Effects of Normobaric Hypoxia and Adrenergic Blockade over 72 h on Cardiac Function in Rats
Abstract
1. Introduction
2. Results
2.1. Nutritional Condition and Blood Analysis
2.2. Complications Prior to or during Hemodynamic Measurements
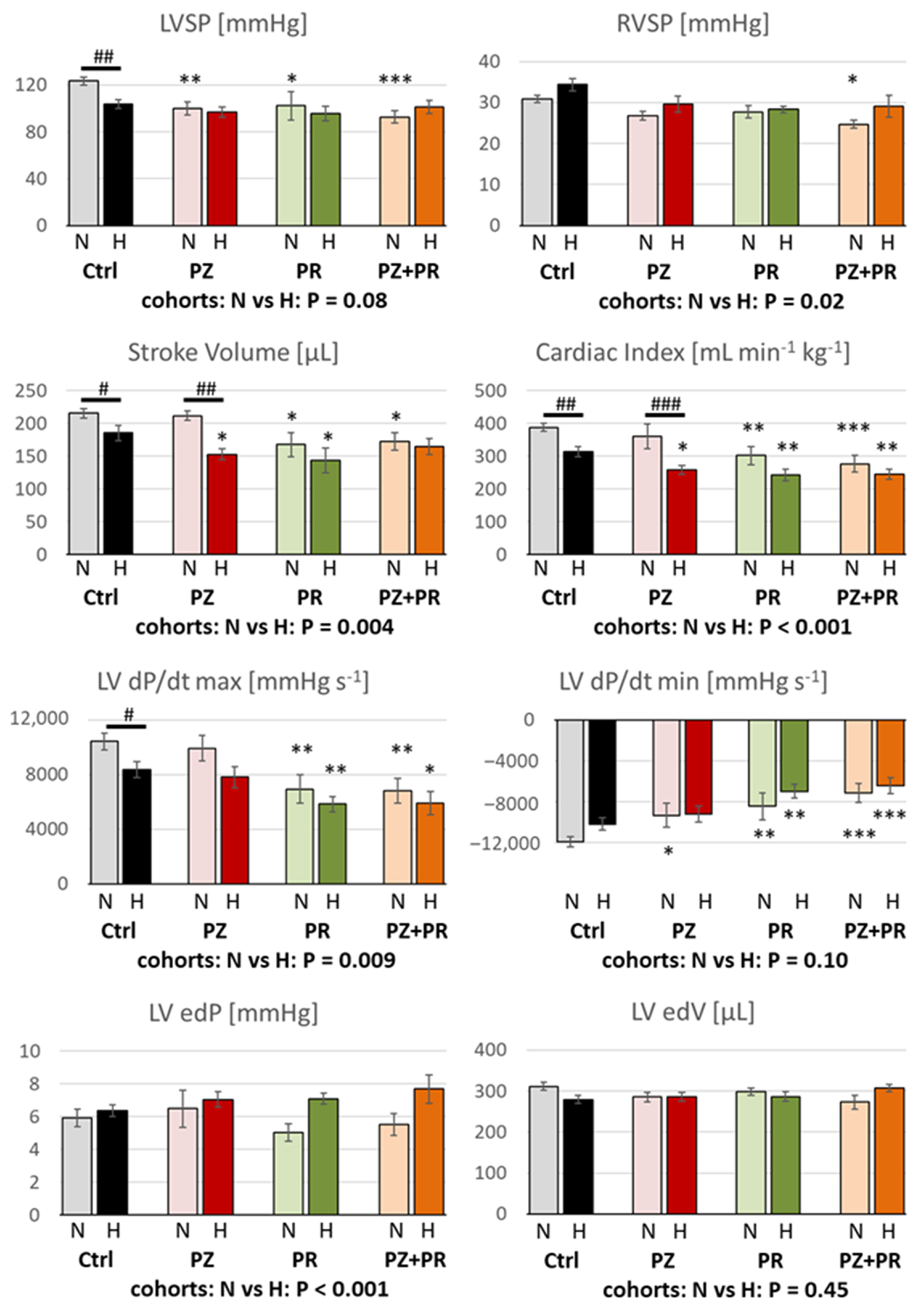
2.3. Results of Hemodynamic Measurements
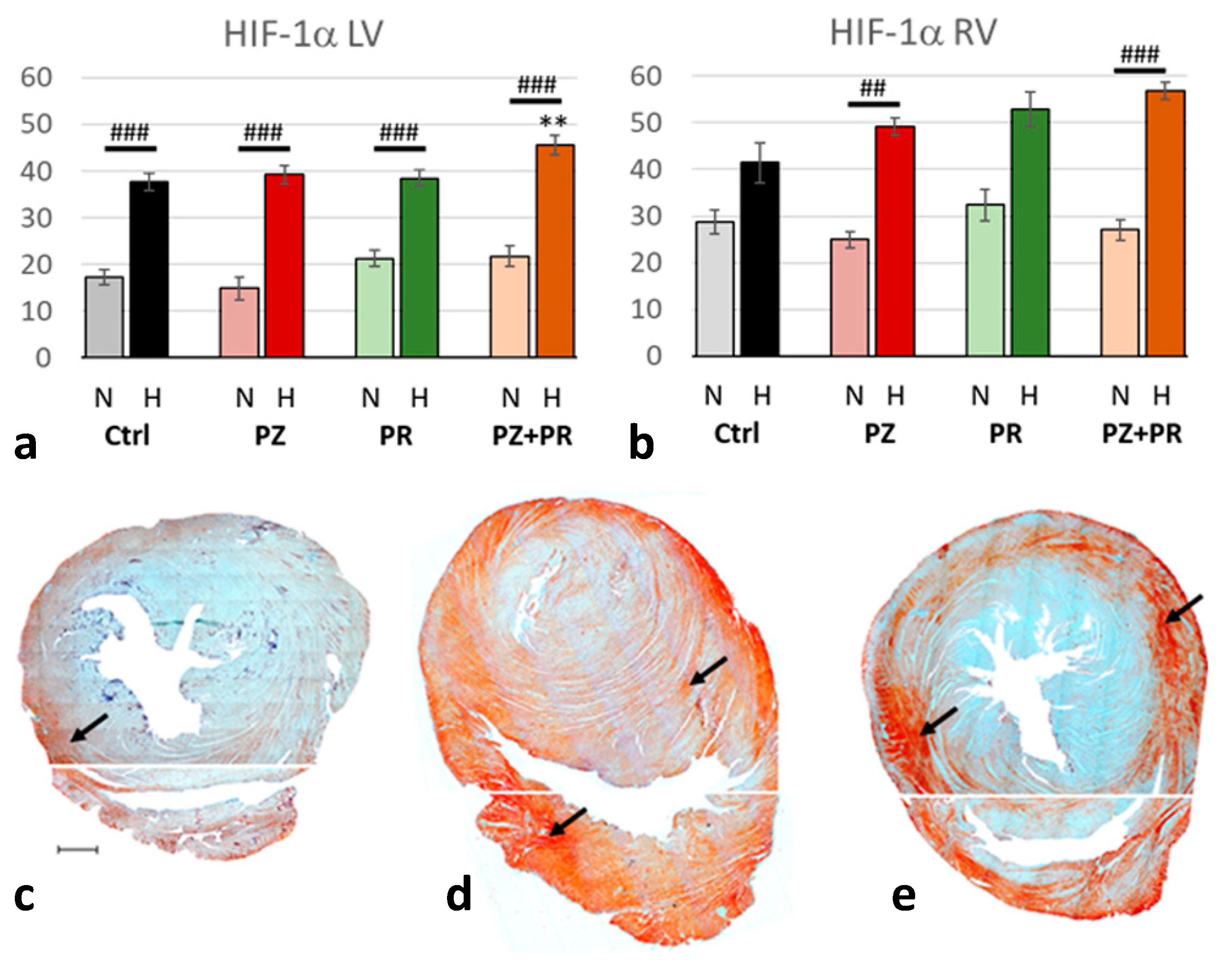
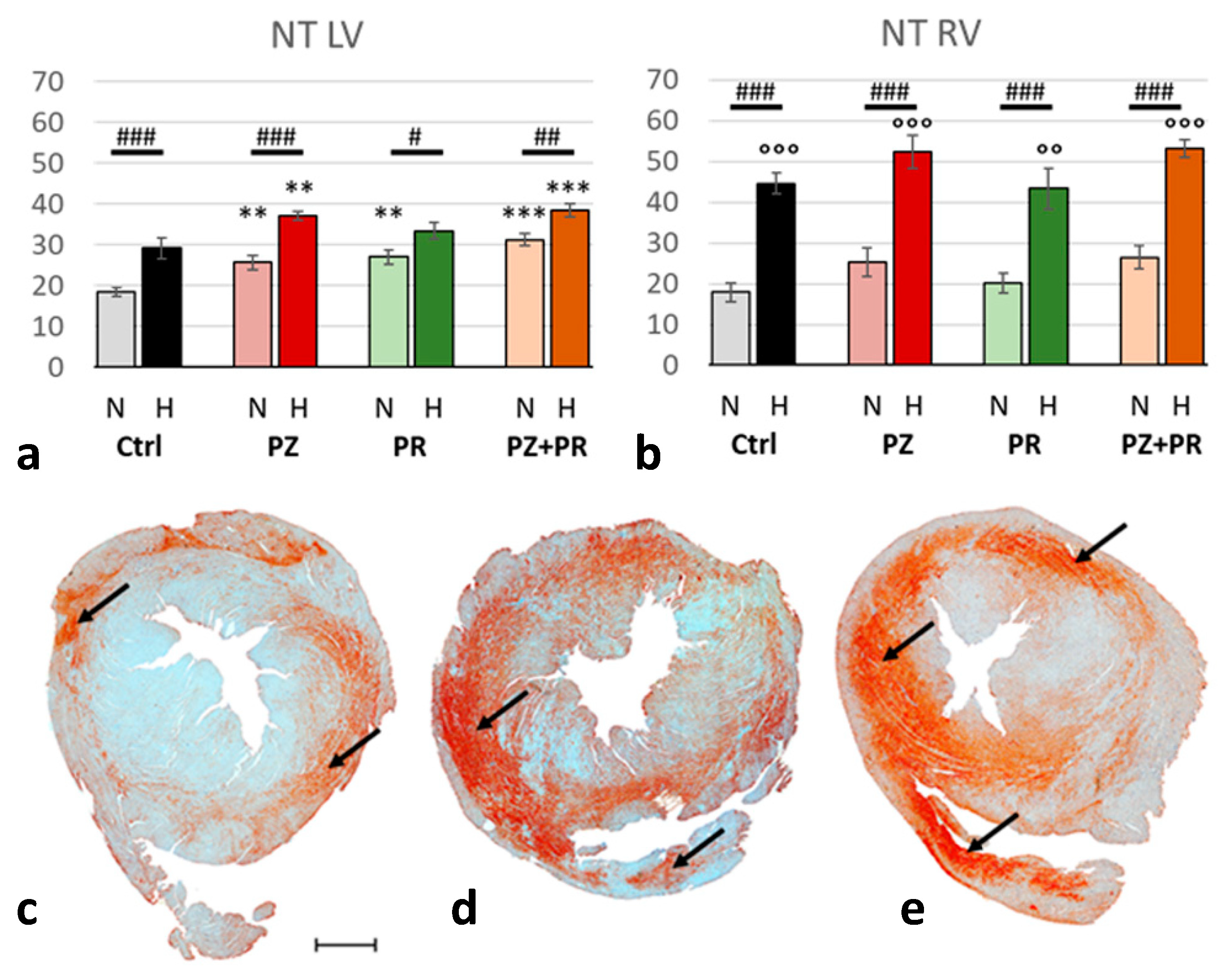
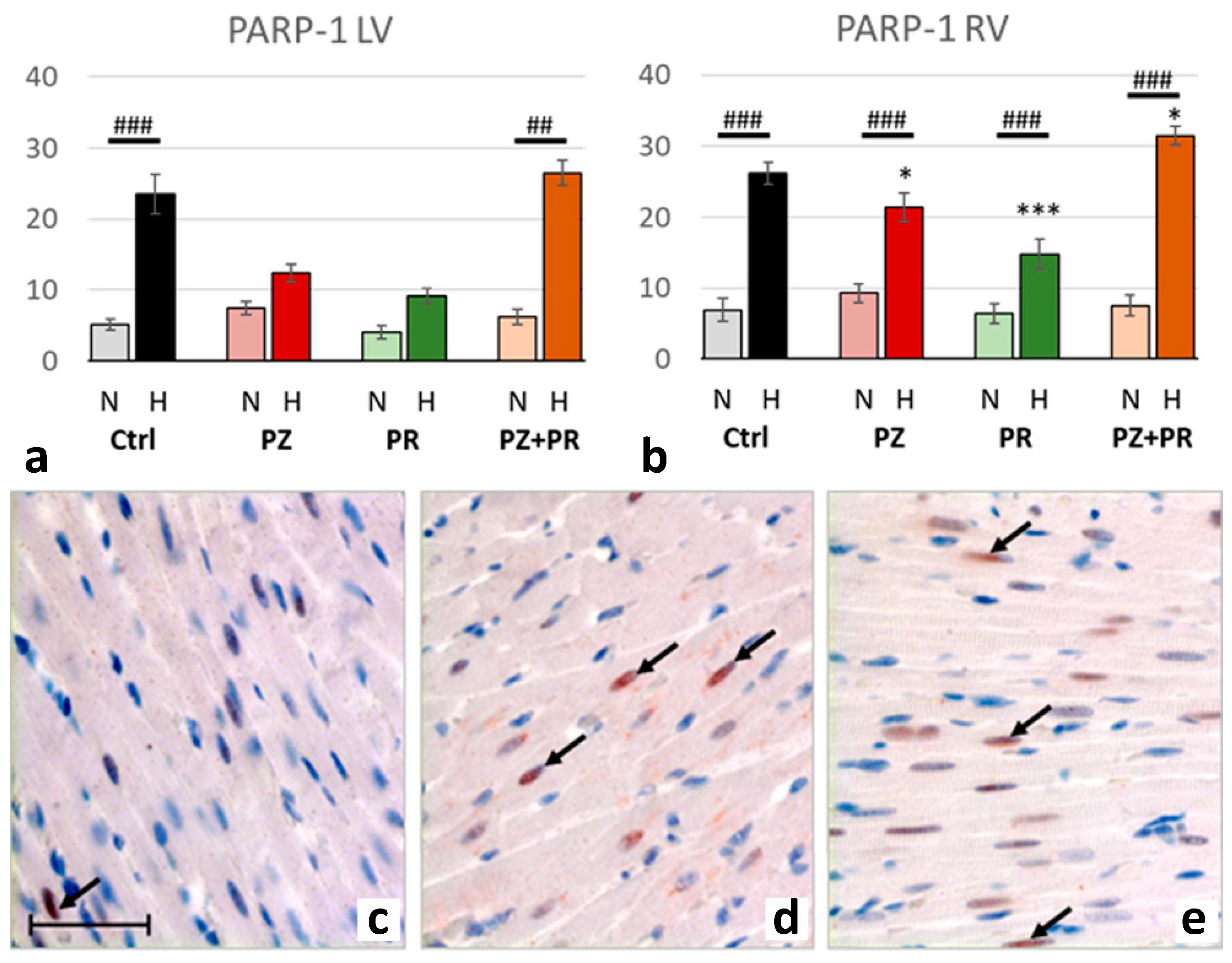
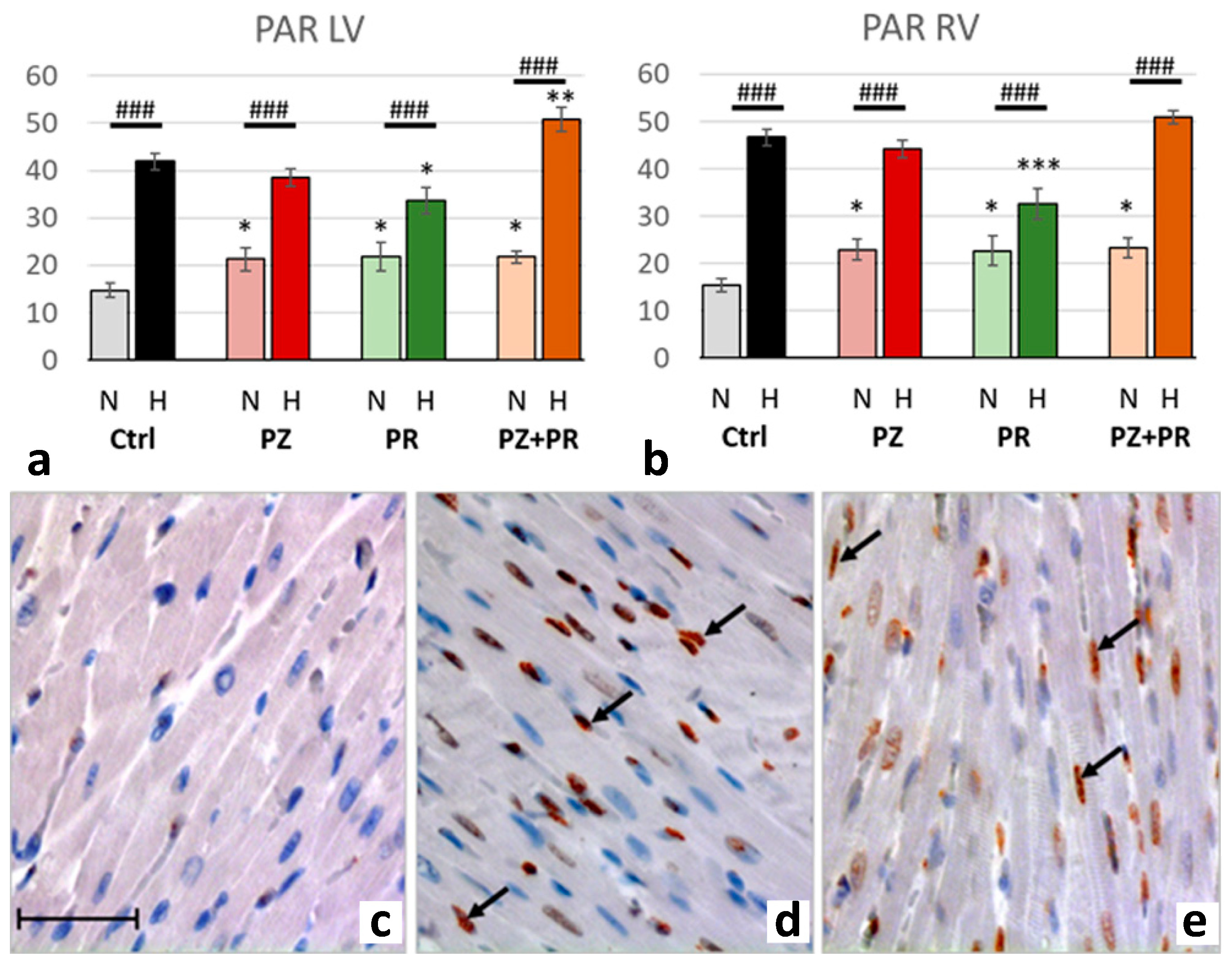
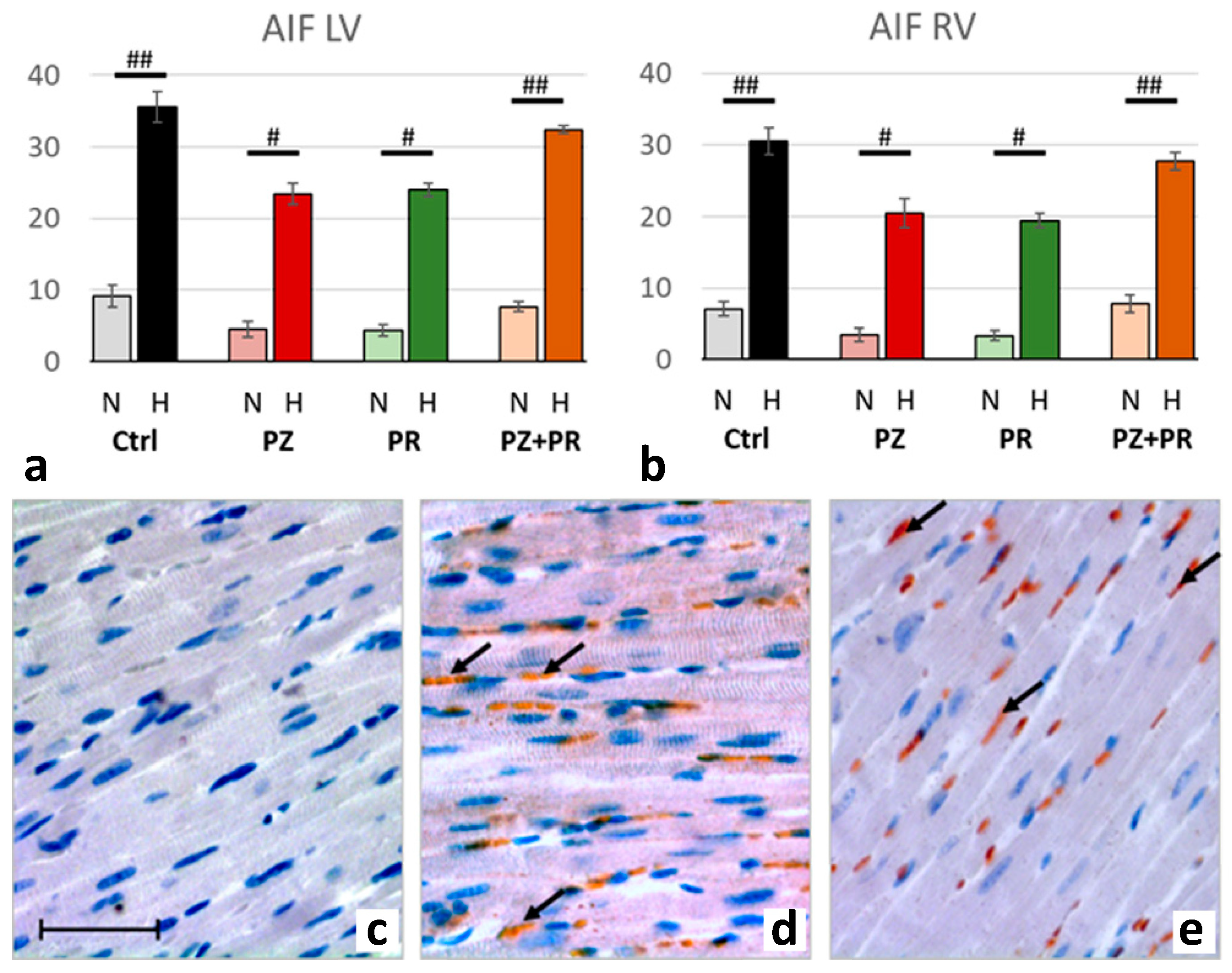
2.4. Results of Immunohistochemical Analyzes
3. Discussion
3.1. Adaptation to Hypoxia
3.2. What Might Account for the LV Depression in Hypoxia?
3.3. Limitations of the Study
4. Materials and Methods
4.1. Animal Model
4.2. Study Protocol
4.3. Hemodynamic Measurements
4.4. Sampling of Materials
4.5. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bölter, C.; Gabriel, P.; Appelt, P.; Salameh, A.; Schierle, K.; Rassler, B. Effects of Adrenergic Agonists and Antagonists on Cardiopulmonary Function during Normobaric Hypoxia in Rat. Front. Physiol. 2019, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Appelt, P.; Gabriel, P.; Bölter, C.; Fiedler, N.; Schierle, K.; Salameh, A.; Rassler, B. Left ventricular depression and pulmonary edema in rats after short-term normobaric hypoxia: Effects of adrenergic blockade and reduced fluid load. Pflug. Arch. 2021, 473, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Rohlicek, C.V.; Matsuoka, T.; Saiki, C. Cardiovascular response to acute hypoxemia in adult rats hypoxemic neonatally. Cardiovasc. Res. 2002, 53, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chaudary, N.; Naydenova, Z.; Shuralyova, I.; Coe, I.R. Hypoxia regulates the adenosine transporter, mENT1, in the murine cardiomyocyte cell line, HL-1. Cardiovasc. Res. 2004, 61, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Mozet, C.; Martin, R.; Welt, K.; Fitzl, G. Cardioprotective effect of EGb 761 on myocardial ultrastructure of young and old rat heart and antioxidant status during acute hypoxia. Aging Clin. Exp. Res. 2009, 21, 14–21. [Google Scholar] [CrossRef]
- Holloway, C.; Cochlin, L.; Codreanu, I.; Bloch, E.; Fatemian, M.; Szmigielski, C.; Atherton, H.; Heather, L.; Francis, J.; Neubauer, S.; et al. Normobaric hypoxia impairs human cardiac energetics. FASEB J. 2011, 25, 3130–3135. [Google Scholar] [CrossRef]
- Essop, M.F.; Razeghi, P.; McLeod, C.; Young, M.E.; Taegtmeyer, H.; Sack, M.N. Hypoxia-induced decrease of UCP3 gene expression in rat heart parallels metabolic gene switching but fails to affect mitochondrial respiratory coupling. Biochem. Biophys. Res. Commun. 2004, 314, 561–564. [Google Scholar] [CrossRef]
- Ashmore, T.; Fernandez, B.O.; Branco-Price, C.; West, J.A.; Cowburn, A.S.; Heather, L.C.; Griffin, J.L.; Johnson, R.S.; Feelisch, M.; Murray, A.J. Dietary nitrate increases arginine availability and protects mitochondrial complex I and energetics in the hypoxic rat heart. J. Physiol. 2014, 592, 4715–4731. [Google Scholar] [CrossRef]
- Rumsey, W.L.; Abbott, B.; Bertelsen, D.; Mallamaci, M.; Hagan, K.; Nelson, D.; Erecinska, M. Adaptation to hypoxia alters energy metabolism in rat heart. Am. J. Physiol. 1999, 276, H71–H80. [Google Scholar] [CrossRef]
- Walley, K.R.; Becker, C.J.; Hogan, R.A.; Teplinsky, K.; Wood, L.D. Progressive hypoxemia limits left ventricular oxygen consumption and contractility. Circ. Res. 1988, 63, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Zöbisch, H.; Schröder, B.; Vigelahn, J.; Jahn, M.; Abraham, G.; Seeger, J.; Dähnert, I.; Dhein, S. Effects of Hypoxia and Acidosis on Cardiac Electrophysiology and Hemodynamics. Is NHE-Inhibition by Cariporide Still Advantageous? Front. Physiol. 2020, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- David, K.K.; Andrabi, S.A.; Dawson, T.M.; Dawson, V.L. Parthanatos, a messenger of death. Front. Biosci. 2009, 14, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Tekin, D.; Dursun, A.D.; Xi, L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacol. Sin. 2010, 31, 1085–1094. [Google Scholar] [CrossRef]
- Heistad, D.D.; Abboud, F.M. Circulatory adjustments to hypoxia. Circulation 1980, 61, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, D.L.; Bastow, H., III; Scruby, A.E. Modulation of human sinus node function by systemic hypoxia. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 570–577. [Google Scholar] [CrossRef]
- Lesske, J.; Fletcher, E.C.; Bao, G.; Unger, T. Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J. Hypertens. 1997, 15, 1593–1603. [Google Scholar] [CrossRef]
- Bärtsch, P.; Gibbs, J.S. Effect of altitude on the heart and the lungs. Circulation 2007, 116, 2191–2202. [Google Scholar] [CrossRef]
- Dedobbeleer, C.; Hadefi, A.; Naeije, R.; Unger, P. Left ventricular adaptation to acute hypoxia: A speckle-tracking echocardiography study. J. Am. Soc. Echocardiogr. 2013, 26, 736–745. [Google Scholar] [CrossRef]
- Burniston, J.G.; Ellison, G.M.; Clark, W.A.; Goldspink, D.F.; Tan, L.B. Relative toxicity of cardiotonic agents: Some induce more cardiac and skeletal myocyte apoptosis and necrosis in vivo than others. Cardiovasc. Toxicol. 2005, 5, 355–364. [Google Scholar] [CrossRef]
- Feng, Y.; Tang, X.Y.; Dai, D.Z.; Dai, Y. Reversal of isoproterenol-induced downregulation of phospholamban and FKBP12.6 by CPU0213-mediated antagonism of endothelin receptors. Acta Pharmacol. Sin. 2007, 28, 1746–1754. [Google Scholar] [CrossRef]
- Ellison, G.M.; Torella, D.; Karakikes, I.; Purushothaman, S.; Curcio, A.; Gasparri, C.; Indolfi, C.; Cable, N.T.; Goldspink, D.F.; Nadal-Ginard, B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J. Biol. Chem. 2007, 282, 11397–11409. [Google Scholar] [CrossRef] [PubMed]
- Goebel, B.; Handrick, V.; Lauten, A.; Fritzenwanger, M.; Schütze, J.; Otto, S.; Figulla, H.R.; Edvardsen, T.; Poerner, T.C.; Jung, C. Impact of acute normobaric hypoxia on regional and global myocardial function: A speckle tracking echocardiography study. Int. J. Cardiovasc. Imaging 2013, 29, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Maggiorini, M.; Mélot, C.; Pierre, S.; Pfeiffer, F.; Greve, I.; Sartori, C.; Lepori, M.; Hauser, M.; Scherrer, U.; Naeije, R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 2001, 103, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Packer, C.S.; Rhoades, R.A. Pulmonary vein contracts in response to hypoxia. Am. J. Physiol. 1993, 265, L87–L92. [Google Scholar] [CrossRef]
- León-Velarde, F.; Bourin, M.C.; Germack, R.; Mohammadi, K.; Crozatier, B.; Richalet, J.P. Differential alterations in cardiac adrenergic signaling in chronic hypoxia or norepinephrine infusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R274–R281. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.P.; Balanos, G.M.; Dorrington, K.L.; Robbins, P.A. Two temporal components within the human pulmonary vascular response to approximately 2 h of isocapnic hypoxia. J. Appl. Physiol. 2005, 98, 1125–1139. [Google Scholar] [CrossRef]
- Yan, B.; Hu, Y.; Ji, H.; Bao, D. The effect of acute hypoxia on left ventricular function during exercise. Eur. J. Appl. Physiol. 2007, 100, 261–265. [Google Scholar] [CrossRef]
- Leuenberger, U.; Gleeson, K.; Wroblewski, K.; Prophet, S.; Zelis, R.; Zwillich, C.; Sinoway, L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am. J. Physiol. 1991, 261, H1659–H1664. [Google Scholar] [CrossRef]
- Salvi, S.S. Alpha1-adrenergic hypothesis for pulmonary hypertension. Chest 1999, 115, 1708–1719. [Google Scholar] [CrossRef]
- Moretta, D.; Papamatheakis, D.G.; Morris, D.P.; Giri, P.C.; Blood, Q.; Murray, S.; Ramzy, M.; Romero, M.; Vemulakonda, S.; Lauw, S.; et al. Long-Term High-Altitude Hypoxia and Alpha Adrenoceptor-Dependent Pulmonary Arterial Contractions in Fetal and Adult Sheep. Front. Physiol. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Ait Mou, Y.; Bollensdorff, C.; Cazorla, O.; Magdi, Y.; de Tombe, P.P. Exploring cardiac biophysical properties. Glob. Cardiol. Sci. Pract. 2015, 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Kuhtz-Buschbeck, J.P.; Drake-Holland, A.; Noble, M.I.M.; Lohff, B.; Schaefer, J. Rediscovery of Otto Frank’s contribution to science. J. Mol. Cell. Cardiol. 2018, 119, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Reissig, C.; Rohling, M.A.; Tannapfel, A.; Wenger, R.H.; Zimmer, H.G. Time-course of hypoxia-induced lung injury in rats. Respir. Physiol. Neurobiol. 2007, 159, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; Bunn, H.F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J. Biol. Chem. 1996, 271, 32253–32259. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.; Lipsic, E.; Henning, R.H.; de Boer, R.A.; Suurmeijer, A.J.; van Veldhuisen, D.J.; van Gilst, W.H. Erythropoietin improves left ventricular function and coronary flow in an experimental model of ischemia-reperfusion injury. Eur. J. Heart Fail. 2004, 6, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, A.F.; Muniyappa, R.; Black, A.D.; Blendea, M.C.; Cohen, I.; Deng, L.; Sowers, J.R.; Cutaia, M.V.; El-Sherif, N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem. Biophys. Res. Commun. 2003, 308, 990–994. [Google Scholar] [CrossRef]
- Shyu, K.G.; Lu, M.J.; Chang, H.; Sun, H.Y.; Wang, B.W.; Kuan, P. Carvedilol modulates the expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor in a rat model of volume-overload heart failure. J. Card. Fail. 2005, 11, 152–159. [Google Scholar] [CrossRef]
- Jochmans-Lemoine, A.; Villalpando, G.; Gonzales, M.; Valverde, I.; Soria, R.; Joseph, V. Divergent physiological responses in laboratory rats and mice raised at high altitude. J. Exp. Biol. 2015, 218, 1035–1043. [Google Scholar] [CrossRef]
- Shao, D.; Tian, R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr. Physiol. 2015, 6, 331–351. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Behrooz, A.; Ismail-Beigi, F. Regulation of glucose transport by hypoxia. Am. J. Kidney Dis. 1999, 34, 189–202. [Google Scholar] [CrossRef]
- Montessuit, C.; Thorburn, A. Transcriptional activation of the glucose transporter GLUT1 in ventricular cardiac myocytes by hypertrophic agonists. J. Biol. Chem. 1999, 274, 9006–9012. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Tokunaga, C.; Kareco, T.; Dorscheid, D.R.; Walley, K.R. Myocardial hypoxia-inducible HIF-1alpha, VEGF, and GLUT1 gene expression is associated with microvascular and ICAM-1 heterogeneity during endotoxemia. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H448–H456. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.C.; Liu, H.; Pinkas, G.A.; Thompson, L.P. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr. Res. 2012, 71, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Connelly, R.; Traber, D.L.; Hamahata, A.; Nakano, Y.; Esechie, A.; Jonkam, C.; von Borzyskowski, S.; Traber, L.D.; Schmalstieg, F.C.; et al. Time course of nitric oxide synthases, nitrosative stress, and poly(ADP ribosylation) in an ovine sepsis model. Crit. Care 2010, 14, R129. [Google Scholar] [CrossRef]
- Yu, S.W.; Andrabi, S.A.; Wang, H.; Kim, N.S.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 18314–18319. [Google Scholar] [CrossRef]
- Ye, H.; Cande, C.; Stephanou, N.C.; Jiang, S.; Gurbuxani, S.; Larochette, N.; Daugas, E.; Garrido, C.; Kroemer, G.; Wu, H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat. Struct. Biol. 2002, 9, 680–684. [Google Scholar] [CrossRef]
- Perez, D.M.; Doze, V.A. Cardiac and neuroprotection regulated by α(1)-adrenergic receptor subtypes. J. Recept. Signal Transduct. Res. 2011, 31, 98–110. [Google Scholar] [CrossRef]
- Iwai-Kanai, E.; Hasegawa, K.; Araki, M.; Kakita, T.; Morimoto, T.; Sasayama, S. alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation 1999, 100, 305–311. [Google Scholar] [CrossRef]
- Communal, C.; Singh, K.; Pimentel, D.R.; Colucci, W.S. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation 1998, 98, 1329–1334. [Google Scholar] [CrossRef]
- Remondino, A.; Kwon, S.H.; Communal, C.; Pimentel, D.R.; Sawyer, D.B.; Singh, K.; Colucci, W.S. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ. Res. 2003, 92, 136–138. [Google Scholar] [CrossRef]
- Myagmar, B.E.; Flynn, J.M.; Cowley, P.M.; Swigart, P.M.; Montgomery, M.D.; Thai, K.; Nair, D.; Gupta, R.; Deng, D.X.; Hosoda, C.; et al. Adrenergic Receptors in Individual Ventricular Myocytes: The Beta-1 and Alpha-1B Are in All Cells, the Alpha-1A Is in a Subpopulation, and the Beta-2 and Beta-3 Are Mostly Absent. Circ. Res. 2017, 120, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Dhein, S.; Blanke, K.; Rastan, A.; Hiyasat, B.; Dietze, A.; Sobiraij, A.; Dähnert, I.; Janousek, J. Right or left ventricular pacing in young minipigs with chronic atrioventricular block: Long-term in vivo cardiac performance, morphology, electrophysiology, and cellular biology. Circulation 2012, 125, 2578–2587. [Google Scholar] [CrossRef]
- Salameh, A.; Keller, M.; Dähnert, I.; Dhein, S. Olesoxime Inhibits Cardioplegia-Induced Ischemia/Reperfusion Injury. A Study in Langendorff-Perfused Rabbit Hearts. Front. Physiol. 2017, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
| Cohort | Normoxic Cohort: | Hypoxic Cohort: | ||||||
|---|---|---|---|---|---|---|---|---|
| Group (n) | N-Ctrl (7–10) | N-PZ (6–8) | N-PR (7–8) | N-PZ+PR (8) | H-Ctrl (8–10) | H-PZ (8–14) | H-PR (13–14) | H-PZ+PR (9–10) |
| BW change (% baseline) | −2.3 (−5.0; 0.5) | −12.7 (−14.7; −9.9) ### | ||||||
| −1.7 (−3.7; 4.4) | −4.2 (−7.7; −2.1) | −1.7 (−3.7; 4.4) | −4.2 (−7.7; −2.1) | −1.7 (−3.7; 4.4) | −4.2 (−7.7; −2.1) | −1.7 (−3.7; 4.4) | −4.2 (−7.7; −2.1) | |
| Total food uptake (g) | 34.8 (23.4; 43.2) | 14.2 (9.2; 19.4) ### | ||||||
| 43.2 (38.6; 47.4) | 24.6 (20.4; 37.6) | 43.2 (38.6; 47.4) | 24.6 (20.4; 37.6) | 43.2 (38.6; 47.4) | 24.6 (20.4; 37.6) | 43.2 (38.6; 47.4) | 24.6 (20.4; 37.6) | |
| c (Glucose) (mmol/L) | 8.6 (8.4; 9.8) | 12.4 (10.0; 14.2) ### | ||||||
| 8.4 (7.4; 8.6) | 9.0 (8.4; 12.0) | 8.4 (7.4; 8.6) | 9.0 (8.4; 12.0) | 8.4 (7.4; 8.6) | 9.0 (8.4; 12.0) | 8.4 (7.4; 8.6) | 9.0 (8.4; 12.0) | |
| Cohort | Normoxic Cohort: | Hypoxic Cohort: | ||||||
|---|---|---|---|---|---|---|---|---|
| Group (n) | N-Ctrl (6–9) | N-PZ (6–8) | N-PR (6–8) | N-PZ+PR (6–8) | H-Ctrl (6–10) | H-PZ (6–10) | H-PR (7–14) | H-PZ+PR (6–9) |
| c (Hb) (g/mL) | 13.5 ± 0.2 | 16.2 ± 0.1 ### | ||||||
| 14.1 ± 0.2 | 13.1 ± 0.6 | 13.5 ± 0.4 | 13.1 ± 0.3 | 15.9 ± 0.2 ### | 16.3 ± 0.3 ### | 15.9 ± 0.3 ### | 16.8 ± 0.3 ### | |
| Hematocrit (%) | 41.5 (39.6; 43.4) | 49.2 (47.6; 50.7) ### | ||||||
| 42.3 (41.2; 44.6) | 39.9 (38.0; 40.4) | 42.3 (41.2; 44.6) | 39.9 (38.0; 40.4) | 42.3 (41.2; 44.6) | 39.9 (38.0; 40.4) | 42.3 (41.2; 44.6) | 39.9 (38.0; 40.4) | |
| SaO2 (%) | 91.8 ± 0.9 | 84.5 ± 1.4 ### | ||||||
| 92.8 ± 0.9 | 92.4 ± 2.4 | 90.2 ± 3.7 | 91.1 ± 1.3 | 82.1 ± 2.1 ## | 80.8 ± 2.8 ## | 89.8 ± 1.9 * | 83.4 ± 3.3 # | |
| Arterial pH | 7.44 (7.42; 7.50) | 7.33 (7.29; 7.38) ### | ||||||
| 7.44 (7.43; 7.49) | 7.47 (7.40; 7.53) | 7.44 (7.43; 7.49) | 7.47 (7.40; 7.53) | 7.44 (7.43; 7.49) | 7.47 (7.40; 7.53) | 7.44 (7.43; 7.49) | 7.47 (7.40; 7.53) | |
| Arterial pCO2 (mmHg) | 38.0 ± 1.1 | 30.1 ± 1.1 ### | ||||||
| 37.4 ± 1.8 | 40.2 ± 2.7 | 33.4 ± 2.7 | 39.9 ± 1.7 | 32.6 ± 2.9 | 31.1 ± 2.0 # | 30.2 ± 1.4 | 25.0 ± 1.4 ### * | |
| Base excess | +2.8 ± 0.3 | −10.4 ± 0.8 ### | ||||||
| +2.4 ± 0.6 | +2.9 ± 0.7 | +2.6 ± 0.1 | +5.1 ± 0.0 | −16.3 ± 1.9 ### | −10.7 ± 0.0 ### * | −9.6 ± 0.8 ### *** | −9.0 ± 0.9 ### *** | |
| Cohort | Normoxic Cohort: | Hypoxic Cohort: | ||||||
|---|---|---|---|---|---|---|---|---|
| Group (n) | N-Ctrl (9–14) | N-PZ (6–7) | N-PR (6–8) | N-PZ + PR (6–8) | H-Ctrl (14–16) | H-PZ (9–13) | H-PR (7–12) | H-PZ + PR (9) |
| EF [%] | 56.9 ± 1.8 | 48.8 ± 1.7 ## | ||||||
| 61.8 ± 1.2 | 62.6 ± 1.5 | 49.5 ± 5.6 * | 50.7 ± 4.3 * | 54.1 ± 2.9 # | 45.9 ± 2.5 ## * | 42.7 ± 4.2 * | 48.4 ± 3.4 | |
| SW [mmHg µL] | 15,001 ± 1146 | 10,954 ± 664 ## | ||||||
| 18512 ± 2174 | 16857 ± 1104 | 12476 ± 1560 * | 10467 ± 1532 *** | 12852 ± 1513 ## | 9577 ± 939 ## | 9151 ± 1164 | 11830 ± 1095 | |
| HR [min−1] | 426.0 ± 7.7 | 390.0 ± 6.2 ### | ||||||
| 441.8 ± 8.1 | 458.1 ± 11.2 | 423.9 ± 9.3 | 374.5 ± 21.1 *** | 410.4 ± 8.3 # | 407.4 ± 11.1 ## | 359.6 ± 11.9 ### *** | 367.7 ± 13.9 ** | |
| LV esE [mmHg/µL] | 0.33 (0.26; 0.50) | 0.36 (0.24; 0.71) | ||||||
| 0.36 (0.24; 0.53) | 0.28 (0.26; 0.34) | 0.44 (0.37; 0.55) | 0.24 (0.17; 0.50) | 0.34 (0.26; 0.66) | 0.57 (0.28; 0.76) | 0.33 (0.25; 0.58) | 0.25 (0.18; 0.72) | |
| RV dP/dtmax [mmHg/s] | 2053 (1602; 2488) | 1919 (1530; 2687) | ||||||
| 2154 (2072; 2448) | 2441 (2001; 3233) | 1888 (1609; 3348) | 1473 (1390; 1719) | 2511 (1823; 3118) | 2238 (1799; 3049) | 1568 (1503; 1919) | 1402 (1158; 1631) * | |
| RV dP/dtmin [mmHg/s] | −1711 (−2145; −1215) | −1618 (−2138; −1274) | ||||||
| −1896 (−2501; −1727) | −1863 (−2164; −1166) | −1409 (−1774; −1228) | −1208 (−1454; −1053) | −2088 (−2384; −1487) | −1856 (−2291; −1236) | −1379 (−1681; −1077) | −1333 (−1778; −1098) | |
| MAP [mmHg] | 96.4 ± 3.5 | 91.0 ± 2.4 | ||||||
| 109.6 ± 3.5 | 91.0 ± 5.7 | 92.7 ± 11.6 | 84.7 ± 5.0 | 91.4 ± 3.6 | 91.2 ± 4.8 | 87.1 ± 5.9 | 95.2 ± 5.7 | |
| TPR [mmHg min kg s−1] | 0.28 (0.25; 0.33) | 0.31 (0.29; 0.41) # | ||||||
| 0.28 (0.25; 0.32) | 0.25 (0.24; 0.28) | 0.28 (0.23; 0.46) | 0.31 (0.26; 0.38) | 0.30 (0.25; 0.33) | 0.30 (0.27; 0.46) | 0.33 (0.30; 0.43) | 0.40 (0.30; 0.51) | |
| Group | Number of Animals |
|---|---|
| N-Ctrl | 14 |
| H-Ctrl | 18 |
| N-PZ | 8 |
| H-PZ | 14 |
| N-PR | 8 |
| H-PR | 14 |
| N-PZ+PR | 8 |
| H-PZ+PR | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubert, E.; Rassler, B.; Hoschke, A.; Raffort, C.; Salameh, A. Effects of Normobaric Hypoxia and Adrenergic Blockade over 72 h on Cardiac Function in Rats. Int. J. Mol. Sci. 2023, 24, 11417. https://doi.org/10.3390/ijms241411417
Neubert E, Rassler B, Hoschke A, Raffort C, Salameh A. Effects of Normobaric Hypoxia and Adrenergic Blockade over 72 h on Cardiac Function in Rats. International Journal of Molecular Sciences. 2023; 24(14):11417. https://doi.org/10.3390/ijms241411417
Chicago/Turabian StyleNeubert, Elias, Beate Rassler, Annekathrin Hoschke, Coralie Raffort, and Aida Salameh. 2023. "Effects of Normobaric Hypoxia and Adrenergic Blockade over 72 h on Cardiac Function in Rats" International Journal of Molecular Sciences 24, no. 14: 11417. https://doi.org/10.3390/ijms241411417
APA StyleNeubert, E., Rassler, B., Hoschke, A., Raffort, C., & Salameh, A. (2023). Effects of Normobaric Hypoxia and Adrenergic Blockade over 72 h on Cardiac Function in Rats. International Journal of Molecular Sciences, 24(14), 11417. https://doi.org/10.3390/ijms241411417







