Quantitative Threshold Determination of Auditory Brainstem Responses in Mouse Models
Abstract
:1. Introduction
- i.
- The approximate curve of the correlation coefficient versus the sound pressure level function should be unified with only the sigmoid function;
- ii.
- Instead of judging whether sigmoid function fitting was possible based on criterion a < criterion < b, 0.005 < d < 0.999 (Equation (1)) after fitting, the cross-covariance with the ideal ABR waveforms (template) was calculated as soon as the ABR waveforms were obtained.
- x = lower level of correlated pairs (in dB SPL); y = correlation coefficient;
- a = minimum value; b = maximum value;
- c = x value at midpoint; d = slope.
2. Results
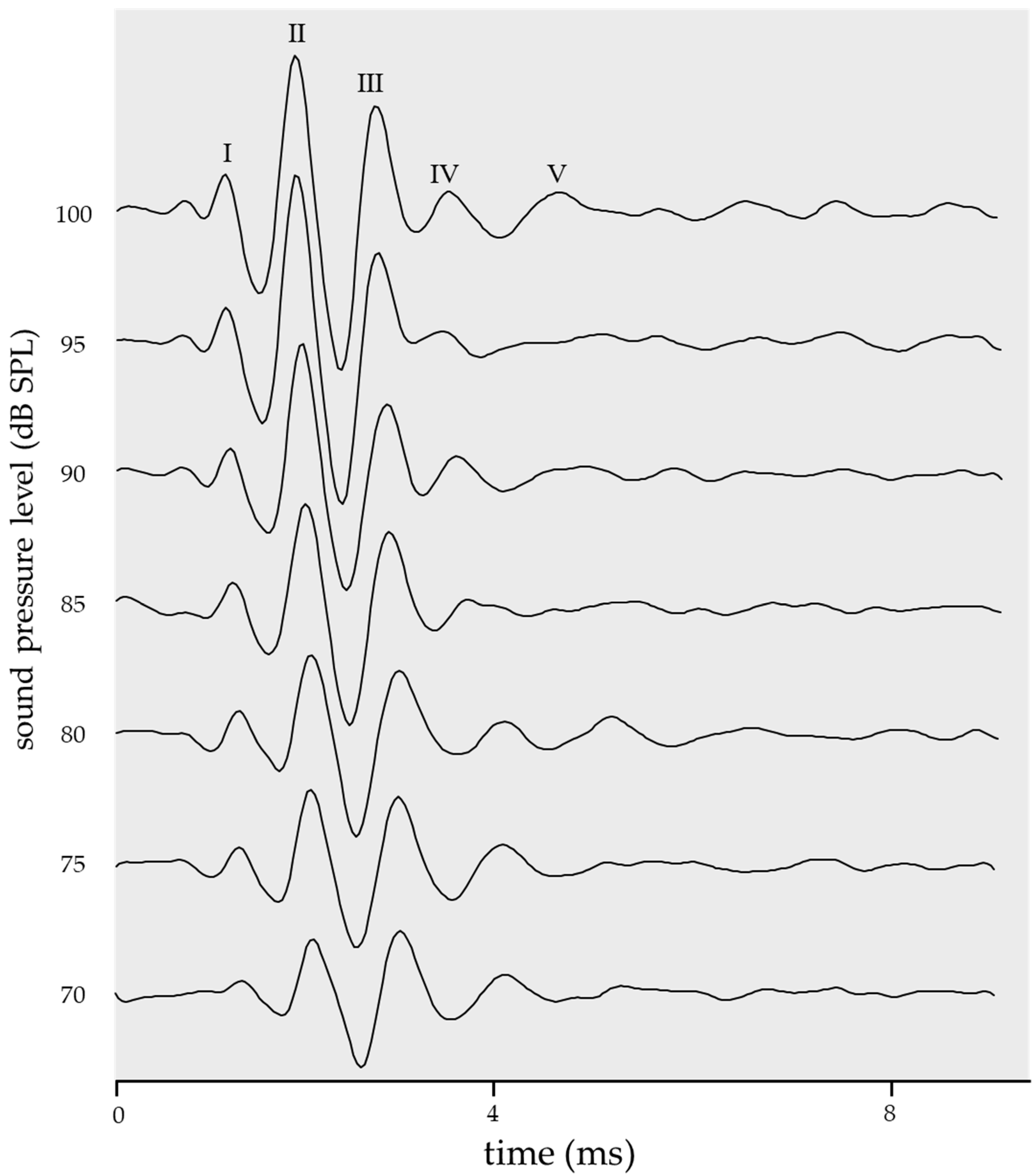
2.1. Ideal ABR Waveform as a Standard Template
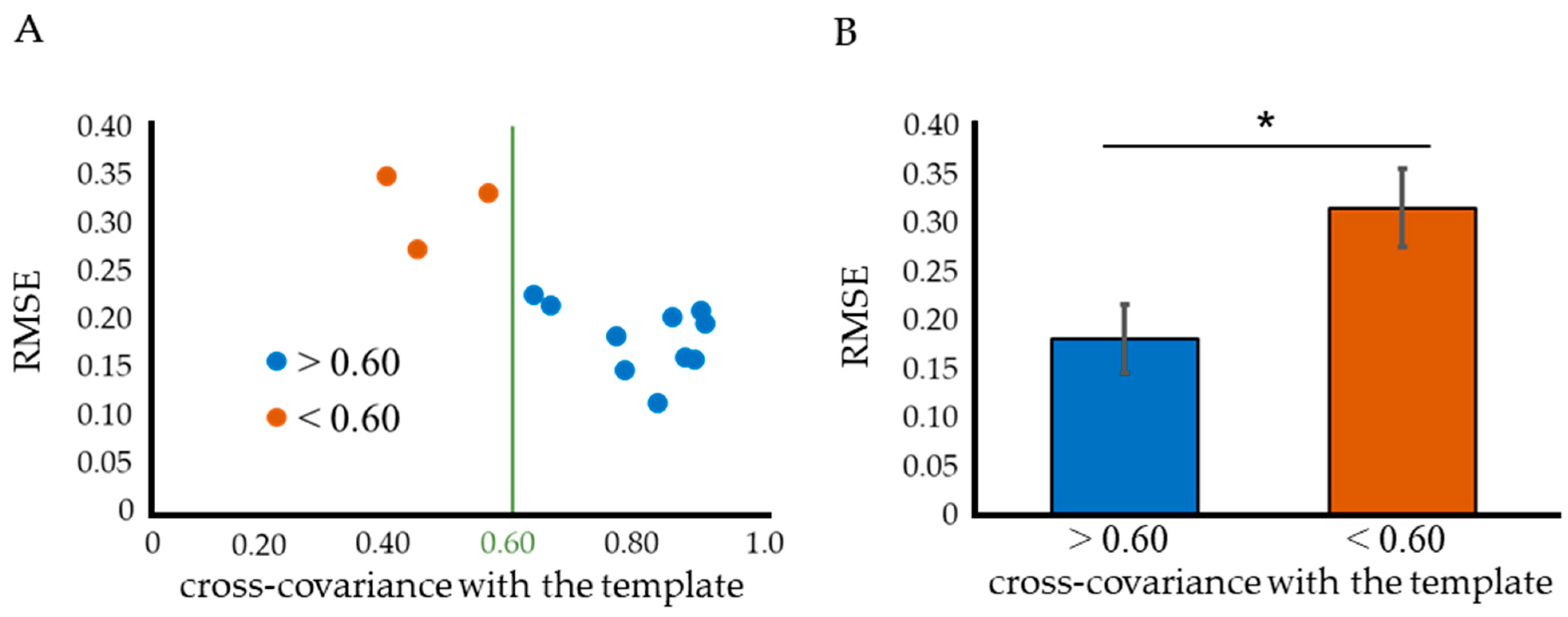
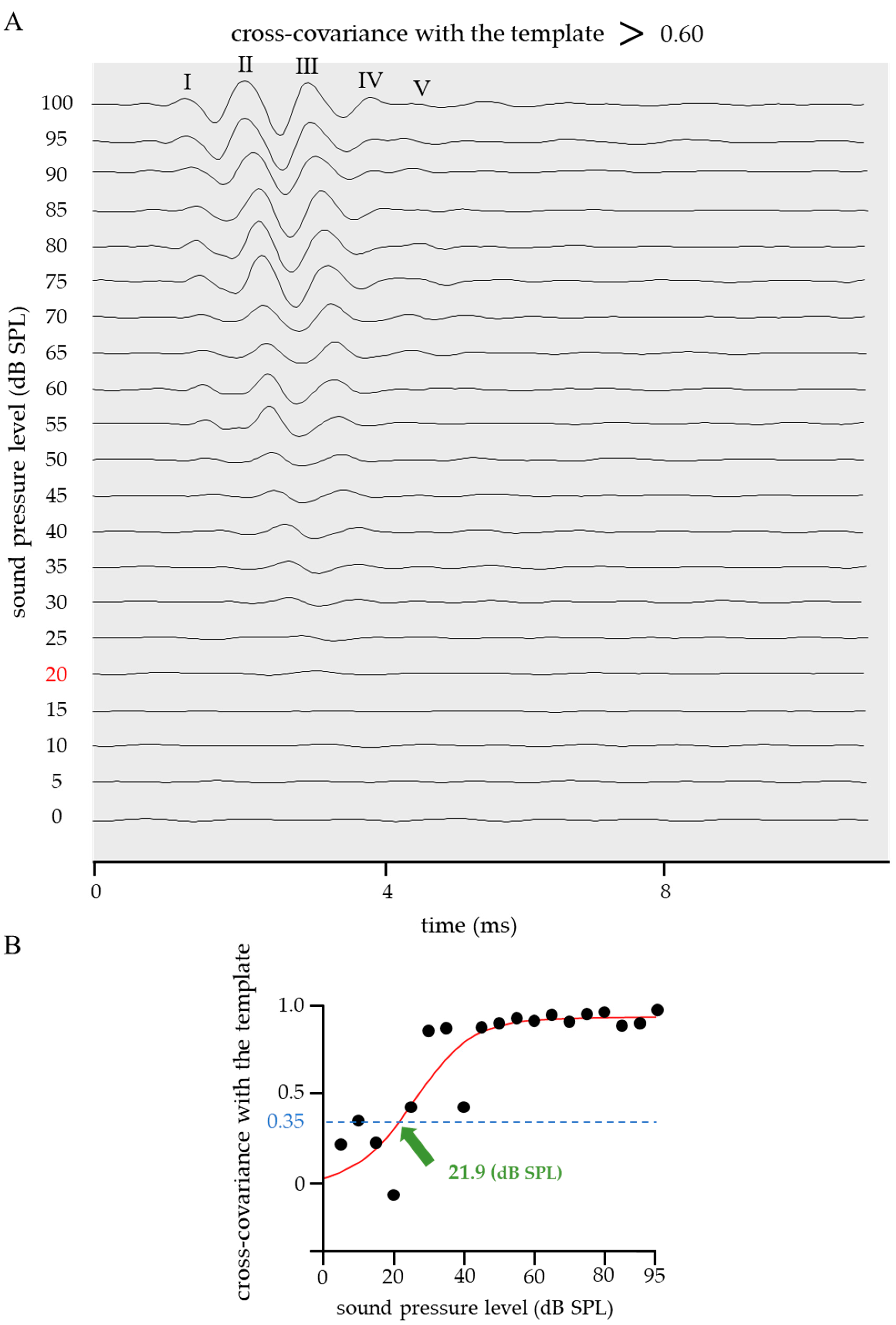
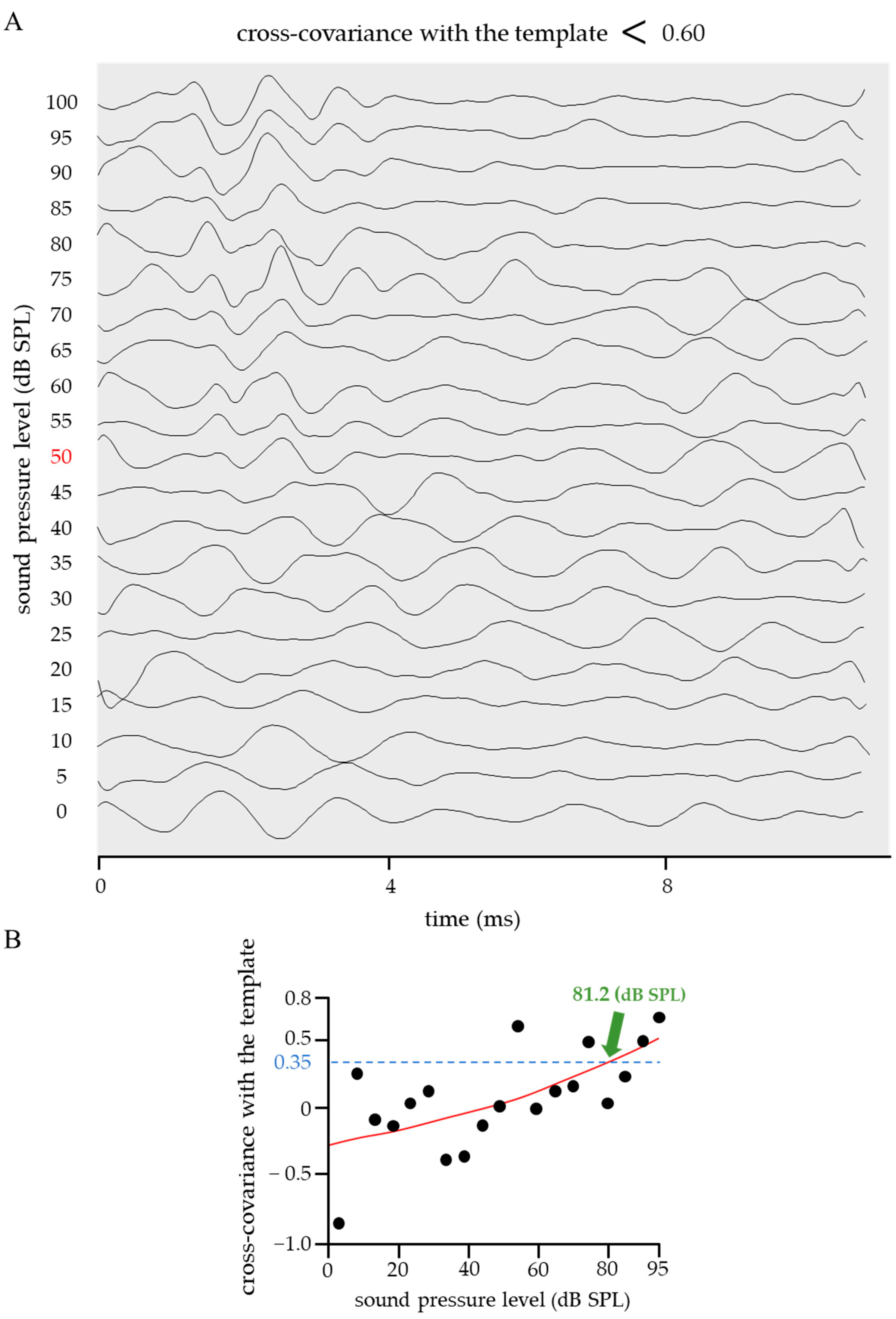
2.2. Threshold in the Cross-Covariance with a Standard Template
2.3. Improved Quantitative ABR Threshold Algorithm
- i.
- Only perform sigmoid function fitting without fitting by a binomial power function;
- ii.
- The ABR waveform disturbance was determined based on the cross-covariance with the template before and after sigmoid function fitting.
3. Discussion
4. Materials and Methods
4.1. Model Mouse
4.2. ABR Testing
4.3. Quantitative Determination of ABR Threshold
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henry, K.R. Auditory brainstem volume-conducted responses: Origins in the laboratory mouse. J. Am. Aud. Soc. 1979, 4, P173–P178. [Google Scholar]
- Melcher, J.R.; Guinan, J.J., Jr.; Knudson, I.M.; Kiang, N.Y. Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hear. Res. 1996, 93, 28–51. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, Y.; Da, P.; Shao, B.; Pan, H.; He, Z.; Cheng, C.; Li, D.; Guo, J.; Wu, X.; et al. Deletion of Limk1 and Limk2 in mice does not alter cochlear development or auditory function. Sci. Rep. 2019, 9, 3357. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, S.; Ito, M.; Takada, Y.; Ogawa, K.; Matsuo, K. Resorption of auditory ossicles and hearing loss in mice lacking osteoprotegerin. Bone 2006, 39, 414–419. [Google Scholar] [CrossRef]
- Vidler, M.; Parkert, D. Auditory brainstem response threshold estimation: Subjective threshold estimation by experienced clinicians in a computer simulation of the clinical test. Int. J. Audiol. 2004, 43, 417–429. [Google Scholar] [CrossRef]
- Weber, B.A. Pitfalls in auditory brain stem response audiometry. Ear. Hear. 1983, 4, 179–184. [Google Scholar] [CrossRef]
- Davey, R.; McCullagh, P.; Lightbody, G.; McAllister, G. Auditory brainstem response classification: A hybrid model using time and frequency features. Artif. Intell. Med. 2007, 40, 1–14. [Google Scholar] [CrossRef]
- Elberling, C. Auditory electrophysiology: The use of templates and cross correlation functions in the analysis of brain stem potentials. Scand. Audiol. 1979, 8, 187–190. [Google Scholar] [CrossRef]
- McCullagh, P.; Wang, H.; Zheng, H.; Lightbody, G.; McAllister, G. A comparison of supervised classification methods for auditory brainstem response determination. Stud. Health Technol. Inform. 2007, 129, 1289–1293. [Google Scholar]
- Bogaerts, S.; Clements, J.D.; Sullivan, J.M.; Oleskevich, S. Automated threshold detection for auditory brainstem responses: Comparison with visual estimation in a stem cell transplantation study. BMC Neurosci. 2009, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Lance, N. Estimating auditory neuronal dynamic range using a fitted function. Hear. Res. 2002, 167, 13–27. [Google Scholar]
- Suthakar, K.; Liberman, M.C. A simple algorithm for objective threshold determination of auditory brainstem responses. Hear. Res. 2019, 381, 107782. [Google Scholar] [CrossRef]
- Arnold, S.A. Objective versus visual detection of the auditory brain stem response. Ear. Hear. 1985, 6, 144–150. [Google Scholar] [CrossRef]
- Wong, P.K.; Bickford, R.G. Brain stem auditory evoked potentials: The use of noise estimate. Electroencephalogr. Clin. Neurophysiol. 1980, 50, 25–34. [Google Scholar] [CrossRef]
- Salvi, R.J.; Ahroon, W.; Saunders, S.S.; Arnold, S.A. Evoked potentials: Computer-automated threshold-tracking procedure using an objective detection criterion. Ear. Hear. 1987, 8, 151–156. [Google Scholar] [CrossRef]
- Xu, Z.M.; De Vel, E.; Vinck, B.; Van Cauwenberge, P. Application of cross-correlation function in the evaluation of objective MLR thresholds in the low and middle frequencies. Scand. Audiol. 1995, 24, 231–236. [Google Scholar]
- Galbraith, G.C.; Brown, W.S. Cross-correlation and latency compensation analysis of click-evoked and frequency-following brain-stem responses in man. Electroencephalogr. Clin. Neurophysiol. 1990, 77, 295–308. [Google Scholar] [CrossRef]
- Ozasa, R.; Ishimoto, T.; Miyabe, S.; Hashimoto, J.; Hirao, M.; Yoshikawa, H.; Nakano, T. Osteoporosis changes collagen/apatite orientation and Young’s modulus in vertebral cortical bone of rat. Calcif. Tissue Int. 2019, 104, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Matsugaki, A.; Kimura, Y.; Watanabe, R.; Nakamura, F.; Takehana, R.; Nakano, T. Impaired alignment of bone matrix microstructure associated with disorganized osteoblast arrangement in malignant melanoma metastasis. Biomolecules 2021, 11, 131. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, T.; Kawahara, K.; Matsugaki, A.; Kamioka, H.; Nakano, T. Quantitative Evaluation of Osteocyte Morphology and Bone Anisotropic Extracellular Matrix in Rat Femur. Calcif. Tissue Int. 2021, 109, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Kamal, B.; Russell, D.; Payne, A.; Constante, D.; Tanner, K.E.; Isaksson, H.; Mathavan, N.; Cobb, S.R. Biomechanical properties of bone in a mouse model of Rett syndrome. Bone 2015, 71, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Takeda, S.; Xu, R.; Ochi, H.; Sunamura, S.; Sato, T.; Shibata, S.; Yoshida, Y.; Gu, Z.; Kimura, A.; et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature 2013, 497, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Oswald, A.B.; Fabella, B.A.; Ren, Y.; Rodriguiz, R.; Trainor, G.; Greenblatt, M.B.; Hilton, M.J.; Pitt, G.S. The CaV1.2 L-type calcium channel regulates bone homeostasis in the middle and inner ear. Bone 2019, 125, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Bai, X.; Chen, S.; Xie, L.; Qiu, Y.; Li, H.; Sun, Y. CCDC154 mutant caused abnormal remodeling of the otic capsule and hearing loss in mice. Front. Cell Dev. Biol. 2021, 9, 637011. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, A.F.; Kristiansen, A.G.; Adams, J.C.; Kujawa, S.G.; Merchant, S.N.; McKenna, M.J. Osteoprotegrin knockout mice demonstrate abnormal remodeling of the otic capsule and progressive hearing loss. Laryngoscope 2006, 116, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsugaki, A.; Harada, T.; Kimura, Y.; Sekita, A.; Nakano, T. Dynamic Collision Behavior between Osteoblasts and Tumor Cells Regulates the Disordered Arrangement of Collagen Fiber/Apatite Crystals in Metastasized Bone. Int. J. Mol. Sci. 2018, 3474, 3474. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Kawaai, K.; Hatano, N.; Wu, Y.; Takano, H.; Nomose, A.; Ishimoto, T.; Nakano, N.; Roschger, P.; Blouin, S.; et al. Hypermineralization of hearing-related bones by a specific osteoblast subtype. J. Bone Miner. Res. 2021, 36, 1535–1547. [Google Scholar] [CrossRef]
- Lee, S.; Matsugaki, A.; Kasuga, T.; Nakano, T. Development of bifunctional oriented bioactive glass/poly(lactic acid) composite scaffolds to control osteoblast alignment and proliferation. J. Bone Miner. Res. A 2019, 107, 1031–1041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s), and not of the MDPI and/or editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Ohara, S.; Matsuzaka, T.; Matsugaki, A.; Ishimoto, T.; Ozasa, R.; Kuroda, Y.; Matsuo, K.; Nakano, T. Quantitative Threshold Determination of Auditory Brainstem Responses in Mouse Models. Int. J. Mol. Sci. 2023, 24, 11393. https://doi.org/10.3390/ijms241411393
Tanaka K, Ohara S, Matsuzaka T, Matsugaki A, Ishimoto T, Ozasa R, Kuroda Y, Matsuo K, Nakano T. Quantitative Threshold Determination of Auditory Brainstem Responses in Mouse Models. International Journal of Molecular Sciences. 2023; 24(14):11393. https://doi.org/10.3390/ijms241411393
Chicago/Turabian StyleTanaka, Kenji, Shuma Ohara, Tadaaki Matsuzaka, Aira Matsugaki, Takuya Ishimoto, Ryosuke Ozasa, Yukiko Kuroda, Koichi Matsuo, and Takayoshi Nakano. 2023. "Quantitative Threshold Determination of Auditory Brainstem Responses in Mouse Models" International Journal of Molecular Sciences 24, no. 14: 11393. https://doi.org/10.3390/ijms241411393
APA StyleTanaka, K., Ohara, S., Matsuzaka, T., Matsugaki, A., Ishimoto, T., Ozasa, R., Kuroda, Y., Matsuo, K., & Nakano, T. (2023). Quantitative Threshold Determination of Auditory Brainstem Responses in Mouse Models. International Journal of Molecular Sciences, 24(14), 11393. https://doi.org/10.3390/ijms241411393






