Fabrication, Structure, and Properties of Nonwoven Silk Fabrics Prepared with Different Cocoon Layers
Abstract
:1. Introduction
2. Results and Discussion
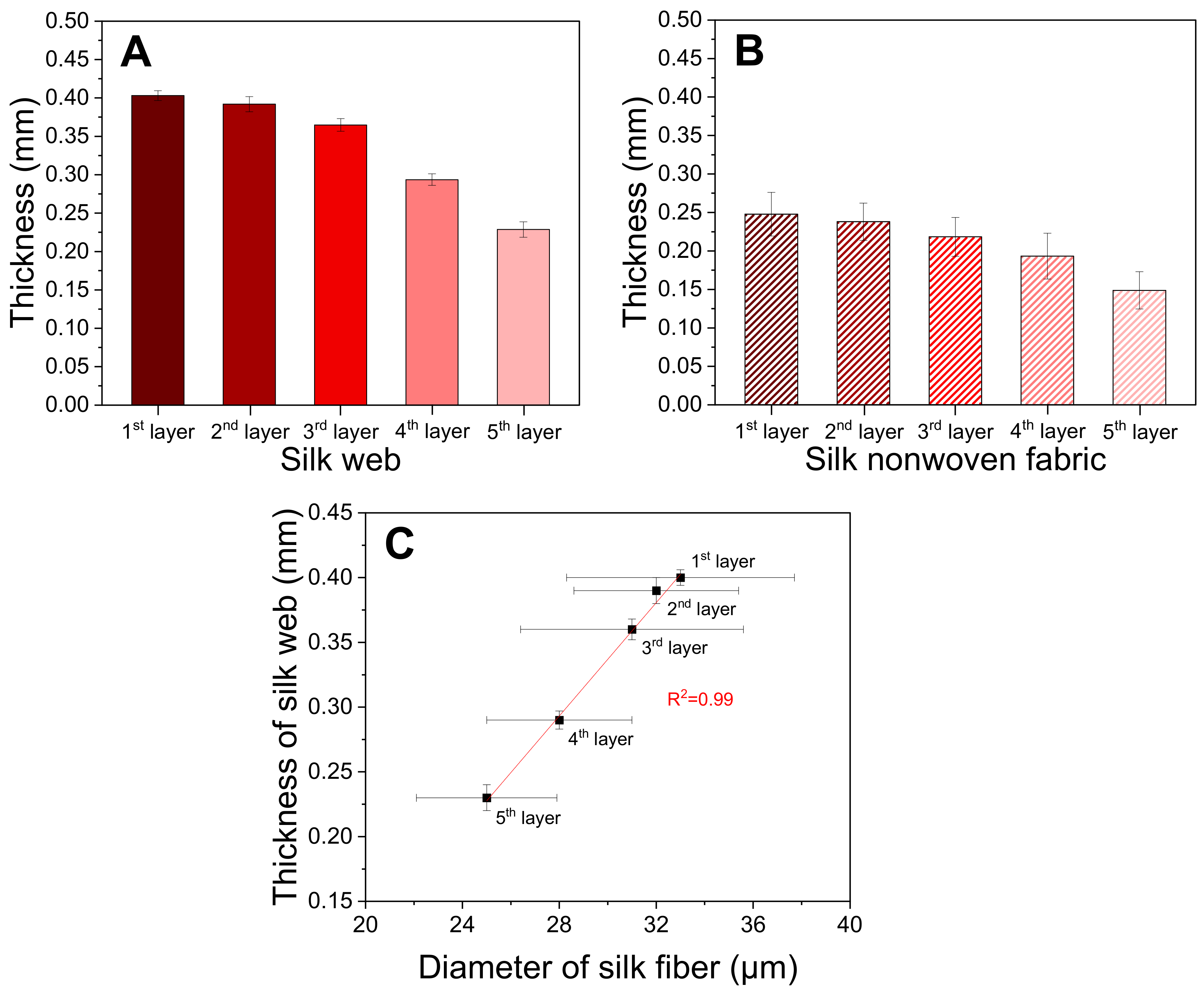
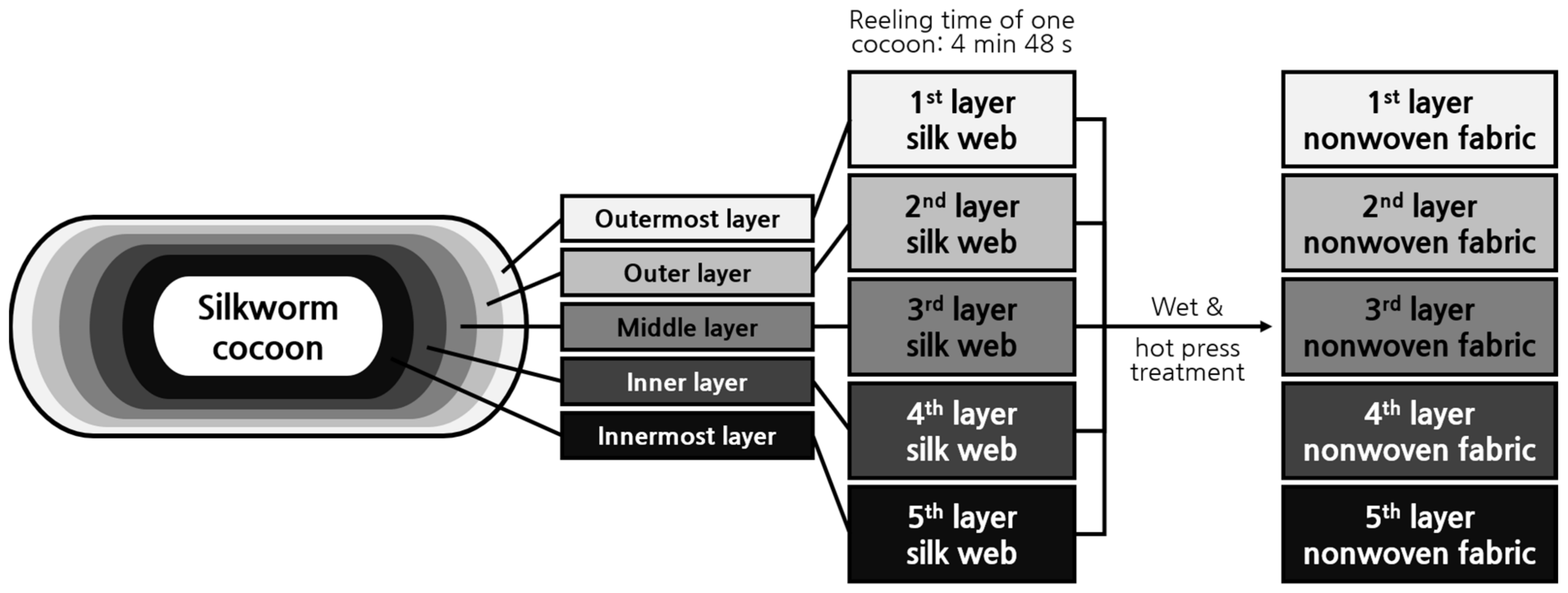
2.1. Morphology of Silk Web and Nonwoven Fabric
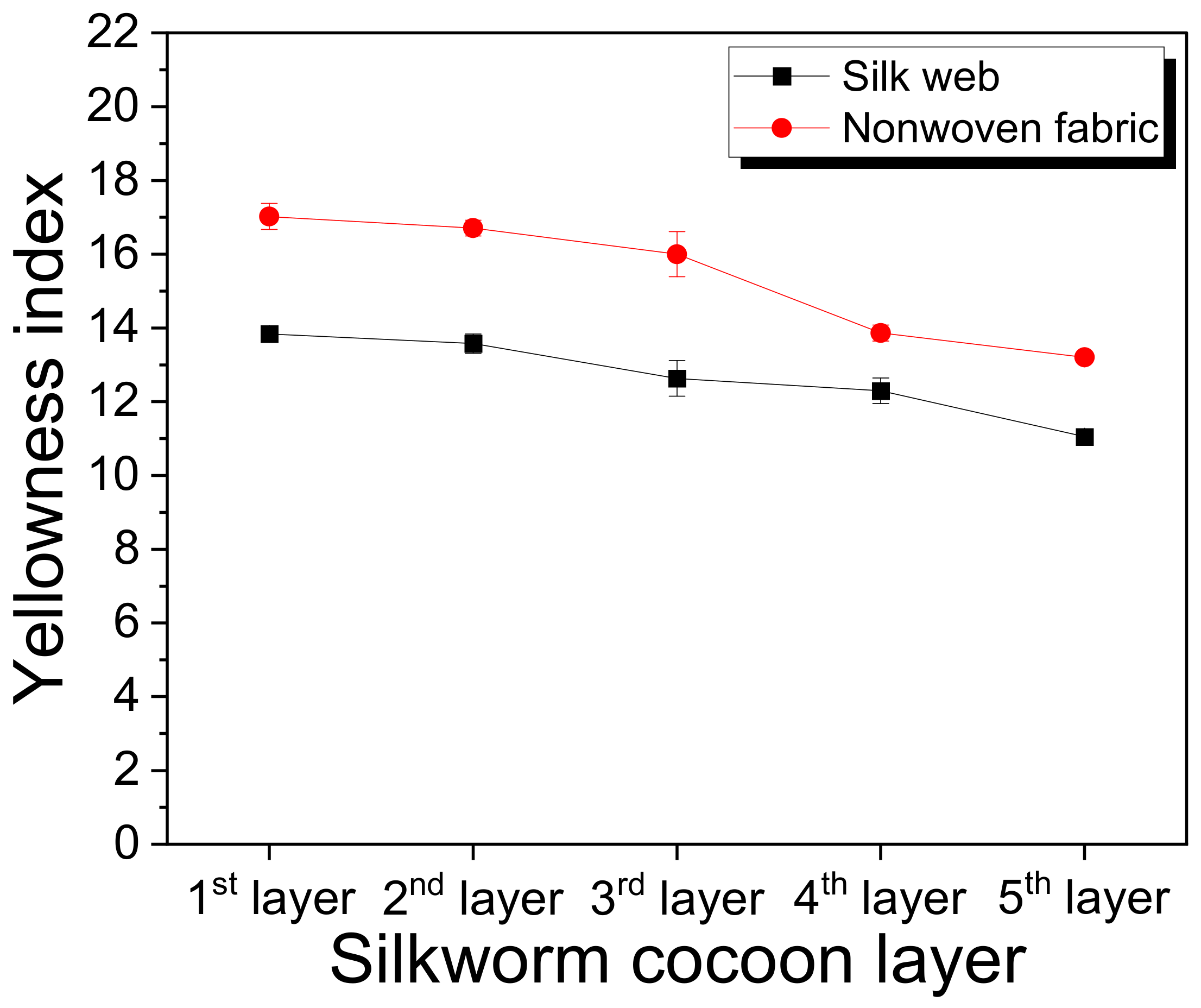
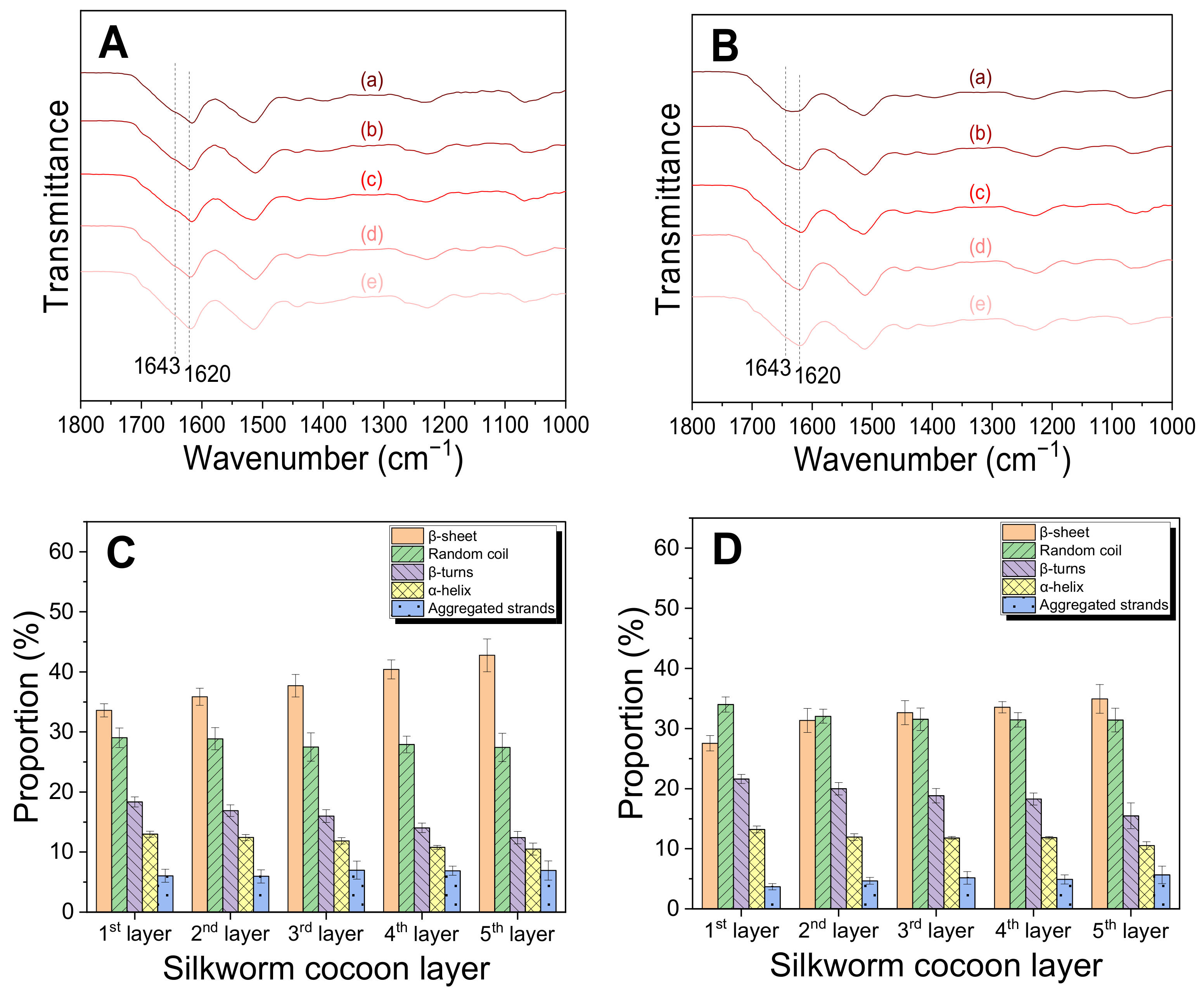
2.2. Structural Characteristics of the Silk Web and Nonwoven Fabric
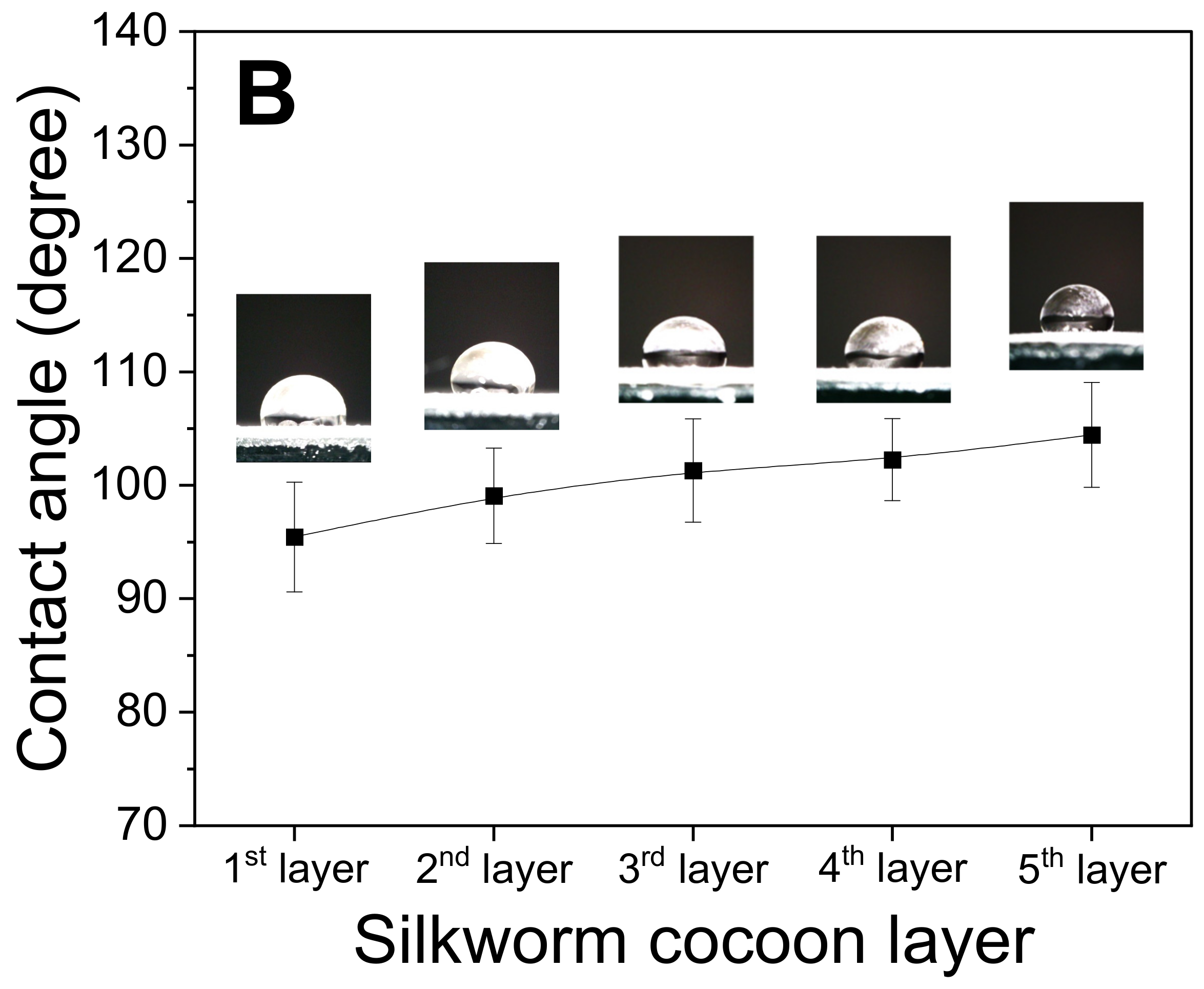
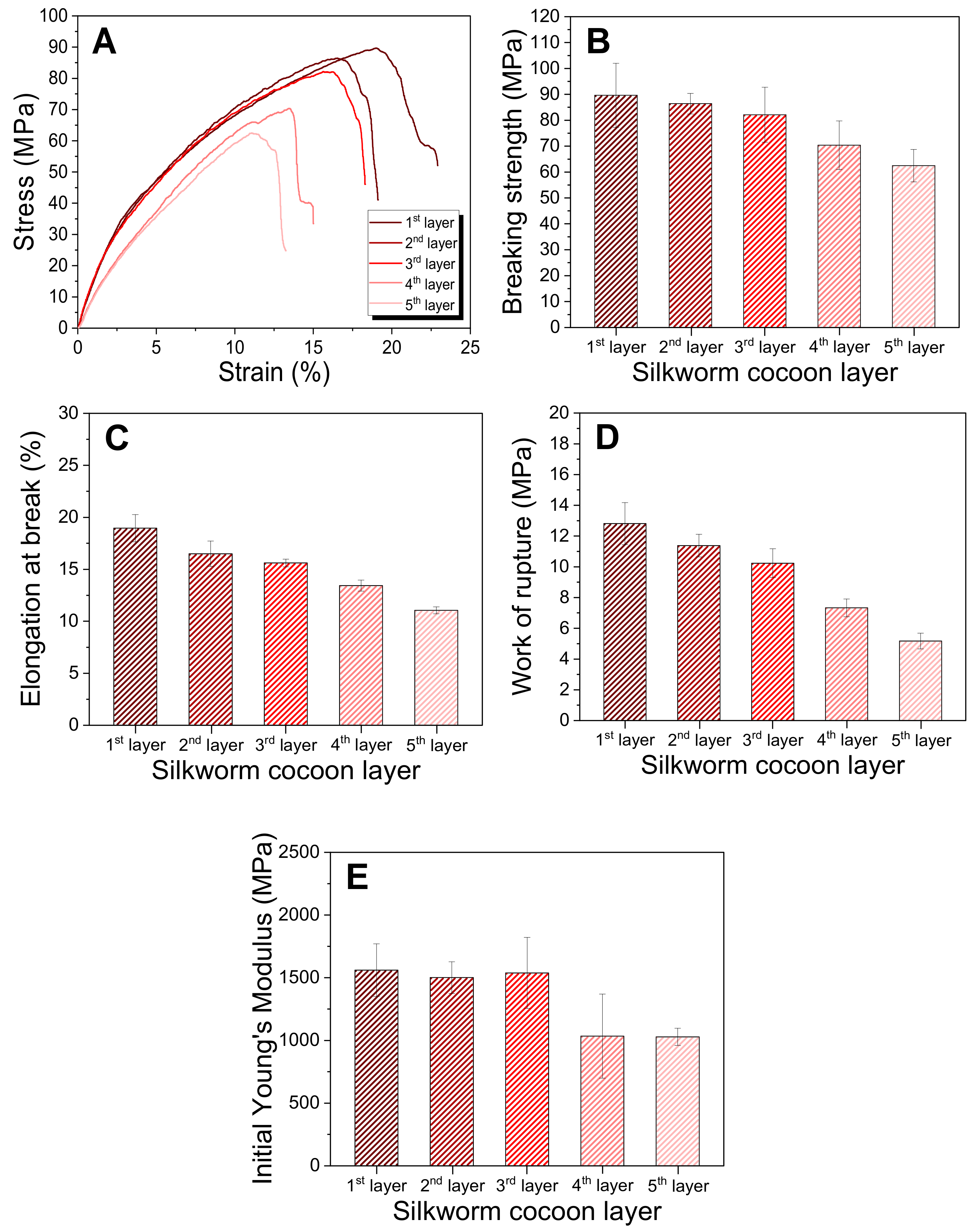
2.3. Thermal and Mechanical Properties of the Silk Web and Nonwoven Silk Fabric
2.4. Cell Viability of the Nonwoven Silk Fabric
3. Materials and Methods
3.1. Materials
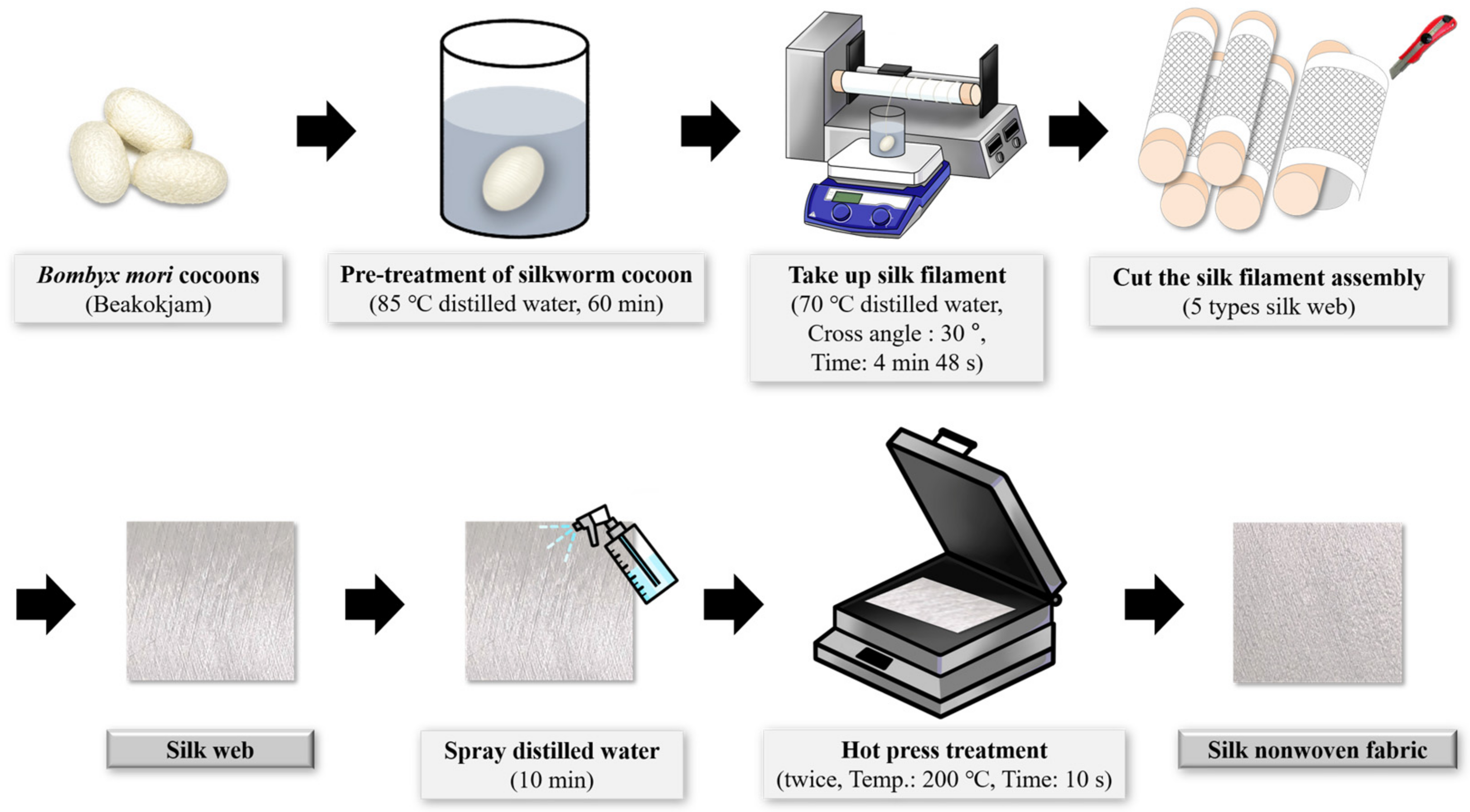
3.2. Preparation of the Silk Web and Nonwoven Silk Fabric
3.3. Measurement and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unger, R.E.; Wolf, M.; Peters, K.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Growth of human cells on a non-woven silk fibroin net: A potential for use in tissue engineering. Biomaterials 2004, 25, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, L.; Apfel, T.; Nuss, K.M.R.; Vogt, R.; Kim, H.Y.; Meinel, L.; Kaplan, D.L.; Auer, J.A.; Merkle, H.P.; Rechenberg, B.V. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur. J. Pharm. Biopharm. 2013, 85, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation of Bombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2004, 91, 2383–2390. [Google Scholar] [CrossRef]
- Zuo, B.; Dai, L.; Wu, Z. Analysis of structure and properties of biodegradable regenerated silk fibroin fibers. J. Mater. Sci. 2006, 41, 3357–3361. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoo, Y.J.; Kim, J.W.; Park, Y.H.; Bae, D.G.; Um, I.C. Effect of molecular weight and storage time on the wet- and electro-spinning of regenerated silk fibroin. Polym. Degrad. Stab. 2012, 97, 1060–1066. [Google Scholar] [CrossRef]
- Sakabe, H.; Ito, H.; Miyamoto, T.; Noishiki, Y.; Ha, W.S. In vivo blood compatibility of regenerated silk fibroin. Sen’i Gakkaishi 1989, 45, 487–490. [Google Scholar] [CrossRef] [Green Version]
- Um, I.C.; Kweon, H.Y.; Hwang, C.M.; Min, B.G.; Park, Y.H. Structural characteristics and properties of silk fibroin/polyurethane blend films. Int. J. Ind. Entomol. 2002, 5, 163–170. [Google Scholar]
- Lu, H.; Jain, M.; Yin, Z.; Xia, K.; Shi, S.; Zhang, M.; Wang, H.; Liang, X.; Ma, W.; Zhang, X.; et al. Silkworm silk fibers with multiple reinforced properties obtained through feeding Ag nanowires. Adv. Fiber Mater. 2022, 4, 547–555. [Google Scholar] [CrossRef]
- Jin, H.J.; Chen, J.; Karageorgious, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibrion mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Marelli, B.; Alessandrino, A.; Farè, S.; Freddi, G.; Mantovani, D.; Tanzi, M.C. Compliant electrospun silk fibroin tubes for small vessel bypass grafting. Acta Biomater. 2010, 6, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.E.; Kim, H.H.; Kim, M.K.; Lee, K.H.; Park, Y.H.; Um, I.C. Effects of different Bombyx mori silkworm varieties on the structural characteristics and properties of silk. Int. J. Biol. Macromol. 2015, 79, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Motta, A.; Freddi, G.; Cannas, M. In vitro evalutation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res. 1999, 16, 382–389. [Google Scholar] [CrossRef]
- Kim, M.K.; Kwak, H.W.; Kim, H.H.; Kwon, T.R.; Kim, S.Y.; Kim, B.J.; Park, Y.H.; Lee, K.H. Surface modification of silk fibroin nanofibrous mat with dextran for wound dressing. Fibers Polym. 2014, 15, 1137–1145. [Google Scholar] [CrossRef]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, L.; Park, H.N.; Shin, S.Y.; Park, W.H.; Lee, S.C.; Kim, T.I.; Park, Y.J.; Seol, Y.J.; Lee, Y.M.; et al. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J. Biotechnol. 2005, 120, 327–339. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, S.G.; Lee, J.W.; Chae, W.S.; Kweon, H.Y.; Jo, Y.Y.; Lee, K.G.; Lee, Y.C.; Choi, J.Y.; Kim, J.Y. Accelerated healing with the use of a silk fibroin membrane for the guided bone regeneration technique. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, e26–e33. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, J.; Chen, C.; Yao, C.; Chung, S.M.; Yao, J.; Lee, I.S.; Kong, X. Silk fibroin membrane used for guided bone tissue regeneration. Mater. Sci. Eng. C. 2017, 70, 148–154. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, Y.; Yao, J.; Shao, Z.; Chen, X. Tamoxifen-loaded silk fibroin electrospun fibers. Mater. Lett. 2016, 178, 31–34. [Google Scholar] [CrossRef]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Lee, K.G.; Kang, S.W.; Kweon, H.Y.; Kim, H.J. Functional recovery guided by an electrospun silk fibroin conduit after sciatic nerve injury in rats. J. Tissue Eng. Regen. Med. 2012, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, B. Development and performance study of a natural silk fiber facial mask paper. J. Eng. Fibers Fabr. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.P.; Feng, X.Q.; Yu, S.W.; Cui, W.Z.; Zou, F.Z. Mechanical properties of silkworm cocoons. Polymer 2005, 46, 9192–9201. [Google Scholar] [CrossRef]
- Chen, F.; Porter, D.; Vollrath, F. Silk cocoon (Bombyx mori): Multi-layer structure and mechanical properties. Acta Biomater. 2012, 8, 2620–2627. [Google Scholar] [CrossRef]
- Chen, F.; Hesselberg, T.; Porter, D.; Vollrath, F. The impact behaviour of silk cocoons. J. Exp. Biol. 2013, 216, 2648–2657. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Kim, M.K.; Kweon, H.Y.; Jo, Y.Y.; Lee, K.G.; Lee, J.K. Comparison of unprocessed silk cocoon and silk cocoon middle layer membranes for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 11. [Google Scholar] [CrossRef] [Green Version]
- Kweon, H.Y.; Jo, Y.Y.; Seok, H.; Kim, S.G.; Chae, W.S.; Sapru, S.; Kundu, S.C.; Kim, D.W.; Park, N.R.; CHe, X.; et al. In vivo bone regeneration ability of different layers of natural silk cocoon processed using an eco-friendly method. Macromol. Res. 2017, 25, 806–816. [Google Scholar] [CrossRef]
- Bae, Y.S.; Um, I.C. Effects of wet and hot press treatments on structure and properties of mechanically fabricated natural silk non-woven fabrics. J. Text. Eng. 2018, 55, 381–389. [Google Scholar] [CrossRef]
- Kim, Y.E.; Bae, Y.J.; Seok, Y.S.; Um, I.C. Effect of hot press time on the structure characteristics and mechanical properties of silk non-woven fabric. Int. J. Ind. Entomol. 2022, 44, 12–20. [Google Scholar] [CrossRef]
- Bae, Y.S.; Um, I.C. Effects of fabrication conditions on structure and properties of mechanically prepared natural silk web and non-woven fabrics. Polymers 2021, 13, 1578. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Um, I.C. Preparation, structural characteristics, and properties of airlaid nonwoven silk fabric. Polym. Korea 2020, 44, 809–816. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, I.C. Preparation, structural characterization, and properties of natural silk non-woven fabrics from different silkworm varieties. Fibers Polym. 2022, 23, 1130–1141. [Google Scholar] [CrossRef]
- Bae, Y.J.; Jang, M.J.; Um, I.C. Silk/rayon webs and nonwoven fabrics: Fabrication, Structural Characteristics, and Properties. Int. J. Mol. Sci. 2022, 23, 7511. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, Y.S.; Kim, S.J.; Song, D.W.; Park, Y.H.; Bae, D.G.; Choi, J.H.; Um, I.C. Preparation of new natural silk non-woven fabrics by using adhesion characteristics of sericin and their characterization. Int. J. Biol. Macromol. 2018, 106, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.R. (Ed.) Quality of cocoon filament. In Silk Reeling; Oxford & IBH Publication Co. Pvt. Ltd.: Oxford, UK, 1998; pp. 58–69. ISBN 9781578080380. [Google Scholar]
- Setoyama, K. Effect of water on the heat-yellowing oh silk fabric and the changes in amino acid composition in the silk fibroin in sealed tubes by heat-treatment. J. Seric. Sci. Jpn. 1982, 51, 365–369. [Google Scholar] [CrossRef]
- Lee, K.G.; Kweon, H.Y.; Yeo, J.H.; Woo, S.O.; Lee, Y.W.; Cho, C.S.; Kim, K.H.; Park, Y.H. Effect of methyl alcohol on the morphology and conformational characteristics of silk sericin. Int. J. Biol. Macromol. 2003, 33, 75–80. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, Y.Q. Effect of regeneration of liquid silk fibroin on its structure and characterization. Soft Matter 2013, 9, 138–145. [Google Scholar] [CrossRef]
- Ude, A.U.; Eshkoor, R.A.; Zulkifili, R.; Ariffin, A.K.; Dzuraidah, A.W.; Azhari, C.H. Bombyx mori silk fibre and its composite: A review of contemporary developments. Mater. Des. 2014, 57, 298–305. [Google Scholar] [CrossRef]
- Chung, D.E.; Um, I.C. Effect of molecular weight and concentration on crystallinity and post drawing of wet spun silk fibroin fiber. Fibers Polym. 2014, 15, 153–160. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, D.W.; Park, Y.H.; Um, I.C. Effect of residual sericin on the structural characteristics and properties of regenerated silk films. Int. J. Biol. Macromol. 2016, 89, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.S.; Ki, C.S.; Um, I.C. Effect of sericin content on the structural characteristics and properties of electro-spun regenerated silk. Fibers Polym. 2018, 19, 507–514. [Google Scholar] [CrossRef]
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef]
- Kim, H.J.; Um, I.C. Effect of degumming ratio on wet spinning and post drawing performance of regenerated silk. Int. J. Biol. Macromol. 2014, 67, 387–393. [Google Scholar] [CrossRef]
- Chen, X.; Shao, Z.; Marinkovic, N.S.; Miller, L.M.; Zhou, P.; Chance, M.R. Conformation transition kinetics of regenerated Bombyx mori silk fibroin membrane monitored by time-resolved FTIR spectroscopy. Biophys. Chem. 2001, 89, 25–34. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Ha, H.C.; Um, I.C.; Park, Y.H. Physical properties of silk fibroin/chitosan blend films. J. Appl. Polym. Sci. 2001, 80, 928–934. [Google Scholar] [CrossRef]
- Park, B.K.; Noh, S.K.; Um, I.C. Molecular conformation and crystallinity of white colored silkworm cocoons with different silkworm varieties. Int. J. Ind. Entomol. 2019, 38, 18–23. [Google Scholar] [CrossRef]
- Park, B.K.; Noh, S.K.; Um, I.C. Crystallinity of yellow colored silkworm variety cocoons. Int. J. Ind. Entomol. 2019, 38, 51–55. [Google Scholar] [CrossRef]
- Choi, H.J.; Noh, S.K.; Um, I.C. Morphology, molecular conformation and moisture regain of cocoons of different silkworm varieties. Int. J. Ind. Entomol. 2020, 40, 6–15. [Google Scholar] [CrossRef]
- Bae, Y.J.; Noh, S.K.; Um, I.C. Crystallinity change of silkworm variety cocoons by heat treatment. Int. J. Ind. Entomol. 2021, 42, 7–13. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, I.C. Effect of silkworm variety on characteristics of raw sericin in silk. Fibers Polym. 2019, 20, 271–279. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, M.K.; Lee, K.H.; Noh, S.K.; Han, M.S.; Um, I.C. Effect of degumming methods on structural characteristics and properties of regenerated silk. Int. J. Biol. Macromol. 2017, 104, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Um, I.C.; Kweon, H.Y.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Magoshi, J.; Nakamura, S. Studies on physical properties and structure of silk. Glass transition and crystallization of silk fibroin. J. Appl. Polym. Sci. 1975, 19, 1013–1015. [Google Scholar] [CrossRef]
- Um, I.C.; Kim, T.H.; Kweon, H.Y.; Ki, C.S.; Park, Y.H. A comparative study on the dielectric and dynamic mechanical relaxation behavior of the regenerated silk fibroin films. Macromol. Res. 2009, 17, 785–790. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, D.G. Alkali Hydrolysis of Insoluble Sericin. J. Seric. Entomol. Sci. 2000, 42, 31–35. [Google Scholar]
- Jo, Y.N.; Park, B.D.; Um, I.C. Effect of storage and drying temperature on the gelation behavior and structural characteristics of sericin. Int. J. Biol. Macromol. 2015, 81, 936–941. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.W.; Kim, K.Y.; Ki, C.S.; Um, I.C. Structural characteristics and properties of cocoon and regenerated silk fibroin from different silkworm strains. Int. J. Mol. Sci. 2023, 24, 4965. [Google Scholar] [CrossRef]
- Jang, M.J.; Um, I.C. Effect of sericin concentration and ethanol content on gelation behavior, rheological properties, and sponge characteristics of silk sericin. Eur. Polym. J. 2017, 93, 761–774. [Google Scholar] [CrossRef]
- Chung, D.E.; Lee, J.H.; Kweon, H.Y.; Lee, K.G.; Um, I.C. Structure and properties of silk sericin obtained from different silkworm varieties. Int. J. Ind. Entomol. 2015, 30, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Real, L.P.; Gardette, J.L. Ageing and characterisation of PVC-based compounds utilised for exterior applications in the building construction field 2: Artificial accelerated ageing with xenon light. Polym. Test. 2001, 20, 789–794. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Kim, H.H.; Song, D.W.; Kim, M.J.; Ryu, S.J.; Um, I.C.; Ki, C.S.; Park, Y.H. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 2016, 90, 26–33. [Google Scholar] [CrossRef]
- Ding, L.; Song, S.; Chen, L.; Shi, J.; Zhao, B.; Teng, G.; Zhang, J. A freeze-thawing method applied to the fabrication of 3-d curdlan/polyvinyl alcohol hydrogels as scaffolds for cell culture. Int. J. Biol. Macromol. 2021, 174, 101–109. [Google Scholar] [CrossRef]




| 1st Layer | 2nd Layer | 3rd Layer | 4th Layer | 5th Layer | |
|---|---|---|---|---|---|
| Silk web |  |  |  |  |  |
| Nonwoven fabric |  |  |  |  |  |
| Nonwoven Fabric | 1st Layer | 2nd Layer | 3rd Layer | 4th Layer | 5th Layer |
|---|---|---|---|---|---|
| Amino Acid (mol %) | |||||
| Aspartic acid | 5.55 | 4.46 | 3.96 | 3.69 | 3.55 |
| Threonine | 3.05 | 2.49 | 2.23 | 2.09 | 1.95 |
| Serine | 14.19 | 12.63 | 12.05 | 11.74 | 11.36 |
| Glutamic acid | 2.11 | 1.81 | 1.70 | 1.61 | 1.66 |
| Glycine | 35.92 | 38.37 | 39.82 | 40.00 | 40.30 |
| Alanine | 23.78 | 25.96 | 26.54 | 27.71 | 27.83 |
| Cysteine | 0.57 | 0.52 | 0.38 | 0.32 | 0.49 |
| Valine | 2.62 | 2.52 | 2.48 | 2.41 | 2.38 |
| Methionine | 0.16 | 0.14 | 0.13 | 0.12 | 0.12 |
| Isoleucine | 0.70 | 0.67 | 0.65 | 0.62 | 0.63 |
| Leucine | 0.70 | 0.62 | 0.59 | 0.55 | 0.56 |
| Tyrosine | 4.36 | 4.50 | 4.53 | 4.54 | 4.58 |
| Phenylalanine | 0.78 | 0.77 | 0.77 | 0.74 | 0.75 |
| Lysine | 1.23 | 0.94 | 0.81 | 0.74 | 0.75 |
| Histidine | 0.58 | 0.46 | 0.40 | 0.38 | 0.38 |
| Arginine | 1.53 | 1.29 | 1.19 | 1.08 | 1.02 |
| Proline | 2.17 | 1.85 | 1.77 | 1.66 | 1.69 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| 1st Layer | 2nd Layer | 3rd Layer | 4th Layer | 5th Layer | |
|---|---|---|---|---|---|
| Silk web |  |  |  |  |  |
| Nonwoven silk fabric |  |  |  |  |  |
| Control | 1st Layer | 2nd Layer | 3rd Layer | 4th Layer | 5th Layer | |
|---|---|---|---|---|---|---|
| 24 h |  |  |  |  |  |  |
| 48 h |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.Y.; Jang, M.J.; Park, B.-D.; Um, I.C. Fabrication, Structure, and Properties of Nonwoven Silk Fabrics Prepared with Different Cocoon Layers. Int. J. Mol. Sci. 2023, 24, 11485. https://doi.org/10.3390/ijms241411485
Choi YY, Jang MJ, Park B-D, Um IC. Fabrication, Structure, and Properties of Nonwoven Silk Fabrics Prepared with Different Cocoon Layers. International Journal of Molecular Sciences. 2023; 24(14):11485. https://doi.org/10.3390/ijms241411485
Chicago/Turabian StyleChoi, Yun Yeong, Mi Jin Jang, Byung-Dae Park, and In Chul Um. 2023. "Fabrication, Structure, and Properties of Nonwoven Silk Fabrics Prepared with Different Cocoon Layers" International Journal of Molecular Sciences 24, no. 14: 11485. https://doi.org/10.3390/ijms241411485
APA StyleChoi, Y. Y., Jang, M. J., Park, B.-D., & Um, I. C. (2023). Fabrication, Structure, and Properties of Nonwoven Silk Fabrics Prepared with Different Cocoon Layers. International Journal of Molecular Sciences, 24(14), 11485. https://doi.org/10.3390/ijms241411485







