datasets
4.4.3. Calculation of Single-Sample Gene Set Enrichment Scores
4.4.4. Abundance Estimation of Tumor Microenvironment Elements
4.4.5. Cluster Analyses
4.4.6. Correlation Analyses
4.4.7. Differential Expression Analyses
4.4.8. Cox Regression Analyses
4.4.9. Selection of Proteins and Genes with Stable and Variable Expression within Tumors
4.4.10. Pathway Analyses
4.4.11. Visualizations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer Statistics. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 9 June 2023).
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasaki, T.; Moore, D.A.; Veeriah, S.; Naceur-Lombardelli, C.; Toncheva, A.; Magno, N.; Ward, S.; Al Bakir, M.; Watkins, T.B.K.; Grigoriadis, K.; et al. Evolutionary characterization of lung adenocarcinoma morphology in TRACERx. Nat. Med. 2023, 29, 833–845. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Roumeliotis, T.I.; Chang, Y.-H.; Chen, C.-T.; Han, C.-L.; Lin, M.-H.; Chen, H.-W.; Chang, G.-C.; Chang, Y.-L.; Wu, C.-T.; et al. Proteogenomics of Non-Smoking Lung Cancer in East Asia Delineates Molecular Signatures of Pathogenesis and Progression. Cell 2020, 182, 226–244.e17. [Google Scholar] [CrossRef] [PubMed]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.-W.; Reva, B.; et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225.e35. [Google Scholar] [CrossRef] [PubMed]
- Lehtiö, J.; Arslan, T.; Siavelis, I.; Pan, Y.; Socciarelli, F.; Berkovska, O.; Umer, H.M.; Mermelekas, G.; Pirmoradian, M.; Jönsson, M.; et al. Proteogenomics of non-small cell lung cancer reveals molecular subtypes associated with specific therapeutic targets and immune-evasion mechanisms. Nat. Cancer 2021, 2, 1224–1242. [Google Scholar] [CrossRef] [PubMed]
- Soltis, A.R.; Bateman, N.W.; Liu, J.; Nguyen, T.; Franks, T.J.; Zhang, X.; Dalgard, C.L.; Viollet, C.; Somiari, S.; Yan, C.; et al. Proteogenomic analysis of lung adenocarcinoma reveals tumor heterogeneity, survival determinants, and therapeutically relevant pathways. Cell Rep. Med. 2022, 3, 100819. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a Kinase Gene, ALK, to a Nucleolar Protein Gene, NPM, in Non-Hodgkin’s Lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, L.; Sun, Y.; Stebbing, J.; Selvaggi, G.; Zhang, Y.; Yu, Z. Targeting ALK Rearrangements in NSCLC: Current State of the Art. Front. Oncol. 2022, 12, 863461. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-Y.; Leem, S.-H.; Lee, J.H.; Kim, H.S. Dual Relationship Between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front. Immunol. 2022, 13, 864739. [Google Scholar] [CrossRef]
- Monkman, J.; Taheri, T.; Warkiani, M.E.; O’leary, C.; Ladwa, R.; Richard, D.; O’byrne, K.; Kulasinghe, A. High-Plex and High-throughput Digital Spatial Profiling of Non-Small-Cell Lung Cancer (NSCLC). Cancers 2020, 12, 3551. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Gupta, S.; Liu, Y.; Fuhrman, K.; Gettinger, S.; Herbst, R.S.; Schalper, K.A.; Rimm, D.L. Biomarkers Associated with Beneficial PD-1 Checkpoint Blockade in Non–Small Cell Lung Cancer (NSCLC) Identified Using High-Plex Digital Spatial Profiling. Clin. Cancer Res. 2020, 26, 4360–4368. [Google Scholar] [CrossRef] [PubMed]
- Larroquette, M.; Guegan, J.-P.; Besse, B.; Cousin, S.; Brunet, M.; Le Moulec, S.; Le Loarer, F.; Rey, C.; Soria, J.-C.; Barlesi, F.; et al. Spatial transcriptomics of macrophage infiltration in non-small cell lung cancer reveals determinants of sensitivity and resistance to anti-PD1/PD-L1 antibodies. J. Immunother. Cancer 2022, 10, e003890. [Google Scholar] [CrossRef] [PubMed]
- Moutafi, M.K.; Molero, M.; Morilla, S.M.; Baena, J.; Vathiotis, I.A.; Gavrielatou, N.; Castro-Labrador, L.; de Garibay, G.R.; Adradas, V.; Orive, D.; et al. Spatially resolved proteomic profiling identifies tumor cell CD44 as a biomarker associated with sensitivity to PD-1 axis blockade in advanced non-small-cell lung cancer. J. Immunother. Cancer 2022, 10, e004757. [Google Scholar] [CrossRef]
- Balbisi, M.; Sugár, S.; Schlosser, G.; Szeitz, B.; Fillinger, J.; Moldvay, J.; Drahos, L.; Szász, A.M.; Tóth, G.; Turiák, L. Inter- and intratumoral proteomics and glycosaminoglycan characterization of ALK rearranged lung adenocarcinoma tissues: A pilot study. Sci. Rep. 2023, 13, 6268. [Google Scholar] [CrossRef]
- Lazar, C.; Gatto, L.; Ferro, M.; Bruley, C.; Burger, T. Accounting for the Multiple Natures of Missing Values in Label-Free Quantitative Proteomics Data Sets to Compare Imputation Strategies. J. Proteome Res. 2016, 15, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.L.; Ocampo, P.S.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef]
- Stewart, P.A.; Parapatics, K.; Welsh, E.A.; Müller, A.C.; Cao, H.; Fang, B.; Koomen, J.M.; Eschrich, S.A.; Bennett, K.L.; Haura, E.B. A Pilot Proteogenomic Study with Data Integration Identifies MCT1 and GLUT1 as Prognostic Markers in Lung Adenocarcinoma. PLoS ONE 2015, 10, e0142162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpnack, M.F.; Ranbaduge, N.; Srivastava, A.; Cerciello, F.; Codreanu, S.G.; Liebler, D.C.; Mascaux, C.; Miles, W.O.; Morris, R.; McDermott, J.E.; et al. Proteogenomic Analysis of Surgically Resected Lung Adenocarcinoma. J. Thorac. Oncol. 2018, 13, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Y.; Zhang, C.; Wang, X.; Zhai, L.; Ma, Y.; Mao, Y.; Qian, K.; Sun, C.; Liu, Z.; Jiang, S.; et al. Integrative Proteomic Characterization of Human Lung Adenocarcinoma. Cell 2020, 182, 245–261.e17. [Google Scholar] [CrossRef] [PubMed]
- Ruggles, K.V.; Krug, K.; Wang, X.; Clauser, K.R.; Wang, J.; Payne, S.H.; Fenyö, D.; Zhang, B.; Mani, D.R. Methods, Tools and Current Perspectives in Proteogenomics. Mol. Cell. Proteom. 2017, 16, 959–981. [Google Scholar] [CrossRef] [Green Version]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Wei, R.; Qiu, H.; Xu, J.; Mo, J.; Liu, Y.; Gui, Y.; Huang, G.; Zhang, S.; Yao, H.; Huang, X.; et al. Expression and prognostic potential of GPX1 in human cancers based on data mining. Ann. Transl. Med. 2020, 8, 124. [Google Scholar] [CrossRef]
- Tian, T.; Qi, C.; Luo, X.; Tu, Q.; Tong, G.; Zhang, Z. Molecular Mechanisms and Prognostic Value of the Selenoprotein Gene Family in Lung Adenocarcinoma and Lung Squamous Cell Carcinoma. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Chen, B.; Shen, Z.; Wu, D.; Xie, X.; Xu, X.; Lv, L.; Dai, H.; Chen, J.; Gan, X. Glutathione Peroxidase 1 Promotes NSCLC Resistance to Cisplatin via ROS-Induced Activation of PI3K/AKT Pathway. BioMed. Res. Int. 2019, 2019, e7640547. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, H.; Zhou, J.; Shao, Q. Glutathione Peroxidase GPX1 and Its Dichotomous Roles in Cancer. Cancers 2022, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhasan, B.; Mikeladze, M.; Guzhova, I.; Margulis, B. Autophagy, molecular chaperones, and unfolded protein response as promoters of tumor recurrence. Cancer Metastasis Rev. 2023, 42, 217–254. [Google Scholar] [CrossRef]
- Pandolfi, P.P. Aberrant mRNA translation in cancer pathogenesis: An old concept revisited comes finally of age. Oncogene 2004, 23, 3134–3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, A.L.; Cox, T.R. The Role of the ECM in Lung Cancer Dormancy and Outgrowth. Front. Oncol. 2020, 10, 1766. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Schneider, J.L.; Lin, J.J.; Shaw, A.T. ALK-positive lung cancer: A moving target. Nat. Cancer 2023, 4, 330–343. [Google Scholar] [CrossRef]
- Pu, D.; Yin, L.; Huang, L.; Qin, C.; Zhou, Y.; Wu, Q.; Li, Y.; Zhou, Q.; Li, L. Cyclooxygenase-2 Inhibitor: A Potential Combination Strategy With Immunotherapy in Cancer. Front. Oncol. 2021, 11, 637504. [Google Scholar] [CrossRef]
- Inamura, K.; Takeuchi, K.; Togashi, Y.; Hatano, S.; Ninomiya, H.; Motoi, N.; Mun, M.; Sakao, Y.; Okumura, S.; Nakagawa, K.; et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod. Pathol. 2009, 22, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Rodig, S.J.; Mino-Kenudson, M.; Dacic, S.; Yeap, B.Y.; Shaw, A.; Barletta, J.A.; Stubbs, H.; Law, K.; Lindeman, N.; Mark, E.; et al. Unique Clinicopathologic Features Characterize ALK-Rearranged Lung Adenocarcinoma in the Western Population. Clin. Cancer Res. 2009, 15, 5216–5223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokoji, R.; Yamasaki, T.; Minami, S.; Komuta, K.; Sakamaki, Y.; Takeuchi, K.; Tsujimoto, M. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J. Clin. Pathol. 2010, 63, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, I.; Ponnusamy, M.P.; Macha, M.A.; Haridas, D.; Majhi, P.D.; Kaur, S.; Jain, M.; Batra, S.K.; Ganti, A.K. Mucins in Lung Cancer: Diagnostic, Prognostic, and Therapeutic Implications. J. Thorac. Oncol. 2015, 10, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.Z.; Liguori, G.; Aquino, G.; La Mantia, E.; Bosari, S.; Ferrero, S.; Rosso, L.; Gaudioso, G.; De Rosa, N.; Scrima, M.; et al. Intratumor Heterogeneity of ALK-Rearrangements and Homogeneity of EGFR-Mutations in Mixed Lung Adenocarcinoma. PLoS ONE 2015, 10, e0139264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int. J. Mol. Sci. 2018, 19, 3448. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Lin, J.J.; Riely, G.J.; Shaw, A.T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov. 2017, 7, 137–155. [Google Scholar] [CrossRef] [Green Version]
- Jena, M.K.; Janjanam, J. Role of extracellular matrix in breast cancer development: A brief update. F1000Research 2018, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Elvira, M.; Sunnerhagen, P. Post-transcriptional regulation during stress. FEMS Yeast Res. 2022, 22, foac025. [Google Scholar] [CrossRef]
- Lin, T.-C.; Yang, C.-H.; Cheng, L.-H.; Chang, W.-T.; Lin, Y.-R.; Cheng, H.-C. Fibronectin in Cancer: Friend or Foe. Cells 2020, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Modugno, F.; Spada, S.; Palermo, B.; Visca, P.; Iapicca, P.; Di Carlo, A.; Antoniani, B.; Sperduti, I.; Di Benedetto, A.; Terrenato, I.; et al. hMENA isoforms impact NSCLC patient outcome through fibronectin/β1 integrin axis. Oncogene 2018, 37, 5605–5617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.-J.; Sung, J.Y.; Kim, S.-H.; Yun, U.-J.; Kim, H.; Jang, E.-J.; Yoo, H.-E.; Hong, E.K.; Goh, S.-H.; Moon, A.; et al. Fibronectin regulates anoikis resistance via cell aggregate formation. Cancer Lett. 2021, 508, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Ishida, T.; Oka, T.; Yasumoto, K.; Sugimachi, K. Distribution of fibronectin in adenocarcinoma of the lung: Classification and prognostic significance. J. Surg. Oncol. 1990, 43, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Kong, Y.; Wang, X.; Li, W.; Chen, S.; Wang, L.; Wang, C.; Zhang, Q. LC-MS/MS-Based Quantitative Proteomics Analysis of Different Stages of Non-Small-Cell Lung Cancer. BioMed. Res. Int. 2021, 2021, e5561569. [Google Scholar] [CrossRef]
- Han, J.-Y.; Kim, H.S.; Lee, S.H.; Park, W.S.; Lee, J.Y.; Yoo, N.J. Immunohistochemical expression of integrins and extracellular matrix proteins in non-small cell lung cancer: Correlation with lymph node metastasis. Lung Cancer 2003, 41, 65–70. [Google Scholar] [CrossRef]
- Sung, W.J.; Park, K.-S.; Kwak, S.G.; Hyun, D.-S.; Jang, J.S.; Park, K.-K. Epithelial-mesenchymal transition in patients of pulmonary adenocarcinoma: Correlation with cancer stem cell markers and prognosis. Int. J. Clin. Exp. Pathol. 2015, 8, 8997–9009. [Google Scholar]
- Maley, C.C.; Koelble, K.; Natrajan, R.; Aktipis, A.; Yuan, Y. An ecological measure of immune-cancer colocalization as a prognostic factor for breast cancer. Breast Cancer Res. 2015, 17, 131. [Google Scholar] [CrossRef]
- De Jong, V.M.T.; Wang, Y.; ter Hoeve, N.D.; Opdam, M.; Stathonikos, N.; Jóźwiak, K.; Hauptmann, M.; Cornelissen, S.; Vreuls, W.; Rosenberg, E.H.; et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy. J. Clin. Oncol. 2022, 40, 2361–2374. [Google Scholar] [CrossRef]
- Turiák, L.; Ozohanics, O.; Tóth, G.; Ács, A.; Révész, Á.; Vékey, K.; Telekes, A.; Drahos, L. High sensitivity proteomics of prostate cancer tissue microarrays to discriminate between healthy and cancerous tissue. J. Proteom. 2018, 197, 82–91. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Bugyi, F.; Tóth, G.; Kovács, K.B.; Drahos, L.; Turiák, L. Comparison of solid-phase extraction methods for efficient purification of phosphopeptides with low sample amounts. J. Chromatogr. A 2022, 1685, 463597. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Foroutan, M.; Bhuva, D.D.; Lyu, R.; Horan, K.; Cursons, J.; Davis, M.J. Single sample scoring of molecular phenotypes. BMC Bioinform. 2018, 19, 404. [Google Scholar] [CrossRef] [Green Version]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [Green Version]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The Reactome Pathway Knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Danaher, P.; Kim, Y.; Nelson, B.; Griswold, M.; Yang, Z.; Piazza, E.; Beechem, J.M. Advances in mixed cell deconvolution enable quantification of cell types in spatial transcriptomic data. Nat. Commun. 2022, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowski, D.; Ben-Shachar, M.S.; Patil, I.; Lüdecke, D. Methods and Algorithms for Correlation Analysis in R. J. Open Source Softw. 2020, 5, 2306. [Google Scholar] [CrossRef]
- Rivellese, F.; Surace, A.E.A.; Goldmann, K.; Sciacca, E.; Çubuk, C.; Giorli, G.; John, C.R.; Nerviani, A.; Fossati-Jimack, L.; Thorborn, G.; et al. Rituximab versus tocilizumab in rheumatoid arthritis: Synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat. Med. 2022, 28, 1256–1268. [Google Scholar] [CrossRef]
- Korotkevich, G.; Sukhov, V.; Budin, N.; Shpak, B.; Artyomov, M.N.; Sergushichev, A. Fast gene set enrichment analysis. bioRxiv 2021, 060012. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]


| Information | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|---|
| Clinical data | Sex | male | male | male | female | female | female | male |
| Age at diagnosis (yrs) | 53.6 | 43.7 | 68.9 | 32.8 | 68.5 | 64.2 | 66.7 | |
| Stage on presentation | NA | NA | NA | 3 | NA | 4 | 3 | |
| Administered ALKi | Crizotinib | Crizotinib | Crizotinib | Crizotinib | Crizotinib | Alectinib | Alectinib | |
| Alive | no | yes | no | yes | yes | yes | yes | |
| OS (yrs) | 2.2 | 6.6 | 4.1 | 13.8 | 7.3 | 4.9 | 6.7 | |
| Proteomic data | Nr. of pROIs | 2 | 2 | 6 | 3 | 4 | 3 | 3 |
| Morphology of pROIs (nr.) | tubular (2) | NAT (1), solid (1) | NAT (3), papillary (2), tubular (1) | papillary (3) | solid (4) | solid (3) | NAT (1), solid (2) | |
| Avr. TIL (%) | 25.00 | 25.00 | 15.00 | 23.33 | 8.75 | 28.33 | 30.00 | |
| Avr. mucin score | 3.00 | 2.00 | 2.00 | 3.00 | 0.00 | 0.00 | 0.50 | |
| Avr. stroma score | 3.00 | 2.00 | 1.67 | 2.33 | 1.75 | 1.00 | 3.00 | |
| Transcriptomic data | Nr. of tROIs | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Morphology of tROIs (nr.) | NAT (1), tubular (11) | NAT (2), solid (10) | NAT (2), papillary (8), tubular (2) | NAT (1), papillary (11) | solid (12) | solid (12) | NAT (2), solid (10) | |
| Avr. immune score | 2.25 | 2.00 | 2.00 | 1.42 | 2.25 | 1.60 | 1.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeitz, B.; Glasz, T.; Herold, Z.; Tóth, G.; Balbisi, M.; Fillinger, J.; Horváth, S.; Mohácsi, R.; Kwon, H.J.; Moldvay, J.; et al. Spatially Resolved Proteomic and Transcriptomic Profiling of Anaplastic Lymphoma Kinase-Rearranged Pulmonary Adenocarcinomas Reveals Key Players in Inter- and Intratumoral Heterogeneity. Int. J. Mol. Sci. 2023, 24, 11369. https://doi.org/10.3390/ijms241411369
Szeitz B, Glasz T, Herold Z, Tóth G, Balbisi M, Fillinger J, Horváth S, Mohácsi R, Kwon HJ, Moldvay J, et al. Spatially Resolved Proteomic and Transcriptomic Profiling of Anaplastic Lymphoma Kinase-Rearranged Pulmonary Adenocarcinomas Reveals Key Players in Inter- and Intratumoral Heterogeneity. International Journal of Molecular Sciences. 2023; 24(14):11369. https://doi.org/10.3390/ijms241411369
Chicago/Turabian StyleSzeitz, Beáta, Tibor Glasz, Zoltán Herold, Gábor Tóth, Mirjam Balbisi, János Fillinger, Szabolcs Horváth, Réka Mohácsi, Ho Jeong Kwon, Judit Moldvay, and et al. 2023. "Spatially Resolved Proteomic and Transcriptomic Profiling of Anaplastic Lymphoma Kinase-Rearranged Pulmonary Adenocarcinomas Reveals Key Players in Inter- and Intratumoral Heterogeneity" International Journal of Molecular Sciences 24, no. 14: 11369. https://doi.org/10.3390/ijms241411369
APA StyleSzeitz, B., Glasz, T., Herold, Z., Tóth, G., Balbisi, M., Fillinger, J., Horváth, S., Mohácsi, R., Kwon, H. J., Moldvay, J., Turiák, L., & Szász, A. M. (2023). Spatially Resolved Proteomic and Transcriptomic Profiling of Anaplastic Lymphoma Kinase-Rearranged Pulmonary Adenocarcinomas Reveals Key Players in Inter- and Intratumoral Heterogeneity. International Journal of Molecular Sciences, 24(14), 11369. https://doi.org/10.3390/ijms241411369






