Combining 16S rRNA Sequencing and Metabolomics Data to Decipher the Interactions between Gut Microbiota, Host Immunity, and Metabolites in Diarrheic Young Small Ruminants
Abstract
:1. Introduction
2. Results
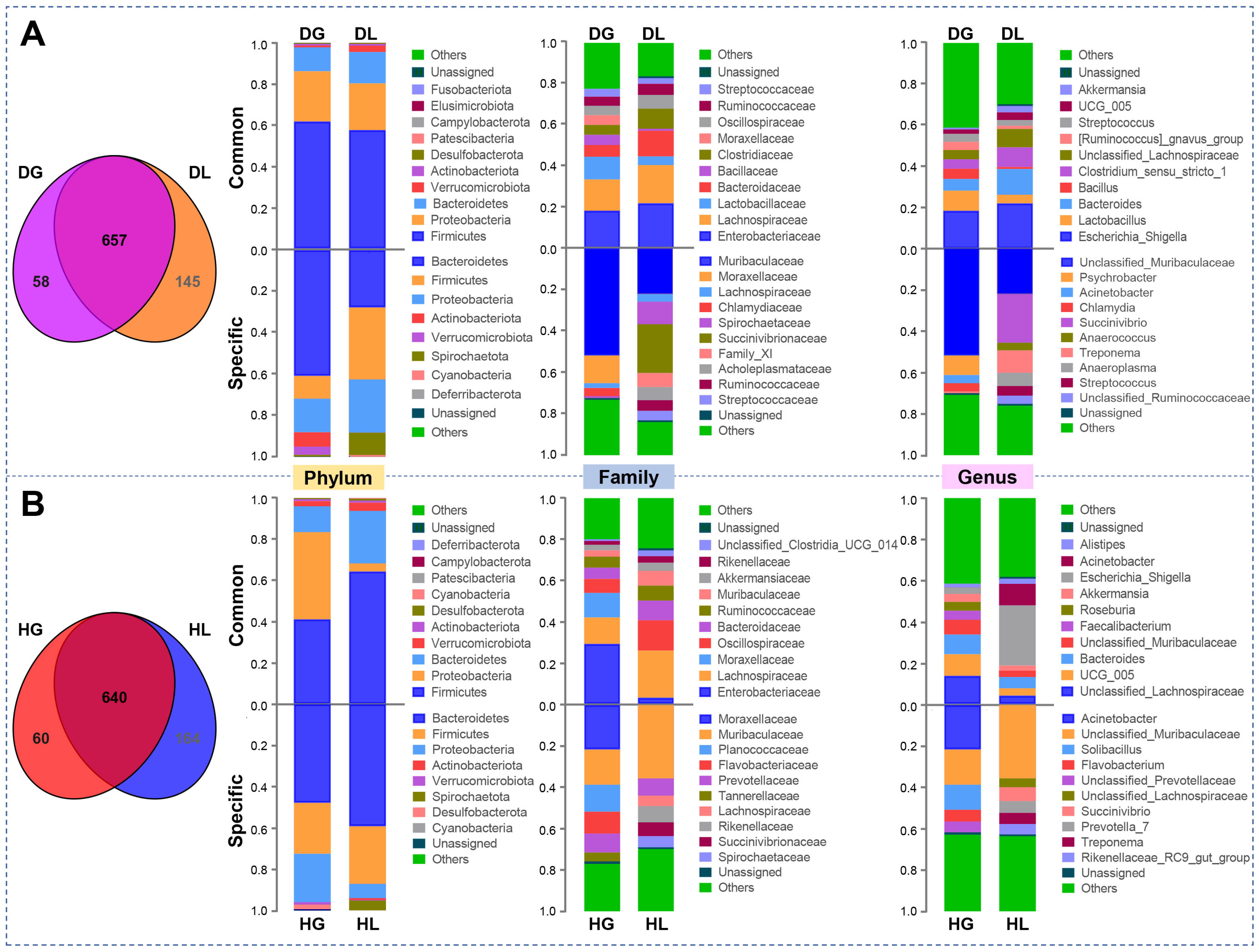
2.1. Diarrhea Alters the Diversity and Composition of the Fecal Microbiota of Lambs and Goat Kids
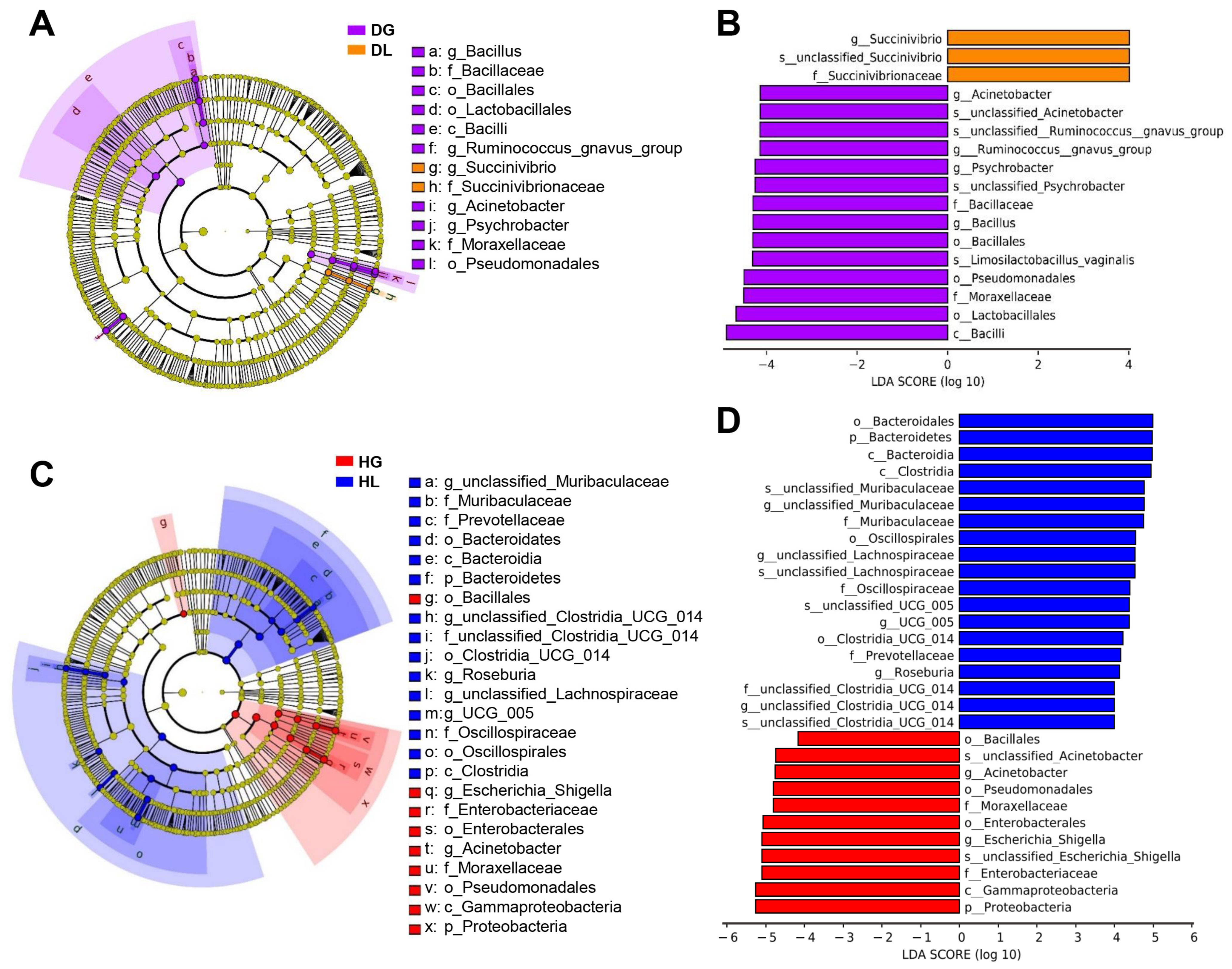
2.2. Diarrhea Shifts of Enriched Genera and Their Potential Functions
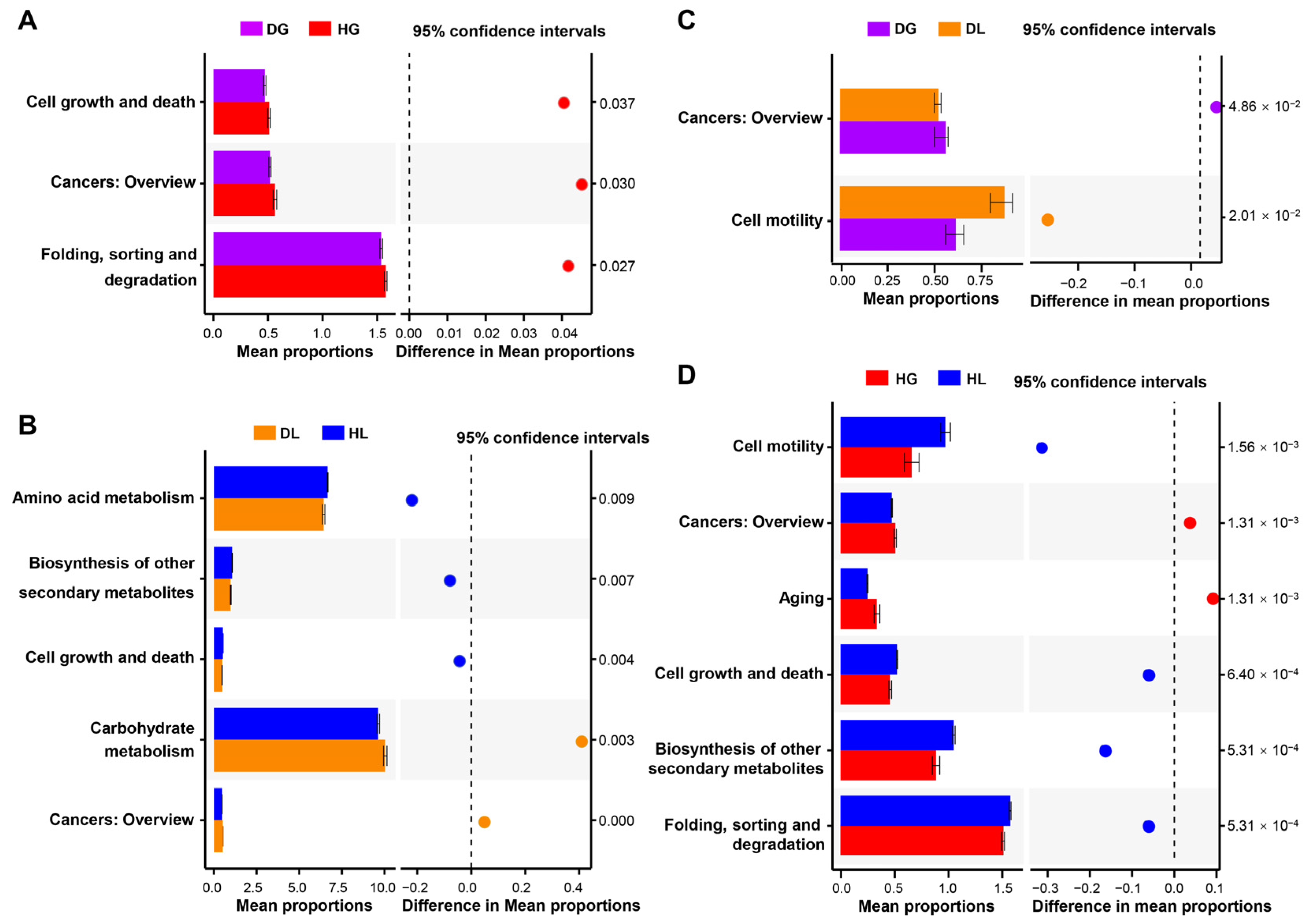
2.3. Diarrhea Changes Biochemical Blood Indexes in Lambs and Goat Kids
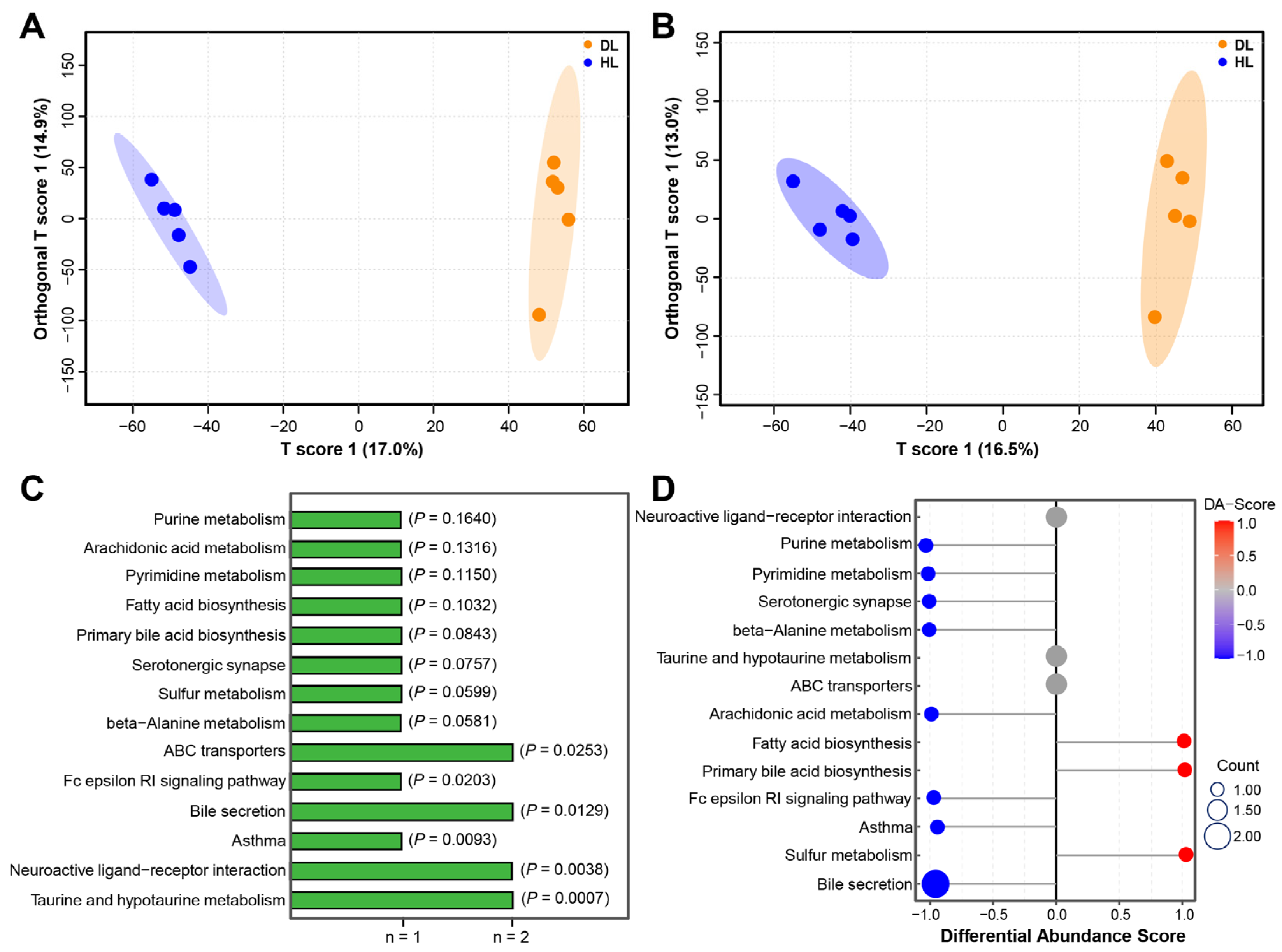
2.4. Diarrhea Alters Serum Metabolism in Both Lambs and Goat Kids
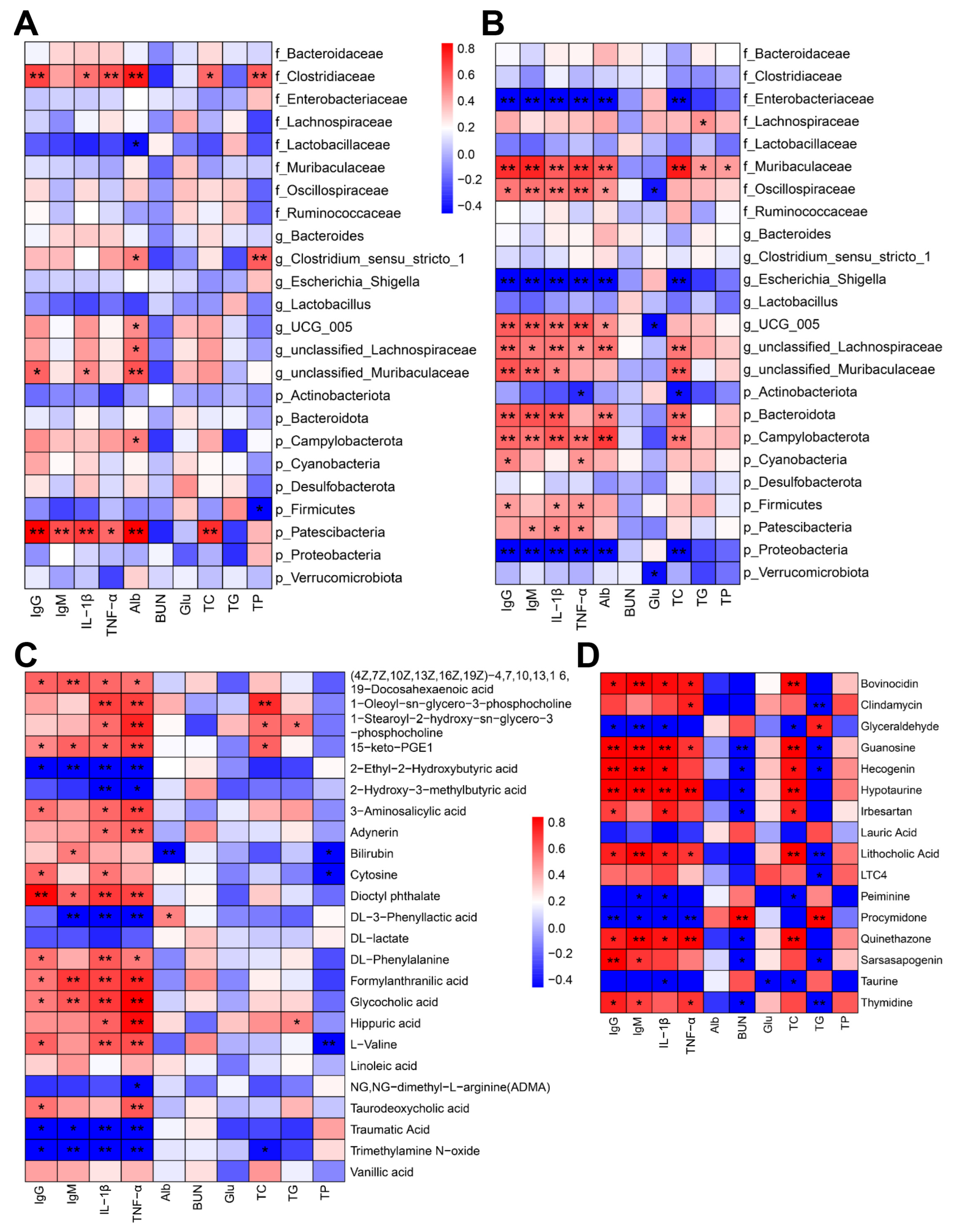
2.5. Associations of Serum Biochemical Indexes with Microbiota and Differential Metabolites
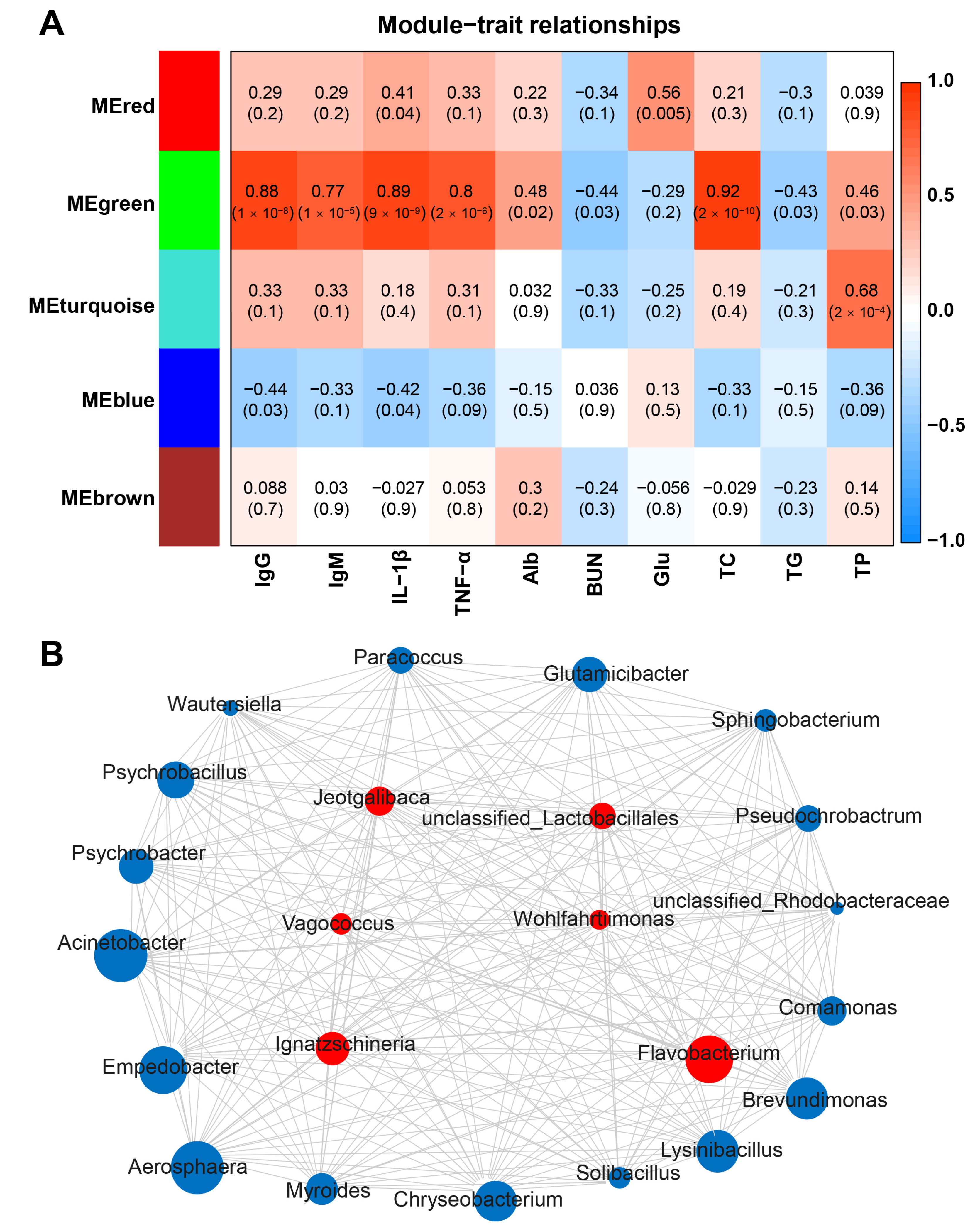
2.6. Hub Microbiota Associated with Serum Factors on Diarrheic Goat Kids and Lambs
3. Discussion
4. Materials and Methods
4.1. Study Location, Samples, and Sampling
4.2. Gut Microbiome Analysis
4.3. Determination of Serum Biochemical Parameters
4.4. Serum Metabolomics and Data Analysis
4.5. Interaction of Gut Microbiome, Host Metabolic and Immune Response
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Zhao, H.; Zhou, Z.; Miao, Y.; Li, R.; Yang, B.; Cao, C.; Xiao, S.; Wang, X.; Liu, H.; et al. Characterization of Extended-Spectrum beta-Lactamase-Producing Escherichia coli Isolates That Cause Diarrhea in Sheep in Northwest China. Microbiol. Spectr. 2022, 10, e0159522. [Google Scholar] [CrossRef] [PubMed]
- Karasova, D.; Crhanova, M.; Babak, V.; Jerabek, M.; Brzobohaty, L.; Matesova, Z.; Rychlik, I. Development of piglet gut microbiota at the time of weaning influences development of postweaning diarrhea—A field study. Res. Vet. Sci. 2021, 135, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, T.; Zhao, G.; Zhang, N.; Tu, Y.; Li, F.; Cui, K.; Bi, Y.; Ding, H.; Diao, Q. Effect of Age and Weaning on Growth Performance, Rumen Fermentation, and Serum Parameters in Lambs Fed Starter with Limited Ewe-Lamb Interaction. Animals 2019, 9, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.; Zou, H.; Li, J.; Wei, H. Landscapes of Enteric Virome Signatures in Early-Weaned Piglets. Microbiol. Spectr. 2022, 10, e0169822. [Google Scholar] [CrossRef]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut Microbiota and Diarrhea: An Updated Review. Front. Cell Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef]
- Landete, J.M.; Arqués, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Muñoz, R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Qin, S. Gut Microbiota Modulation on Intestinal Mucosal Adaptive Immunity. J. Immunol. Res. 2019, 2019, 4735040. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef]
- Whon, T.W.; Kim, H.S.; Shin, N.R.; Sung, H.; Kim, M.S.; Kim, J.Y.; Kang, W.; Kim, P.S.; Hyun, D.W.; Seong, H.J.; et al. Calf Diarrhea Caused by Prolonged Expansion of Autochthonous Gut Enterobacteriaceae and Their Lytic Bacteriophages. mSystems 2021, 6, e00816-20. [Google Scholar] [CrossRef]
- Mi, R.; Wang, X.; Huang, Y.; Mu, G.; Zhang, Y.; Jia, H.; Zhang, X.; Yang, H.; Wang, X.; Han, X.; et al. Sheep as a Potential Source of Zoonotic Cryptosporidiosis in China. Appl. Environ. Microbiol. 2018, 8, e00868-18. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhou, B.; Zhu, W. Pathogenic Escherichia coli-Specific Bacteriophages and Polyvalent Bacteriophages in Piglet Guts with Increasing Coliphage Numbers after Weaning. Appl. Environ. Microbiol. 2021, 87, e0096621. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Wang, C.; Wang, X.; Freitas-de-Melo, A.; Zeng, B.; Zhao, Q.; Zhan, S.; Wang, L.; Cao, J.; Dai, D.; et al. Early Weaning and Milk Substitutes Affect the Gut Microbiome, Metabolomics, and Antibody Profile in Goat Kids Suffering From Diarrhea. Front. Microbiol. 2022, 13, 904475. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Wang, Y.; Wang, X.; Freitas-de-Melo, A.; Li, H.; Zhan, S.; Wang, L.; Cao, J.; Dai, D.; Guo, J.; et al. Diarrhea in suckling lambs is associated with changes in gut microbiota, serum immunological and biochemical parameters in an intensive production system. Front. Microbiol. 2022, 13, 1020657. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.C.; Wang, B.; Wang, Y.M.; Hu, R.G.; Atiewin, A.; Gao, D.; Gao, Y.H.; Ma, H.X. Characterization of bacterial community changes and antibiotic resistance genes in lamb manure of different incidence. Sci. Rep. 2019, 9, 10101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhao, L.; Zhai, Z.; Zhao, W.; Ding, J.; Dai, R.; Sun, T.; Meng, H. Porcine Epidemic Diarrhea Virus Infection Induced the Unbalance of Gut Microbiota in Piglets. Curr. Microbiol. 2015, 71, 643–649. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ma, Y.; Yang, S.; Zhang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum β-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Zoetendal, E.G.; Akkermans, A.D.L.; Vliet, A.V.; de Visser, J.A.; de Vos, W.M. The Host Genotype Affects the Bacterial Community in the Human Gastronintestinal Tract. Microb. Ecol. Health. Dis. 2001, 13, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jiang, N.; Zhang, W.; Lv, Z.; Liu, J.; Shi, H. Bacillus amyloliquefaciens-9 Reduces Somatic Cell Count and Modifies Fecal Microbiota in Lactating Goats. Mar. Drugs 2021, 19, 404. [Google Scholar] [CrossRef]
- Xu, B.; Qin, W.; Xu, Y.; Yang, W.; Chen, Y.; Huang, J.; Zhao, J.; Ma, L. Dietary Quercetin Supplementation Attenuates Diarrhea and Intestinal Damage by Regulating Gut Microbiota in Weanling Piglets. Oxidative Med. Cell. Longev. 2021, 2021, 6221012. [Google Scholar] [CrossRef]
- Islam, J.; Tanimizu, M.; Shimizu, Y.; Goto, Y.; Ohtani, N.; Sugiyama, K.; Tatezaki, E.; Sato, M.; Makino, E.; Shimada, T.; et al. Development of a rational framework for the therapeutic efficacy of fecal microbiota transplantation for calf diarrhea treatment. Microbiome 2022, 10, 31. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Xu, T.; Qian, M.; Yang, Z.; Zhan, X.; Han, X. Early-Life Intervention Using Exogenous Fecal Microbiota Alleviates Gut Injury and Reduce Inflammation Caused by Weaning Stress in Piglets. Front. Microbiol. 2021, 12, 671683. [Google Scholar] [CrossRef]
- Shankar, V.; Hamilton, M.J.; Khoruts, A.; Kilburn, A.; Unno, T.; Paliy, O.; Sadowsky, M.J. Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation. Microbiome 2014, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Whon, T.W.; Sung, H.; Jeong, Y.S.; Jung, E.S.; Shin, N.R.; Hyun, D.W.; Kim, P.S.; Lee, J.Y.; Lee, C.H.; et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat. Commun. 2021, 12, 161. [Google Scholar] [CrossRef]
- Zhang, W.; Xin, H.; Jiang, N.; Lv, Z.; Shu, J.; Shi, H. Bacillus Amyloliquefaciens-9 as an Alternative Approach to Cure Diarrhea in Saanen Kids. Animals 2021, 11, 592. [Google Scholar] [CrossRef]
- Fei, H.; Lin, G.D.; Zheng, C.C.; Huang, M.M.; Qian, S.C.; Wu, Z.J.; Sun, C.; Shi, Z.G.; Li, J.Y.; Han, B.N. Effects of Bacillus amyloliquefaciens and Yarrowia lipolytica lipase 2 on immunology and growth performance of Hybrid sturgeon. Fish Shellfish Immunol. 2018, 82, 250–257. [Google Scholar] [CrossRef]
- Choi, K.S.; Kang, J.H.; Cho, H.C.; Yu, D.H.; Park, J. Changes in serum protein electrophoresis profiles and acute phase proteins in calves with diarrhea. Can. J. Vet. Res. 2021, 85, 45–50. [Google Scholar]
- Vega, C.G.; Bok, M.; Ebinger, M.; Rocha, L.A.; Rivolta, A.A.; González Thomas, V.; Muntadas, P.; D’Aloia, R.; Pinto, V.; Parreño, V.; et al. A new passive immune strategy based on IgY antibodies as a key element to control neonatal calf diarrhea in dairy farms. BMC Vet. Res. 2020, 16, 264. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Elsohaby, I.; Mkrtchyan, H.V.; Alhaider, A. Changes in blood biomarkers in Arabian horses with Clostridium difficile-induced enterocolitis. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101525. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. Colonic microflora: Nutrition and health. Nutrition 1997, 13, 476–478. [Google Scholar] [CrossRef]
- Luo, T.; Li, Y.; Zhang, W.; Liu, J.; Shi, H. Rumen and fecal microbiota profiles associated with immunity of young and adult goats. Front. Immunol. 2022, 13, 978402. [Google Scholar] [CrossRef]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Deng, X.; Yang, X.; Wang, J.; Li, T.; Hua, G.; Han, D.; Da, L.; Li, R.; Rong, W.; et al. Characteristics of Bacterial Microbiota in Different Intestinal Segments of Aohan Fine-Wool Sheep. Front. Microbiol. 2022, 13, 874536. [Google Scholar] [CrossRef] [PubMed]
- Shabana, I.I.; Albakri, N.N.; Bouqellah, N.A. Metagenomic investigation of faecal microbiota in sheep and goats of the same ages. J. Taibah. Univ. Sci. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Langda, S.; Zhang, C.; Zhang, K.; Gui, B.; Ji, D.; Deji, C.; Cuoji, A.; Wang, X.; Wu, Y. Diversity and Composition of Rumen Bacteria, Fungi, and Protozoa in Goats and Sheep Living in the Same High-Altitude Pasture. Animals 2020, 10, 186. [Google Scholar] [CrossRef] [Green Version]
- Xin, J.; Chai, Z.; Zhang, C.; Zhang, Q.; Zhu, Y.; Cao, H.; Zhong, J.; Ji, Q. Comparing the Microbial Community in Four Stomach of Dairy Cattle, Yellow Cattle and Three Yak Herds in Qinghai-Tibetan Plateau. Front. Microbiol. 2019, 10, 1547. [Google Scholar] [CrossRef] [Green Version]
- Mahayri, T.M.; Fliegerová, K.O.; Mattiello, S.; Celozzi, S.; Mrázek, J.; Mekadim, C.; Sechovcová, H.; Kvasnová, S.; Atallah, E.; Moniello, G. Host Species Affects Bacterial Evenness, but Not Diversity: Comparison of Fecal Bacteria of Cows and Goats Offered the Same Diet. Animals 2022, 12, 2011. [Google Scholar] [CrossRef]
- Kwon, M.S.; Jo, H.E.; Lee, J.; Choi, K.S.; Yu, D.; Oh, Y.S.; Park, J.; Choi, H.J. Alteration of the gut microbiota in post-weaned calves following recovery from bovine coronavirus-mediated diarrhea. J. Anim. Sci. Technol. 2021, 63, 125–136. [Google Scholar] [CrossRef]
- Li, A.; Yang, Y.; Qin, S.; Lv, S.; Jin, T.; Li, K.; Han, Z.; Li, Y. Microbiome analysis reveals gut microbiota alteration of early-weaned Yimeng black goats with the effect of milk replacer and age. Microb. Cell Fact. 2021, 20, 78. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Zhang, W.; Kulyar, M.F.; Ullah, K.; Han, Z.; Qin, J.; Bi, C.; Wang, Y.; Li, K. Comparative analysis of gut microbiota in healthy and diarrheic yaks. Microb. Cell Fact. 2022, 21, 111. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Raabis, S.; Li, W.; Cersosimo, L. Effects and immune responses of probiotic treatment in ruminants. Vet. Immunol. Immunopathol. 2019, 208, 58–66. [Google Scholar] [CrossRef]
- Gomez, D.E.; Galvao, K.N.; Rodriguez-Lecompte, J.C.; Costa, M.C. The Cattle Microbiota and the Immune System: An Evolving Field. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 485–505. [Google Scholar] [CrossRef]
- Fan, P.; Kim, M.; Liu, G.; Zhai, Y.; Liu, T.; Driver, J.D.; Jeong, K.C. The Gut Microbiota of Newborn Calves and Influence of Potential Probiotics on Reducing Diarrheic Disease by Inhibition of Pathogen Colonization. Front. Microbiol. 2021, 12, 772863. [Google Scholar] [CrossRef]
- Dong, H.; Liu, B.; Li, A.; Iqbal, M.; Mehmood, K.; Jamil, T.; Chang, Y.F.; Zhang, H.; Wu, Q. Microbiome analysis reveals the attenuation effect of lactobacillus from yaks on diarrhea via modulation of gut microbiota. Front. Cell Infect. Microbiol. 2021, 10, 610781. [Google Scholar] [CrossRef]
- Fernández, S.; Fraga, M.; Castells, M.; Colina, R.; Zunino, P. Effect of the administration of Lactobacillus spp. strains on neonatal diarrhoea, immune parameters and pathogen abundance in pre-weaned calves. Benef. Microbes 2020, 11, 477–488. [Google Scholar] [CrossRef]
- Jia, D.; Wang, Y.; Wang, J.; Liu, J.; Li, H.; Liu, A.; Wang, J.; Guan, G.; Luo, J.; Yin, H.; et al. Lactobacillus animalis pZL8a: A potential probiotic isolated from pig feces for further research. 3 Biotech 2021, 11, 132. [Google Scholar] [CrossRef]
- Dutta, S.K.; Girotra, M.; Garg, S.; Dutta, A.; von Rosenvinge, E.C.; Maddox, C.; Song, Y.; Bartlett, J.G.; Vinayek, R.; Fricke, W.F. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin. Gastroenterol. Hepatol. 2014, 12, 1572–1576. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef]
- Abe, J.; Tomigahara, Y.; Tarui, H.; Omori, R.; Kawamura, S. Identification of Metabolism and Excretion Differences of Procymidone between Rats and Humans Using Chimeric Mice: Implications for Differential Developmental Toxicity. J. Agric. Food Chem. 2018, 66, 1955–1963. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, R.; Xiong, S.; Zhang, C.; Zhang, Y. Protective effects of curcumin against rat intestinal inflammation-related motility disorders. Mol. Med. Rep. 2021, 23, 391. [Google Scholar] [CrossRef]
- Yun, S.W.; Kim, J.K.; Han, M.J.; Kim, D.H. Lacticaseibacillus paracasei NK112 mitigates Escherichia coli-induced depression and cognitive impairment in mice by regulating IL-6 expression and gut microbiota. Benef. Microbes 2021, 12, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-κB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [Green Version]
- Peterson, K.M.; Shu, J.; Duggal, P.; Haque, R.; Mondal, D.; Petri, W.A., Jr. Association between TNF-alpha and Entamoeba histolytica diarrhea. Am. J. Trop. Med. Hyg. 2010, 82, 620–625. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]

| Item | DL | DG | HL | HG | p Value | |
|---|---|---|---|---|---|---|
| DL vs. DG | HL vs. HG | |||||
| IgM (g/L) | 0.087 | 0.098 | 0.092 | 0.103 | 3.079 × 10−9 | 9.902 × 10−14 |
| IgG (g/L) | 0.699 | 0.988 | 0.763 | 1.020 | 4.596 × 10−20 | 7.838 × 10−21 |
| IL−1β | 65.064 | 112.521 | 78.027 | 161.361 | 1.038 × 10−9 | 6.836 × 10−10 |
| TNF-α | 124.148 | 185.495 | 146.731 | 208.137 | 2.283 × 10−9 | 4.580 × 10−10 |
| TP (g/L) | 46.609 | 54.580 | 44.924 | 50.180 | 0.007 | 0.016 |
| Alb (g/L) | 23.573 | 27.670 | 21.812 | 27.170 | 0.007 | 3.427 × 10−6 |
| Glu (mmol/L) | 3.514 | 3.057 | 4.001 | 2.941 | 0.398 | 0.014 |
| TC (mmol/L) | 1.110 | 4.286 | 1.418 | 5.131 | 6.624 × 10−11 | 7.876 × 10−11 |
| TG (mmol/L) | 0.740 | 0.48 | 0.340 | 0.574 | 0.021 | 0.004 |
| BUN (mmol/L) | 7.454 | 4.861 | 5.084 | 5.958 | 0.001 | 0.262 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Niu, L.; Wang, Y.; Zhan, S.; Wang, L.; Dai, D.; Cao, J.; Guo, J.; Li, L.; Zhang, H.; et al. Combining 16S rRNA Sequencing and Metabolomics Data to Decipher the Interactions between Gut Microbiota, Host Immunity, and Metabolites in Diarrheic Young Small Ruminants. Int. J. Mol. Sci. 2023, 24, 11423. https://doi.org/10.3390/ijms241411423
Wang X, Niu L, Wang Y, Zhan S, Wang L, Dai D, Cao J, Guo J, Li L, Zhang H, et al. Combining 16S rRNA Sequencing and Metabolomics Data to Decipher the Interactions between Gut Microbiota, Host Immunity, and Metabolites in Diarrheic Young Small Ruminants. International Journal of Molecular Sciences. 2023; 24(14):11423. https://doi.org/10.3390/ijms241411423
Chicago/Turabian StyleWang, Xinlu, Lili Niu, Yaxuan Wang, Siyuan Zhan, Linjie Wang, Dinghui Dai, Jiaxue Cao, Jiazhong Guo, Li Li, Hongping Zhang, and et al. 2023. "Combining 16S rRNA Sequencing and Metabolomics Data to Decipher the Interactions between Gut Microbiota, Host Immunity, and Metabolites in Diarrheic Young Small Ruminants" International Journal of Molecular Sciences 24, no. 14: 11423. https://doi.org/10.3390/ijms241411423
APA StyleWang, X., Niu, L., Wang, Y., Zhan, S., Wang, L., Dai, D., Cao, J., Guo, J., Li, L., Zhang, H., & Zhong, T. (2023). Combining 16S rRNA Sequencing and Metabolomics Data to Decipher the Interactions between Gut Microbiota, Host Immunity, and Metabolites in Diarrheic Young Small Ruminants. International Journal of Molecular Sciences, 24(14), 11423. https://doi.org/10.3390/ijms241411423






