Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Phosphotungstic Acid Encapsulated in MIL-101 (Cr, Sn) Catalyst in Deep Eutectic Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalyst
2.2. Effect of Solvents
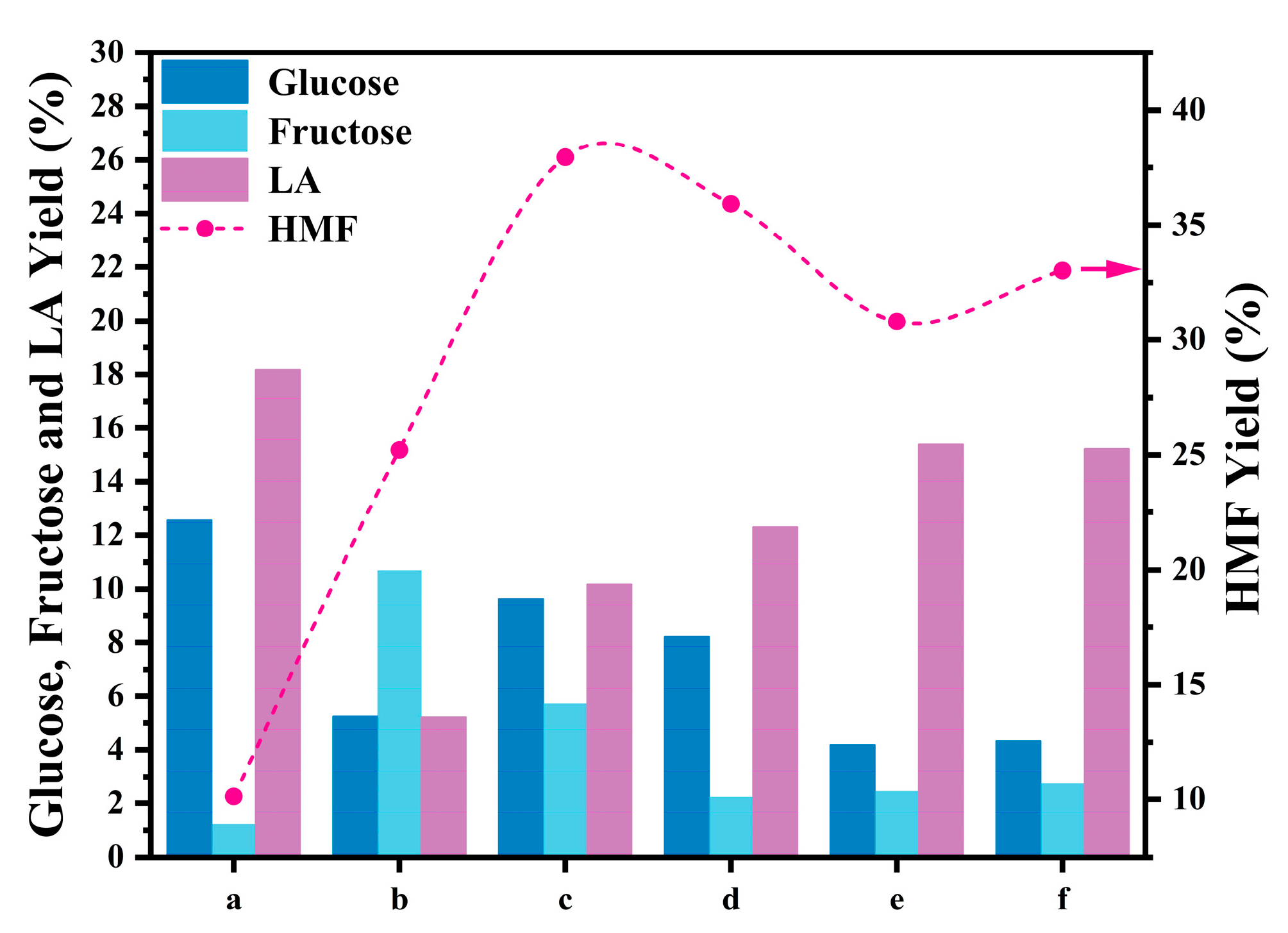
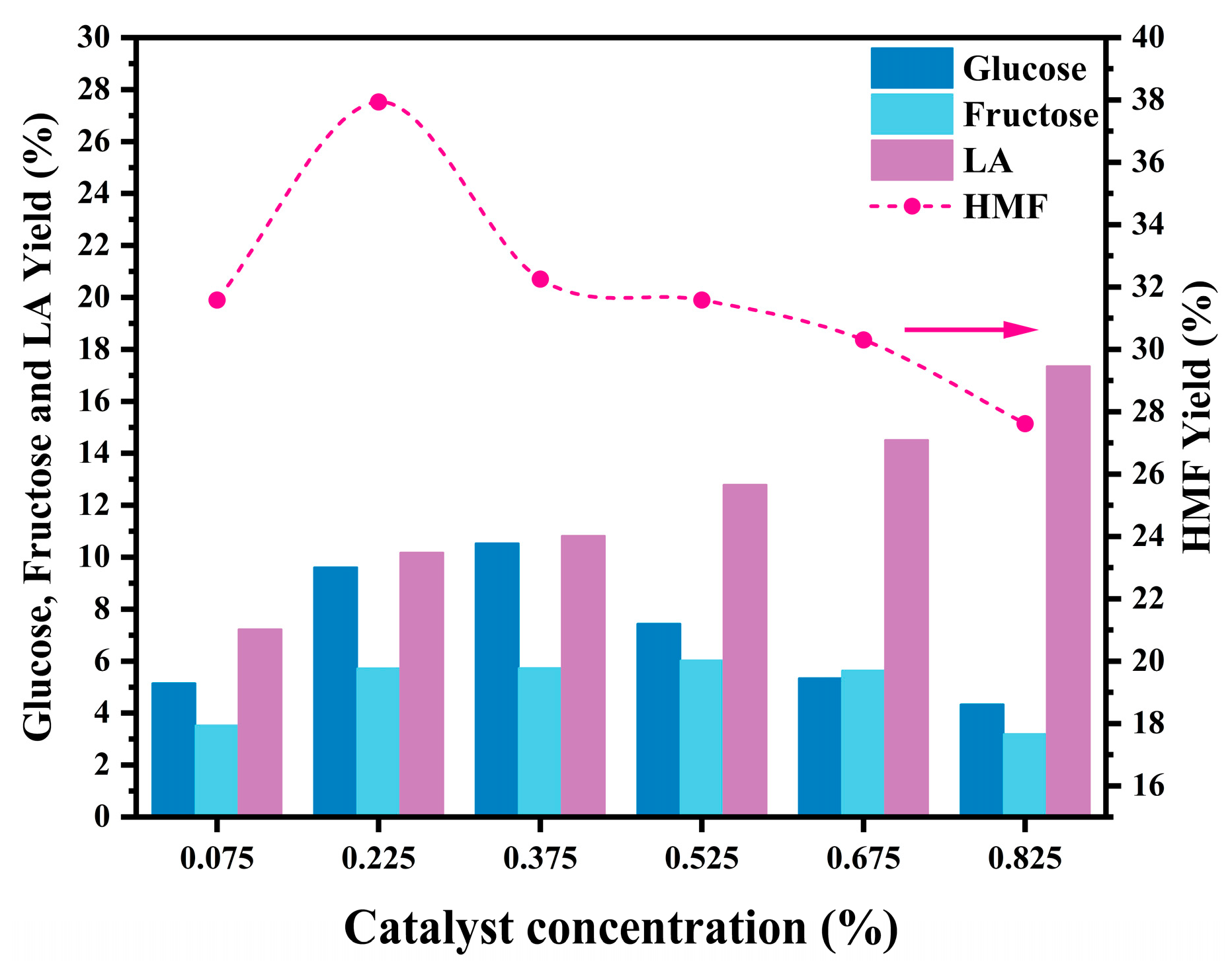
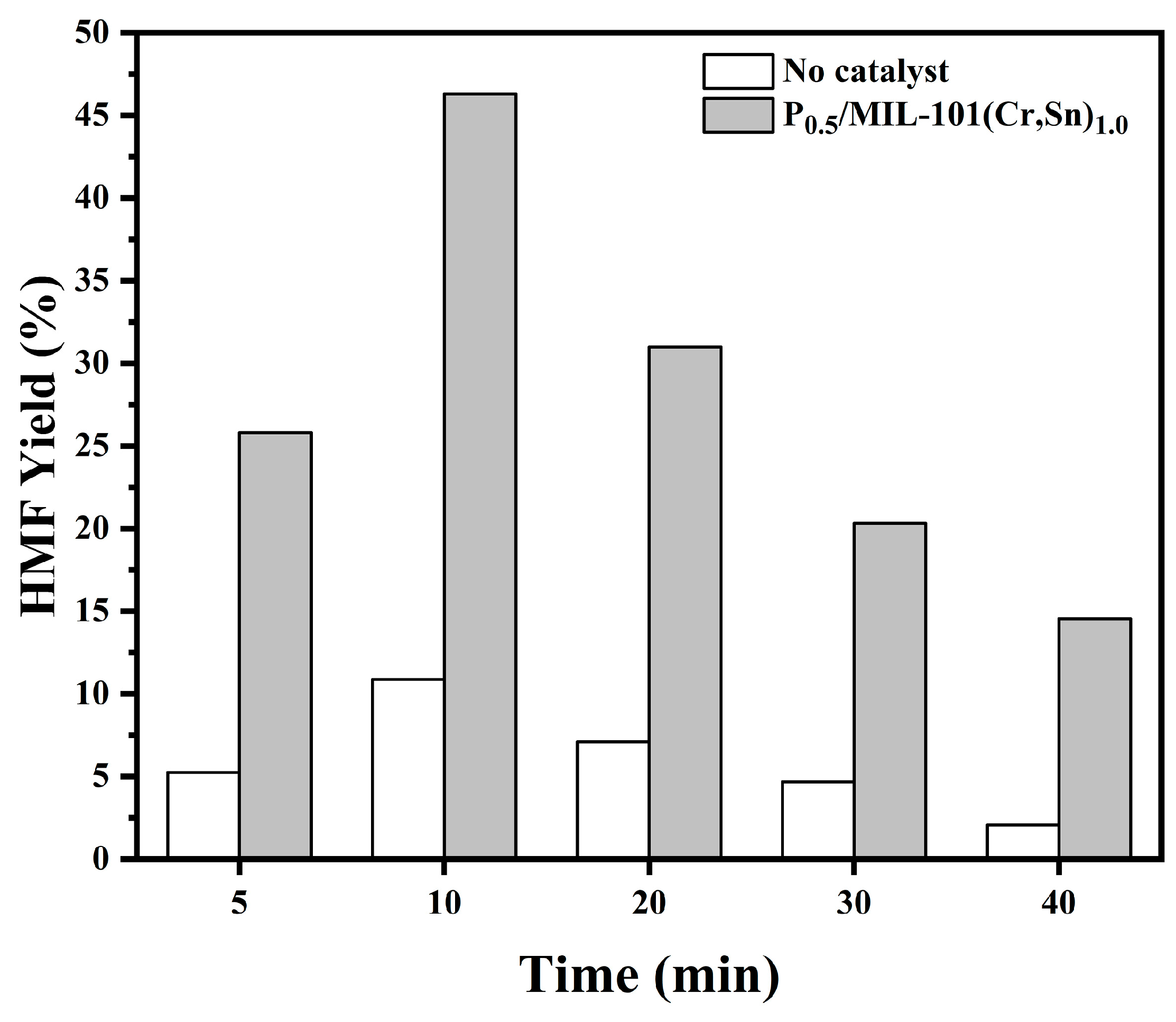
2.3. Catalytic Performance
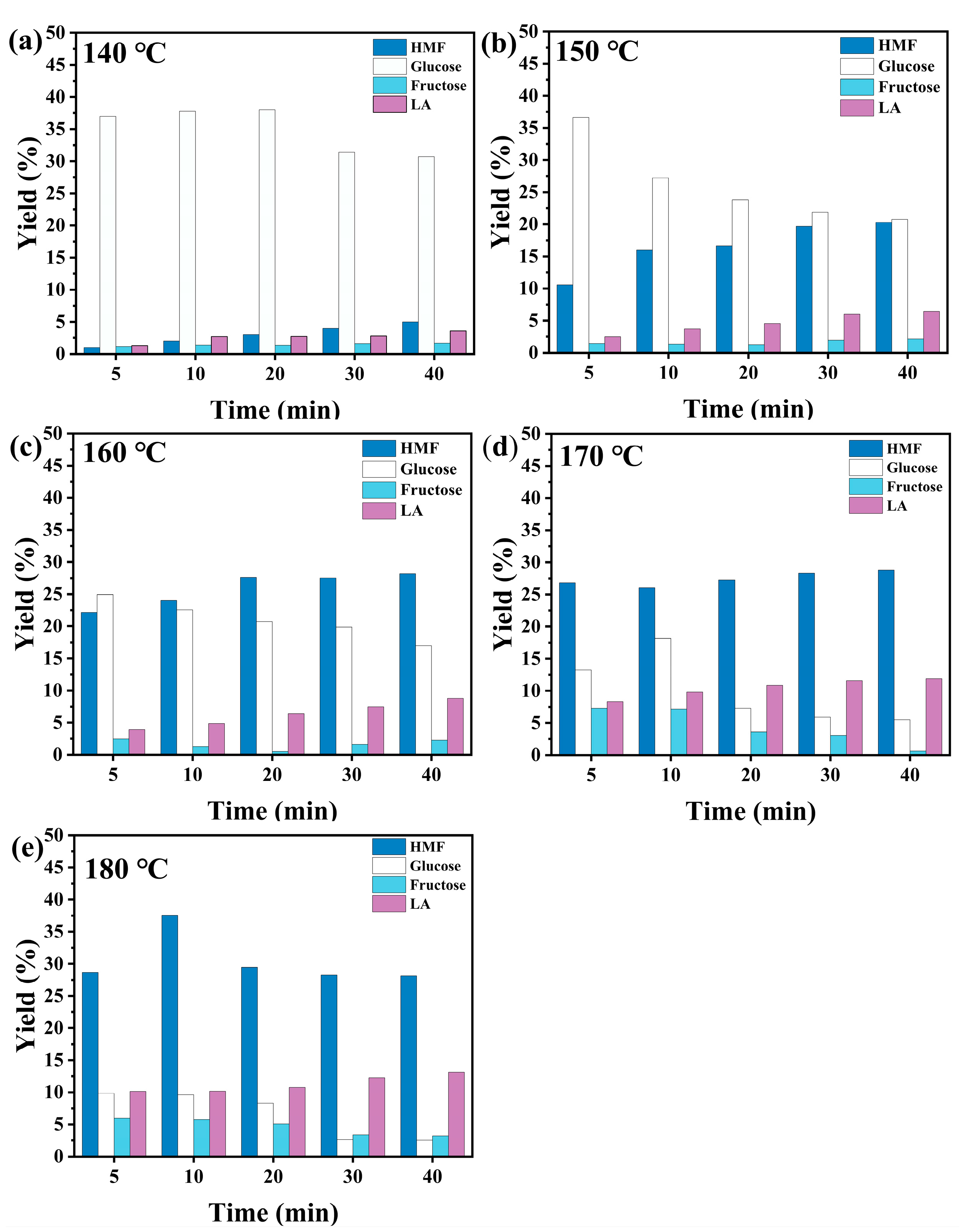
2.4. Effects of Reaction Time and Temperature on the Yield of HMF
2.5. Preparation of HMF by Catalytic Conversion of Glucose
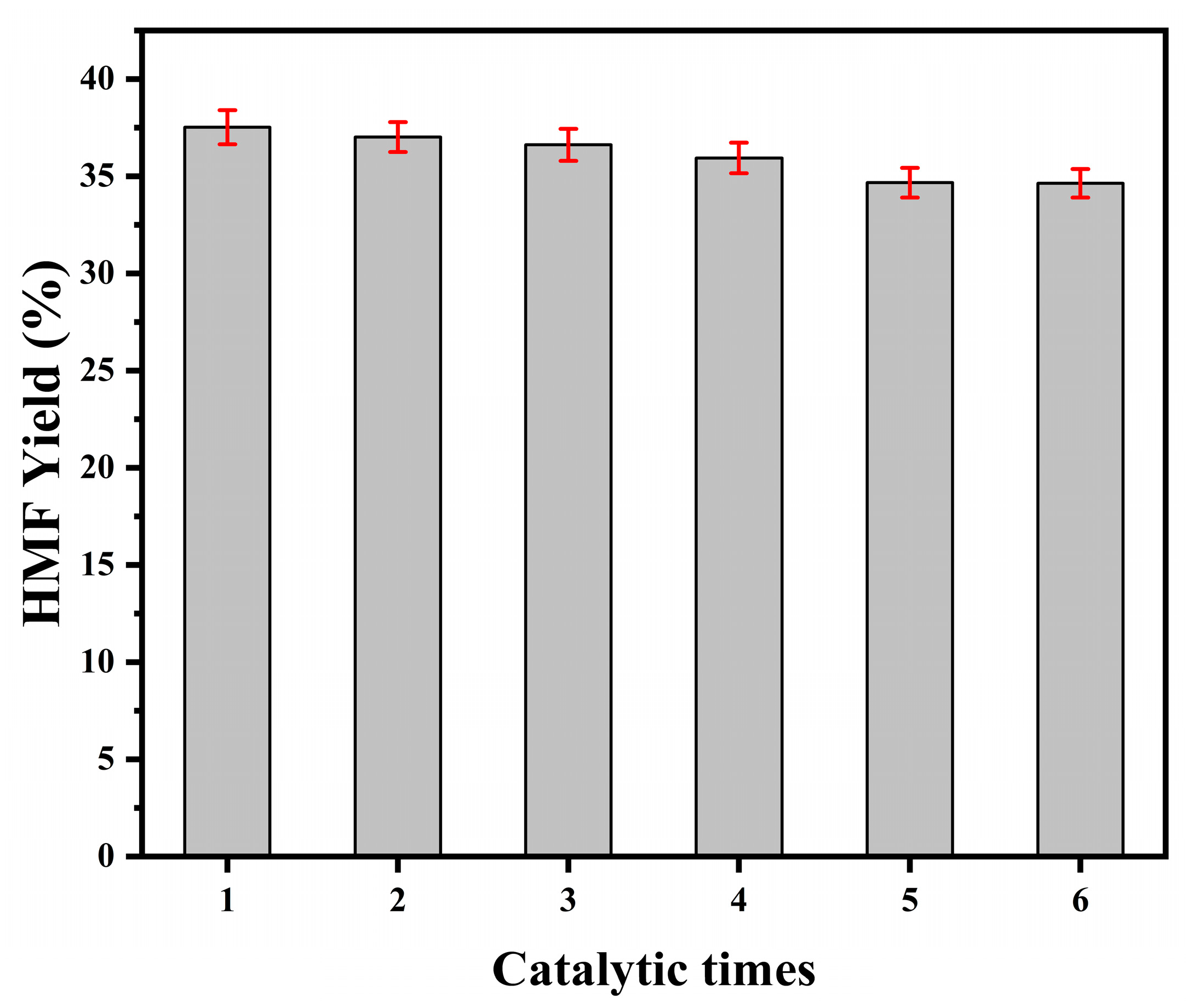
2.6. Recycling of Catalyst
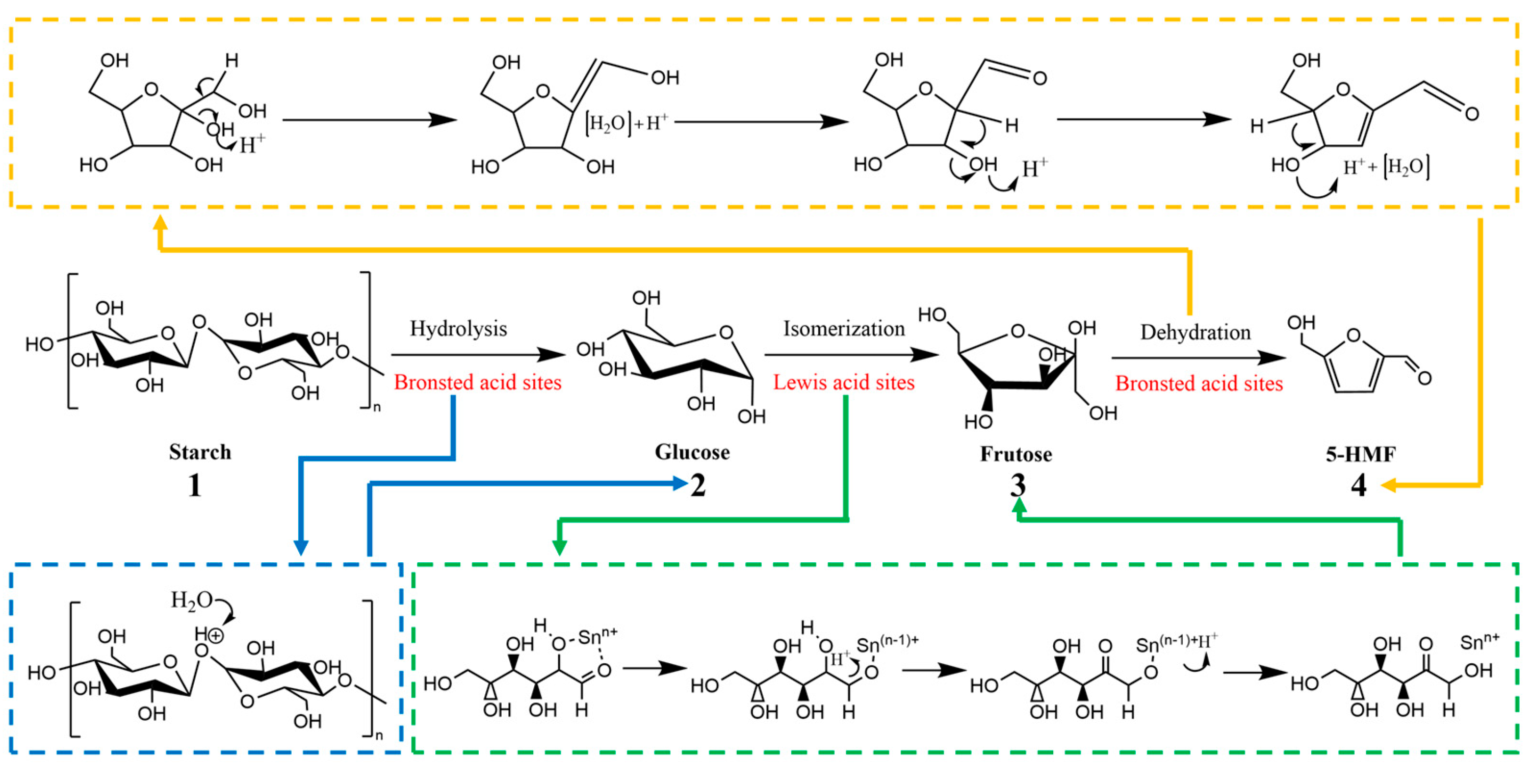
2.7. Chemical Pathway of Starch Conversion to HMF
3. Materials and Methods
3.1. Materials
3.2. Preparation of DESs
3.3. Catalyst Preparation
3.4. Characterization
3.5. Acid Strength and Density of Catalysts
3.6. Dehydration of Starch to HMF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portillo Perez, G.; Mukherjee, A.; Dumont, M.-J. Insights into HMF catalysis. J. Ind. Eng. Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Soetedjo, J.N.M.; Pidko, E.A.; van der Waal, J.C.; Hensen, E.J.M.; de Jong, E.; Heeres, H.J. Dehydration of Different Ketoses and Aldoses to 5-Hydroxymethylfurfural. Chemsuschem 2013, 6, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Raw plant-based biorefinery: A new paradigm shift towards biotechnological approach to sustainable manufacturing of HMF. Biotechnol. Adv. 2019, 37, 107422. [Google Scholar] [CrossRef]
- Chen, L.F.; Xiong, Y.H.; Qin, H.; Qi, Z.W. Advances of Ionic Liquids and Deep Eutectic Solvents in Green Processes of Biomass-Derived 5-Hydroxymethylfurfural. Chemsuschem 2022, 15, e202102635. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Li, X.Y.; Xu, R.; Yang, J.X.; Nie, S.X.; Liu, D.; Liu, Y.; Si, C.L. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind. Crops Prod. 2019, 130, 184–197. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chheda, J.N.; Roman-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Nie, Y.F.; Hou, Q.D.; Bai, C.Y.L.; Qian, H.L.; Bai, X.Y.; Ju, M.T. Transformation of carbohydrates to 5-hydroxymethylfurfural with high efficiency by tandem catalysis. J. Clean. Prod. 2020, 274, 123023. [Google Scholar] [CrossRef]
- Hou, Q.D.; Zhen, M.N.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.Q.; Li, W.Z.; Ju, M.T. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B-Environ. 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Kim, Y.; Mittal, A.; Robichaud, D.J.; Pilath, H.M.; Etz, B.D.; St John, P.C.; Johnson, D.K.; Kim, S. Prediction of Hydroxymethylfurfural Yield in Glucose Conversion through Investigation of Lewis Acid and Organic Solvent Effects. ACS Catal. 2020, 10, 14707–14721. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.J.; Cherestes, A. Production of 5-Hydroxymethylfurfural from Starch Through an Environmentally-Friendly Synthesis Pathway. Catal. Lett. 2019, 149, 283–291. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Navarro-Jaén, S.; Drault, F.; Ivanova, S. Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics. Catalysts 2021, 11, 1395. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Liu, X.; Xia, Q.; Wang, Y. Comprehensive Understanding of the Role of Brønsted and Lewis Acid Sites in Glucose Conversion into 5-Hydromethylfurfural. ChemCatChem 2017, 9, 2739–2746. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Zhao, S.; Wang, X.; Wang, S. High selective production of 5-hydroymethylfurfural from fructose by a solid heteropolyacid catalyst. Fuel 2011, 90, 2289–2293. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Tulaphol, S.; Hossain, M.A.; Jasinski, J.B.; Sun, N.; George, A.; Simmons, B.A.; Maihom, T.; Crocker, M.; Sathitsuksanoh, N. Cooperative Brønsted-Lewis acid sites created by phosphotungstic acid encapsulated metal–organic frameworks for selective glucose conversion to 5-hydroxymethylfurfural. Fuel 2021, 310, 122459. [Google Scholar] [CrossRef]
- Zhu, Z.R.; Tain, R.; Rhodes, C. A study of the decomposition behaviour of 12-tungstophosphate heteropolyacid in solution. Can. J. Chem. 2003, 81, 1044–1050. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Mirhosseini-Eshkevari, B.; Tavakoli, M.; Zamani, F. Metal–organic frameworks: Advanced tools for multicomponent reactions. Green Chem. 2020, 22, 7265–7300. [Google Scholar] [CrossRef]
- Hu, Z.G.; Zhao, D. Metal-organic frameworks with Lewis acidity: Synthesis, characterization, and catalytic applications. Crystengcomm 2017, 19, 4066–4081. [Google Scholar] [CrossRef]
- Feng, D.W.; Gu, Z.Y.; Li, J.R.; Jiang, H.L.; Wei, Z.W.; Zhou, H.C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal-Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef]
- Johnson, J.A.; Luo, J.; Zhang, X.; Chen, Y.S.; Morton, M.D.; Echeverria, E.; Torres, F.E.; Zhang, J. Porphyrin-Metalation-Mediated Tuning of Photoredox Catalytic Properties in Metal-Organic Frameworks. ACS Catal. 2015, 5, 5283–5291. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. Selective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives. New J. Chem. 2016, 40, 7958–7967. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.C.; Yaghi, O.M. Bronsted Acidity in Metal-Organic Frameworks. Chem. Rev. 2015, 115, 6966–6997. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Wang, Y.; Ma, Y. Synthesis of phosphotungstic acid-supported versatile metal–organic framework PTA@MIL-101(Fe)–NH2–Cl. RSC Adv. 2015, 5, 97589–97597. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Juan-Alcaniz, J.; Ramos-Fernandez, E.V.; Lafont, U.; Gascon, J.; Kapteijn, F. Building MOF bottles around phosphotungstic acid ships: One-pot synthesis of bi-functional polyoxometalate-MIL-101 catalysts. J. Catal. 2010, 269, 229–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Degirmenci, V.; Li, C.; Hensen, E.J.M. Phosphotungstic Acid Encapsulated in Metal–Organic Framework as Catalysts for Carbohydrate Dehydration to 5-Hydroxymethylfurfural. ChemSusChem 2011, 4, 59–64. [Google Scholar] [CrossRef]
- Hao, J.; Mao, W.; Ye, G.; Xia, Y.; Wei, C.; Zeng, L.; Zhou, J. Tin–chromium bimetallic metal–organic framework MIL-101 (Cr, Sn) as a catalyst for glucose conversion into HMF. Biomass Bioenergy 2022, 159, 106395. [Google Scholar] [CrossRef]
- Liu, B.T.; Vikrant, V.; Kim, K.H.; Kumar, V.; Kailasa, S.K. Critical role of water stability in metal-organic frameworks and advanced modification strategies for the extension of their applicability. Environ. Sci.-Nano 2020, 7, 1319–1347. [Google Scholar] [CrossRef]
- Zhao, T.; Jeremias, F.; Boldog, I.; Nguyen, B.; Henninger, S.K.; Janiak, C. High-yield, fluoride-free and large-scale synthesis of MIL-101(Cr). Dalton Trans. 2015, 44, 16791–16801. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Zeng, L.Y.; Mao, W.; Hao, J.W.; Ye, G.R.; Song, X.P.; Zeng, L.; Wang, S.F.; Zhou, J.H. Structure and functionality of cassava starch in different deep eutectic solvents/water mixtures: A comparative study. Ind. Crops Prod. 2022, 177. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Len, C. Various carbohydrate precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Wan, J.; Ma, Y. Catalytic hydrolysis of cellulose by phosphotungstic acid–supported functionalized metal-organic frameworks with different electronegative groups. Environ. Sci. Pollut. Res. 2019, 26, 15345–15353. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-Y.; Zhang, Y.-F.; Liu, Y.; Qin, F.-X.; Ren, H.-T.; Wu, S.-H. Adsorptive removal of dibenzothiophene from model fuels over one-pot synthesized PTA@MIL-101(Cr) hybrid material. J. Hazard. Mater. 2013, 262, 589–597. [Google Scholar] [CrossRef]
- Leng, K.; Sun, Y.; Li, X.; Sun, S.; Xu, W. Rapid Synthesis of Metal–Organic Frameworks MIL-101(Cr) Without the Addition of Solvent and Hydrofluoric Acid. Cryst. Growth Des. 2016, 16, 1168–1171. [Google Scholar] [CrossRef]
- Wang, X.; Liang, F.; Huang, C.; Li, Y.; Chen, B. Highly active tin(iv) phosphate phase transfer catalysts for the production of lactic acid from triose sugars. Catal. Sci. Technol. 2015, 5, 4410–4421. [Google Scholar] [CrossRef]
- Schütz, C.; Dwars, T.; Schnorpfeil, C.; Radnik, J.; Menzel, M.; Kragl, U. Selective polymerization of propylene oxide by a tin phosphate coordination polymer. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3032–3041. [Google Scholar] [CrossRef]
- Matsumiya, H.; Hara, T. Conversion of glucose into 5-hydroxymethylfurfural with boric acid in molten mixtures of choline salts and carboxylic acids. Biomass Bioenergy 2015, 72, 227–232. [Google Scholar] [CrossRef]
- Zuo, M.; Le, K.; Li, Z.; Jiang, Y.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Green process for production of 5-hydroxymethylfurfural from carbohydrates with high purity in deep eutectic solvents. Ind. Crops Prod. 2017, 99, 1–6. [Google Scholar] [CrossRef]
- Gomes, G.R.; Pastre, J.C. Microwave-assisted HMF production from water-soluble sugars using betaine-based natural deep eutectic solvents (NADES). Sustain. Energy Fuels 2020, 4, 1891–1898. [Google Scholar] [CrossRef]
- Yang, H.Y.; Lang, J.Y.; Lu, J.L.; Lan, P.; Zhang, H. Study on Catalytic Conversion of Cellulose to 5-Hydroxymethyl Furfural by Directional Degradation in Deep Eutectic Solvents. Bioresources 2020, 15, 3344–3355. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2, 5-dimethylfuran. Renew. Sustain. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]
- Cai, C.M.; Nagane, N.; Kumar, R.; Wyman, C.E. Coupling metal halides with a co-solvent to produce furfural and 5-HMF at high yields directly from lignocellulosic biomass as an integrated biofuels strategy. Green Chem. 2014, 16, 3819–3829. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. THF co-solvent enhances hydrocarbon fuel precursor yields from lignocellulosic biomass. Green Chem. 2013, 15, 3140–3145. [Google Scholar] [CrossRef]
- Tang, J.; Guo, X.; Zhu, L.; Hu, C. Mechanistic Study of Glucose-to-Fructose Isomerization in Water Catalyzed by [Al(OH)2(aq)]+. ACS Catal. 2015, 5, 5097–5103. [Google Scholar] [CrossRef]
- Akiyama, G.; Matsuda, R.; Sato, H.; Kitagawa, S. Catalytic Glucose Isomerization by Porous Coordination Polymers with Open Metal Sites. Chem. Asian J. 2014, 9, 2772–2777. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, G.; Tang, X.; Wu, Z.; Xu, J.X.; Lin, L.; Liu, S.J. Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural over cellulose-derived carbonaceous catalyst in ionic liquid. Bioresour. Technol. 2013, 148, 501–507. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, C.; Liu, B.; Zhang, M.; Li, Y.; Yang, W.; Lin, Y.; Pan, Y.; Sun, D.; Liu, Y. The encapsulation of POM clusters into MIL-101(Cr) at molecular level: LaW10O36@MIL-101(Cr), an efficient catalyst for oxidative desulfurization. Microporous Mesoporous Mater. 2021, 311, 110694. [Google Scholar] [CrossRef]
- Peng, Y.; Li, X.; Gao, T.; Li, T.; Yang, W. Preparation of 5-methylfurfural from starch in one step by iodide mediated metal-free hydrogenolysis. Green Chem. 2019, 21, 4169–4177. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Mjalli, F.S.; AlNashef, I.M. A new processing route for cleaner production of biodiesel fuel using a choline chloride based deep eutectic solvent. J. Clean. Prod. 2014, 65, 246–251. [Google Scholar] [CrossRef]
- Jagadeeswaraiah, K.; Kumar, C.R.; Prasad, P.S.S.; Lingaiah, N. Incorporation of Zn2+ ions into the secondary structure of heteropoly tungstate: Catalytic efficiency for synthesis of glycerol carbonate from glycerol and urea. Catal. Sci. Technol. 2014, 4, 2969–2977. [Google Scholar] [CrossRef]
- Hao, J.; Song, X.; Jia, S.; Mao, W.; Yan, Y.; Zhou, J. Catalytic Conversion of Starch to 5-Hydroxymethylfurfural by Tin Phosphotungstate. Front. Energy Res. 2021, 9, 679709. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Zhang, L.; Yu, X.; Liu, H.; Yagoub, A.E.A.; Zhou, C. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crops Prod. 2020, 149, 112361. [Google Scholar] [CrossRef]



| Catalysts | Initial Potential | Acid Density | Acid Density and B/L Acid Ratio a | Surface Atomic Content b | PTA Loading c | |||
|---|---|---|---|---|---|---|---|---|
| (mV) | (mmol/g) | (μmol/g) | B/L | ω (O)% | ω (P)% | ω (W)% | (wt%) | |
| PTA | 588 | 6.47 | 20.92 | 2.82 | 22.22 | 1.08 | 76.67 | 100 |
| BM1.0 | −45 | 0.03 | 5.15 | 0.02 | 33.57 | N/A | N/A | 0 |
| P0.5/BM1.0 | 142 | 1.16 | 6.15 | 0.29 | 35.97 | 0.45 | 1.55 | 9.71 |
| P1.0/BM1.0 | 180 | 1.82 | 6.45 | 0.48 | 35.41 | 0.56 | 2.04 | 12.78 |
| P2.0/BM1.0 | 195 | 2.12 | 7.20 | 0.93 | 39.98 | 1.04 | 3.28 | 20.59 |
| P3.0/BM1.0 | 193 | 1.98 | 6.82 | 0.73 | 38.80 | 0.90 | 3.03 | 18.98 |
| Samples | SBET a | Vtotal b | Pore Size c |
|---|---|---|---|
| (m2/g) | (cm3/g) | (nm) | |
| BM1.0 | 2826 | 1.31 | 4.02 |
| P0.5/BM1.0 | 1379 | 0.84 | 3.41 |
| P1.0/BM1.0 | 1171 | 0.74 | 3.97 |
| P2.0/BM1.0 | 1134 | 0.62 | 2.66 |
| P3.0/BM1.0 | 895 | 0.48 | 2.58 |
| DESs/EAC (v/v) | HMF in EAC (mg) | HMF in DES (mg) | HMF Yield (%) a | Partition Coefficient (%) b |
|---|---|---|---|---|
| 2/18 | 17.69 | 4.75 | 21.94 | 76.78 |
| 4/16 | 16.86 | 11.56 | 27.09 | 59.25 |
| 6/14 | 18.54 | 19.45 | 36.18 | 48.81 |
| 8/12 | 14.92 | 24.91 | 37.94 | 37.46 |
| 10/10 | 14.46 | 22.26 | 34.97 | 39.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, W.; Hao, J.; Zeng, L.; Wang, H.; Xu, H.; Zhou, J. Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Phosphotungstic Acid Encapsulated in MIL-101 (Cr, Sn) Catalyst in Deep Eutectic Solvents. Int. J. Mol. Sci. 2023, 24, 11480. https://doi.org/10.3390/ijms241411480
Mao W, Hao J, Zeng L, Wang H, Xu H, Zhou J. Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Phosphotungstic Acid Encapsulated in MIL-101 (Cr, Sn) Catalyst in Deep Eutectic Solvents. International Journal of Molecular Sciences. 2023; 24(14):11480. https://doi.org/10.3390/ijms241411480
Chicago/Turabian StyleMao, Wei, Jiawen Hao, Lingyu Zeng, Hao Wang, Hao Xu, and Jinghong Zhou. 2023. "Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Phosphotungstic Acid Encapsulated in MIL-101 (Cr, Sn) Catalyst in Deep Eutectic Solvents" International Journal of Molecular Sciences 24, no. 14: 11480. https://doi.org/10.3390/ijms241411480
APA StyleMao, W., Hao, J., Zeng, L., Wang, H., Xu, H., & Zhou, J. (2023). Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Phosphotungstic Acid Encapsulated in MIL-101 (Cr, Sn) Catalyst in Deep Eutectic Solvents. International Journal of Molecular Sciences, 24(14), 11480. https://doi.org/10.3390/ijms241411480






