Therapeutical Options in ROS1—Rearranged Advanced Non Small Cell Lung Cancer
Abstract
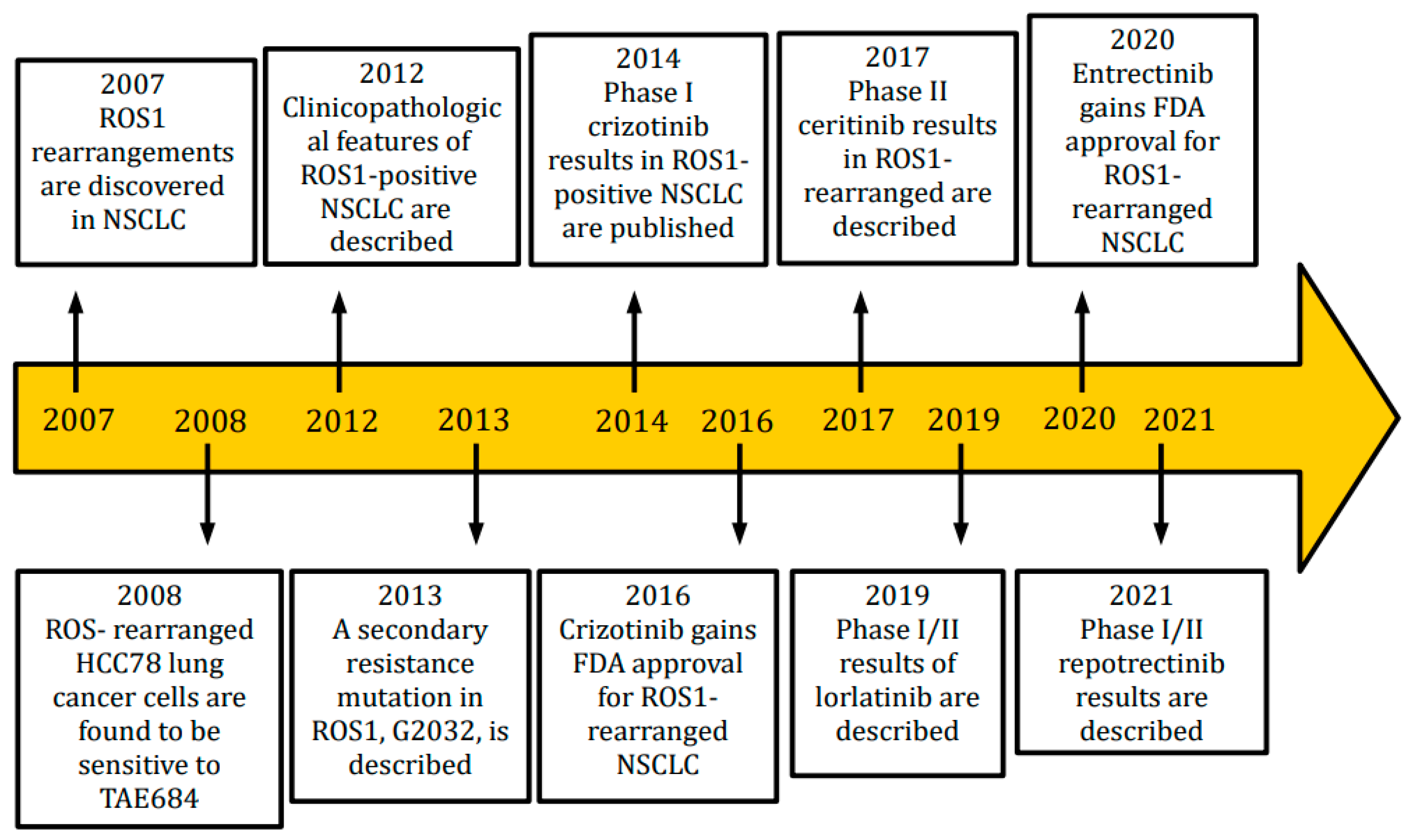
:1. Introduction
2. Crizotinib
3. Entrectinib
4. Entrectinib vs. Crizotinib
5. Ceritinib
6. Lorlatinib
7. Cabozantinib
8. Brigatinib
9. Repotrectinib
10. Taletrectinib
11. Ensartinib
12. Foretinib
13. Other Treatments
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Pikor, L.A.; Ramnarine, V.R.; Lam, S.; Lam, W.L. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013, 82, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquaviva, J.; Wong, R.; Charest, A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim. Biophys. Acta 2009, 1795, 37–52. [Google Scholar] [CrossRef]
- Davies, K.D.; Doebele, R.C. Molecular Pathways: ROS1 Fusion Proteins in Cancer. Clin. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charest, A.; Wilker, E.W.; McLaughlin, M.E.; Lane, K.; Gowda, R.; Coven, S.; McMahon, K.; Kovach, S.; Feng, Y.; Yaffe, M.B.; et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glio-blastoma in mice. Cancer Res. 2006, 66, 7473–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, H.J.; Johnson, H.; Bronson, R.T.; De Feraudy, S.; White, F.; Charest, A. The Oncogenic Lung Cancer Fusion Kinase CD74-ROS Activates a Novel Invasiveness Pathway through E-Syt1 Phosphorylation. Cancer Res. 2012, 72, 3764–3774. [Google Scholar] [CrossRef] [Green Version]
- Wiesweg, M.; Eberhardt, W.E.; Reis, H.; Ting, S.; Savvidou, N.; Skiba, C.; Herold, T.; Christoph, D.C.; Meiler, J.; Worm, K.; et al. High Prevalence of Concomitant Oncogene Mutations in Prospectively Identified Patients with ROS1-Positive Metastatic Lung Cancer. J. Thorac. Oncol. 2016, 12, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Bergethon, K.; Shaw, A.T.; Ou, S.-H.I.; Katayama, R.; Lovly, C.M.; McDonald, N.T.; Massion, P.P.; Siwak-Tapp, C.; Gonzalez, A.; Fang, R.; et al. ROS1 Rearrangements Define a Unique Molecular Class of Lung Cancers. J. Clin. Oncol. 2012, 30, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Choi, Y.-L.; Ahn, M.-J.; Han, J. Histopathologic characteristics of advanced-stage ROS1-rearranged non-small cell lung cancers. Pathol. Res. Pract. 2019, 215, 152441. [Google Scholar] [CrossRef]
- Drilon, A.; Jenkins, C.; Iyer, S.; Schoenfeld, A.; Keddy, C.; Davare, M.A. ROS1-dependent cancers—Biology, diagnostics and therapeutics. Nat. Rev. Clin. Oncol. 2021, 18, 35–55. [Google Scholar] [CrossRef]
- Patil, T.; Smith, D.E.; Bunn, P.A.; Aisner, D.L.; Le, A.T.; Hancock, M.; Purcell, W.T.; Bowles, D.W.; Camidge, D.R.; Doebele, R.C. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non–Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J. Thorac. Oncol. 2018, 13, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Zhu, V.W.; Zhao, J.J.; Gao, Y.; Syn, N.L.; Zhang, S.S.; Ou, S.-H.I.; Bauer, K.A.; Nagasaka, M. Thromboembolism in ALK+ and ROS1+ NSCLC patients: A systematic review and meta-analysis. Lung Cancer 2021, 157, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.T.; Bernardo, R.J.; Berry, G.J.; Kudelko, K.; Wakelee, H.A. Two Cases of Pulmonary Tumor Thrombotic Microangi-opathy Associated with ROS1-Rearranged Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, e153–e156. [Google Scholar] [CrossRef] [PubMed]
- Woodford, R.; Lu, M.; Beydoun, N.; Cooper, W.; Liu, Q.; Lynch, J.; Kasherman, L. Disseminated intravascular coagulation complicating diagnosis of ROS1 -mutant non-small cell lung cancer: A case report and literature review. Thorac. Cancer 2021, 12, 2400–2403. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [PubMed] [Green Version]
- Wang, W.; Cheng, G.; Zhang, G.; Song, Z. Evaluation of a new diagnostic immunohistochemistry approach for ROS1 rearrangement in non-small cell lung cancer. Lung Cancer 2020, 146, 224–229. [Google Scholar] [CrossRef]
- Bubendorf, L.; Büttner, R.; Al-Dayel, F.; Dietel, M.; Elmberger, G.; Kerr, K.; López-Ríos, F.; Marchetti, A.; Öz, B.; Pauwels, P.; et al. Testing for ROS1 in non-small cell lung cancer: A review with recommendations. Virchows Arch. 2016, 469, 489–503. [Google Scholar] [CrossRef]
- Sholl, L.M.; Weremowicz, S.; Gray, S.W.; Wong, K.-K.; Chirieac, L.R.; Lindeman, N.I.; Hornick, J.L. Combined use of ALK immuno-histochemistry and FISH for optimal detection of ALK-rearranged lung adenocarcinomas. J. Thorac. Oncol. 2013, 8, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Zito Marino, F.; Rossi, G.; Cozzolino, I.; Montella, M.; Micheli, M.; Bogina, G.; Munari, E.; Brunelli, M.; Franco, R. Multiplex fluorescence in situ hybridisation to detect anaplastic lymphoma kinase and ROS proto-oncogene 1 receptor tyrosine kinase rearrangements in lung cancer cy-tological samples. J. Clin. Pathol. 2020, 73, 96–101. [Google Scholar] [CrossRef]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef]
- Wong, S.Q.; Li, J.; Tan, A.Y.-C.; Vedururu, R.; Pang, J.-M.B.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; MacArthur, G.A.; et al. Sequence artefacts in a prospective series of forma-lin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genom. 2014, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, L.; Lian, F.; Guo, L.; Qiu, T.; Ling, Y.; Ying, J.; Lin, D. Detection of ROS1 Gene Rearrangement in Lung Adenocarcinoma: Comparison of IHC, FISH and Real-Time RT-PCR. PLoS ONE 2015, 10, e0120422. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, D.M.; Schmidt, R.L.; Furtado, L.V.; Matynia, A.P.; Deftereos, G.; Sirohi, D. Histologic and Molecular Characterization of Non−Small Cell Lung Carcinoma with Discordant ROS1 Immunohistochemistry and Fluorescence In Situ Hybridization. Appl. Immunohistochem. Mol. Morphol. 2021, 30, 19–26. [Google Scholar] [CrossRef]
- Davies, K.D.; Le, A.T.; Sheren, J.; Nijmeh, H.; Gowan, K.; Jones, K.L.; Varella-Garcia, M.; Aisner, D.L.; Doebele, R.C. Comparison of Molecular Testing Modalities for Detection of ROS1 Rearrangements in a Cohort of Positive Patient Samples. J. Thorac. Oncol. 2018, 13, 1474–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. 2022, 29, 641–658. [Google Scholar] [CrossRef]
- Guaitoli, G.; Bertolini, F.; Bettelli, S.; Manfredini, S.; Maur, M.; Trudu, L.; Aramini, B.; Masciale, V.; Grisendi, G.; Dominici, M.; et al. Deepening the Knowledge of ROS1 Rearrangements in Non-Small Cell Lung Cancer: Diagnosis, Treatment, Resistance and Concomitant Alterations. Int. J. Mol. Sci. 2021, 22, 12867. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.; Riely, G.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.; Solomon, B.; Varella-Garcia, M.; Iafrate, A.; Shapiro, G.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef]
- Michels, S.; Massutí, B.; Schildhaus, H.-U.; Franklin, J.; Sebastian, M.; Felip, E.; Grohé, C.; Rodriguez-Abreu, D.; Abdulla, D.S.; Bischoff, H.; et al. Safety and Efficacy of Crizotinib in Patients with Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. J. Thorac. Oncol. 2019, 14, 1266–1276. [Google Scholar] [CrossRef]
- Landi, L.; Chiari, R.; Tiseo, M.; D’Incà, F.; Dazzi, C.; Chella, A.; Delmonte, A.; Bonanno, L.; Giannarelli, D.; Cortinovis, D.L.; et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non-Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin. Cancer Res. 2019, 25, 7312–7319. [Google Scholar] [CrossRef] [Green Version]
- Moro-Sibilot, D.; Cozic, N.; Pérol, M.; Mazières, J.; Otto, J.; Souquet, P.; Bahleda, R.; Wislez, M.; Zalcman, G.; Guibert, S.; et al. Crizotinib in c-MET- or ROS1-positive NSCLC: Results of the AcSé phase II trial. Ann. Oncol. 2019, 30, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wakelee, H.A.; Neal, J.W. Relationship of Driver Oncogenes to Long-Term Pemetrexed Response in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 16, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, A.; Cardona, A.F.; Caglevic, C.; Manca, P.; Ruiz-Patiño, A.; Arrieta, O.; Rolfo, C. Overcoming TKI resistance in fusion-driven NSCLC: New generation inhibitors and rationale for combination strategies. Transl. Lung Cancer Res. 2020, 9, 2581–2598. [Google Scholar] [CrossRef] [PubMed]
- Mengoli, M.C.; Barbieri, F.; Bertolini, F.; Tiseo, M.; Rossi, G. K-RAS mutations indicating primary resistance to crizotinib in ALK-rearranged adenocarcinomas of the lung: Report of two cases and review of the literature. Lung Cancer 2016, 93, 55–58. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, T.; Li, X.; Wang, Y.; Zhao, C.; Zhao, S.; Xi, L.; Zhang, S.; Liu, X.; Jia, Y.; et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer 2017, 123, 2927–2935. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.M.; Katayama, R.; McTigue, M.; Liu, W.; Deng, Y.; Brooun, A.; Friboulet, L.; Huang, D.; Falk, M.D.; Timofeevski, T.; et al. Acquired resistance to crizotinib from a mutation in CD74–ROS1. N. Engl. J. Med. 2013, 368, 2395–2401. [Google Scholar] [CrossRef] [Green Version]
- Gainor, J.F.; Tseng, D.; Yoda, S.; Dagogo-Jack, I.; Friboulet, L.; Lin, J.J.; Hubbeling, H.G.; Dardaei, L.; Farago, A.F.; Schultz, K.R.; et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis. Oncol. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Drilon, A.; Somwar, R.; Wagner, J.P.; Vellore, N.A.; Eide, C.A.; Zabriskie, M.S.; Arcila, M.E.; Hechtman, J.F.; Wang, L.; Smith, R.S.; et al. A Novel Crizotinib-Resistant Solvent-Front Mutation Responsive to Cabozantinib Therapy in a Patient with ROS1-Rearranged Lung Cancer. Clin. Cancer Res. 2016, 22, 2351–2358. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, F.; Loriot, Y.; Kuo, M.S.; Mahjoubi, L.; Lacroix, L.; Planchard, D.; Besse, B.; Farace, F.; Auger, N.; Remon, J.; et al. CrizotinibResistant ROS1 Mutations Reveal a Predictive Kinase Inhibitor Sensitivity Model for ROS1- and ALK-Rearranged Lung Cancers. Clin. Cancer Res. 2016, 22, 5983–5991. [Google Scholar] [CrossRef] [Green Version]
- McCoach, C.E.; Le, A.T.; Gowan, K.; Jones, K.; Schubert, L.; Doak, A.; Estrada-Bernal, A.; Davies, K.D.; Merrick, D.T.; Bunn, P.A., Jr.; et al. Resistance Mechanisms to Targeted Therapies in ROS1(+) and ALK(+) Non-small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 3334–3347. [Google Scholar] [CrossRef] [Green Version]
- Song, A.; Kim, T.M.; Kim, D.-W.; Kim, S.; Keam, B.; Lee, S.-H.; Heo, D.S. Molecular Changes Associated with Acquired Resistance to Crizotinib in. Clin. Cancer Res. 2015, 21, 2379–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drilon, A.; Ou, S.-H.I.; Cho, B.C.; Kim, D.-W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Ahn, M.-J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef] [Green Version]
- Katayama, R.; Kobayashi, Y.; Friboulet, L.; Lockerman, E.L.; Koike, S.; Shaw, A.T.; Engelman, J.A.; Fujita, N. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin. Cancer Res. 2015, 21, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.D.; Mahale, S.; Astling, D.P.; Aisner, D.L.; Le, A.T.; Hinz, T.K.; Vaishnavi, A.; Bunn, P.A.; Heasley, L.E.; Tan, A.-C.; et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS ONE 2013, 8, e82236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Gureasko, J.; Shen, K.; Cole, P.A.; Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006, 125, 1137–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cargnelutti, M.; Corso, S.; Pergolizzi, M.; Mévellec, L.; Aisner, D.L.; Dziadziuszko, R.; Varella-Garcia, M.; Comoglio, P.M.; Doebele, R.C.; Vialard, J.; et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget 2015, 6, 5182–5194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziadziuszko, R.; Le, A.T.; Wrona, A.; Jassem, J.; Camidge, D.R.; Varella-Garcia, M.; Aisner, D.L.; Doebele, R.C. An Activating KIT Mutation Induces Crizotinib Resistance in ROS1-Positive Lung Cancer. J. Thorac. Oncol. 2016, 11, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Ardini, E.; Menichincheri, M.; Banfi, P.; Bosotti, R.; De Ponti, C.; Pulci, R.; Ballinari, D.; Ciomei, M.; Texido, G.; Degrassi, A.; et al. Entrectinib, a Pan–TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol. Cancer Ther. 2016, 15, 628–639. [Google Scholar] [CrossRef] [Green Version]
- Rolfo, C.; Ruiz, R.; Giovannetti, E.; Gil-Bazo, I.; Russo, A.; Passiglia, F.; Giallombardo, M.; Peeters, M.; Raez, L. Entrectinib: A potent new TRK, ROS1, and ALK inhibitor. Expert Opin. Investig. Drugs 2015, 24, 1493–1500. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.-H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and antitumor activity of the multitargeted pan-Trk, ROS1, and ALK inhibitor Entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; De Braud, F.; Rolfo, C.; Ahn, M.J.; Wolf, J.; et al. Entrectinib in ROS1 fusion- positive non-small-cell lung cancer: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Dziadziusko, R.; Krebs, M.G.; De Braud, F.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion–Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Robinson, G.W.; Gauvain, K.; Basu, E.M.; Macy, M.E.; Maese, L.; Whipple, N.S.; Sabnis, A.J.; Foster, J.H.; Shusterman, S.; et al. Entrectinib in children and young adults with solid or primary CNS tumors harboring NTRK, ROS1 or ALK aberrations (STARTRK-NG). Neuro-Oncology 2022, 24, 1776–1789. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Bahcall, M.; Capelletti, M.; Kosaka, T.; Ercan, D.; Sim, T.; Sholl, L.M.; Nishino, M.; Johnson, B.E.; Gray, N.S.; et al. Identification of Existing Drugs That Effectively Target NTRK1 and ROS1 Rearrangements in Lung Cancer. Clin. Cancer Res. 2017, 23, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Clinicaltrials.gov. Randomized, Open Label, Multicenter, Phase III Study of Entrectinib versus Crizotinib in Patients with Locally-Advanced or Metastatic Non-Small Cell Lung Cancer Harboring ROS1 Gene Rearrangements with and without Central Nervous System Metastases. 2021. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04603807 (accessed on 15 June 2023).
- Tremblay, G.; Groff, M.; Iadeluca, L.; Daniele, P.; Wilner, K.; Wiltshire, R.; Bartolome, L.; Usari, T.; Cappelleri, J.C.; Camidge, D.R. Effectiveness of crizotinib versus entrectinib in ROS1-positive non-small-cell lung cancer using clinical and real-world data. Future Oncol. 2022, 18, 2063–2074. [Google Scholar] [CrossRef]
- Chu, P.; Antoniou, M.; Bhutani, M.K.; Aziez, A.; Daigl, M. Matching-adjusted indirect comparison: Entrectinib versus crizotinib in ROS1 fusion-positive non-small cell lung cancer. J. Comp. Eff. Res. 2020, 9, 861–876. [Google Scholar] [CrossRef]
- Friboulet, L.; Li, N.; Katayama, R.; Lee, C.C.; Gainor, J.F.; Crystal, A.S.; Michellys, P.-Y.; Awad, M.M.; Yanagitani, N.; Kim, S.; et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014, 4, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Zou, H.Y.; Friboulet, L.; Kodack, D.P.; Engstrom, L.D.; Li, Q.; West, M.; Tang, R.W.; Wang, H.; Tsaparikos, K.; Wang, J.; et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015, 28, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.Y.; Li, Q.; Engstrom, L.D.; West, M.; Appleman, V.; Wong, K.A.; McTigue, M.; Deng, Y.L.; Liu, W.; Brooun, A.; et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 3493–3498. [Google Scholar] [CrossRef]
- Shaw, A.T.; Felip, E.; Bauer, T.M.; Besse, B.; Navarro, A.; Postel-Vinay, S.; Gainor, J.F.; Johnson, M.; Dietrich, J.; James, L.P.; et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017, 18, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.-C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; A Soo, R.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ruiz-Garcia, A.; James, L.P.; Peltz, G.; Thurm, H.; Clancy, J.; Hibma, J. Lorlatinib Exposure-Response Analyses for Safety and Efficacy in a Phase I/II Trial to Support Benefit–Risk Assessment in Non-Small Cell Lung Cancer. Clin. Pharmacol. Ther. 2021, 110, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Tiseo, M.; Heukamp, L.C.; Menon, R.; Spitaleri, G.; Cortinovis, D.; Delmonte, A.; Galetta, D.; D’arcangelo, M.; D’incà, F.; et al. Secondary ROS1 mutations and lorlatinib sensitivity in crizotinib-refractory ROS1 positive NSCLC: Results of the prospective PFROST trial. Ann. Oncol. 2019, 30, v602–v660. [Google Scholar] [CrossRef]
- Sun, T.Y.; Niu, X.; Chakraborty, A.; Neal, J.W.; Wakelee, H.A. Lengthy Progression-Free Survival and Intracranial Activity of Cabozantinib in Patients with Crizotinib and Ceritinib-Resistant ROS1-Positive Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 14, e21–e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Anjum, R.; Squillace, R.; Nadworny, S.; Zhou, T.; Keats, J.; Ning, Y.; Wardwell, S.D.; Miller, D.; Song, Y.; et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin. Cancer Res. 2016, 22, 5527–5538. [Google Scholar] [CrossRef] [Green Version]
- Dudnik, E.; Agbarya, A.; Grinberg, R.; Cyjon, A.; Bar, J.; Moskovitz, M.; Peled, N.; the Israel Lung Cancer Group. Clinical activity of brigatinib in ROS1-rearranged non-small cell lung cancer. Clin. Transl. Oncol. 2020, 22, 2303–2311. [Google Scholar] [CrossRef]
- Cho, B.C.; Drilon, A.E.; Doebele, R.C.; Kim, D.; Lin, J.J.; Lee, J.; Ahn, M.; Zhu, V.W.; Ejadi, S.; Camidge, D.R.; et al. Safety and preliminary clinical activity of repotrectinib (TPX-0005), a ROS1/TRK/ALK inhibitor, in advanced ROS1 fusion-positive NSCLC. J. Clin. Oncol. 2019, 37, 9011. [Google Scholar] [CrossRef]
- Drilon, A.; Zhai, D.; Deng, W.; Zhang, X.; Lee, D.; Rogers, E.; Whitten, J.; Huang, Z.; Graber, A.; Liu, J.; et al. Repotrectinib, a next generation TRK inhibitor, overcomes TRK resistance mutations including solvent front, gatekeeper and compound mutations. In Proceedings of the AACR Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019. [Google Scholar]
- Yun, M.R.; Kim, D.H.; Kim, S.-Y.; Joo, H.-S.; Lee, Y.W.; Choi, H.M.; Park, C.W.; Heo, S.G.; Na Kang, H.; Lee, S.S.; et al. Repotrectinib Exhibits Potent Antitumor Activity in Treatment-Naïve and Solvent-Front–Mutant ROS1-Rearranged Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 3287–3295. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.C.; Doebele, R.C.; Lin, J.; Nagasaka, M.; Baik, C.; Van Der Wekken, A.; Velcheti, V.; Lee, K.H.; Liu, S.; Solomon, B.; et al. Phase 1/2 TRIDENT-1 Study of Repotrectinib in Patients with ROS1+ or NTRK+ Advanced Solid Tumors. J. Thorac. Oncol. 2021, 16, S174–S175. [Google Scholar] [CrossRef]
- Katayama, R.; Gong, B.; Togashi, N.; Miyamoto, M.; Kiga, M.; Iwasaki, S.; Kamai, Y.; Tominaga, Y.; Takeda, Y.; Kagoshima, Y.; et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat. Commun. 2019, 10, 3604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, K.P.; Borazanci, E.; Shaw, A.T.; Katayama, R.; Shimizu, Y.; Zhu, V.W.; Sun, T.Y.; Wakelee, H.A.; Madison, R.; Schrock, A.B.; et al. U.S. Phase I First-in-human Study of Taletrectinib (DS-6051b/AB-106), a ROS1/TRK Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.I.; Fujiwara, Y.; Shaw, A.T.; Yamamoto, N.; Nakagawa, K.; Fan, F.; Hao, Y.; Gao, Y.; Jänne, P.A.; Seto, T. Efficacy of Taletrectinib (AB-106/DS-6051b) in ROS1+ NSCLC: An Updated Pooled Analysis of U.S. and Japan Phase 1 Studies. JTO Clin. Res. Rep. 2020, 2, 100108. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Infante, J.R.; Reckamp, K.L.; Blumenschein, G.R.; Leal, T.A.; Waqar, S.N.; Gitlitz, B.J.; Sanborn, R.E.; Whisenant, J.G.; Du, L.; et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin. Cancer Res. 2018, 24, 2771–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, X.; Wang, Q.; Cheng, Y.; Liu, X.; Cao, L.; Chen, J.; Dong, X.; Zhou, J.; Fan, Y.; Huang, C.; et al. Safety but Limited Efficacy of Ensartinib in ROS1-Positive NSCLC: A Single-Arm, Multicenter Phase 2 Study. J. Thorac. Oncol. 2021, 16, 1959–1963. [Google Scholar] [CrossRef]
- Davare, M.A.; Saborowski, A.; Eide, C.A.; Tognon, C.; Smith, R.L.; Elferich, J.; Agarwal, A.; Tyner, J.W.; Shinde, U.P.; Lowe, S.W.; et al. Foretinib is a potent inhibitor of oncogenic ROS1 fusion proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 19519–19524. [Google Scholar] [CrossRef]
- Chen, Y.F.; Hsieh, M.S.; Wu, S.G.; Chang, Y.L.; Yu, C.J.; Yang, J.C.H.; Shih, J.Y. Efficacy of pemetrexed-based chemotherapy in patients with ROS1 fusion-positive lung adenocarcinoma compared with in patients harboring other driver mutations in east asian populations. J. Thorac. Oncol. 2016, 11, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Qiang, T.; Li, Z.; Ding, D.; Yu, Y.; Lu, S. First-line crizotinib versus platinum-pemetrexed chemotherapy in patients with advanced ROS1-rearranged non-small-cell lung cancer. Cancer Med. 2020, 9, 3310–3318. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Su, H.; Zhang, Y. Patients with ROS1 rearrangement-positive non-small-cell lung cancer benefit from pemetrexed-based chemotherapy. Cancer Med. 2016, 5, 2688–2693. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Schneider, J.L.; Patil, T.; Zhu, V.W.; Goldman, D.A.; Yang, S.-R.; Falcon, C.J.; Do, A.; Nie, Y.; Plodkowski, A.J.; et al. Response to Immune Checkpoint Inhibition as Monotherapy or in Combination with Chemotherapy in Metastatic ROS1- Rearranged Lung Cancers. JTO Clin. Res. Rep. 2021, 2, 100187. [Google Scholar] [PubMed]
- Jiang, L.; Su, X.; Zhang, T.; Yin, X.; Zhang, M.; Fu, H.; Han, H.; Sun, Y.; Dong, L.; Qian, J.; et al. PD-L1 expression and its relationship with oncogenic drivers in non-small cell lung cancer (NSCLC). Oncotarget 2017, 8, 26845–26857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazieres, J.; Drilon, A.; Lusque, A.B.; Mhanna, L.; Cortot, A.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Watanabe, H.; Ichihara, E.; Kayatani, H.; Makimoto, G.; Ninomiya, K.; Nishii, K.; Higo, H.; Ando, C.; Okawa, S.; Nakasuka, T.; et al. VEGFR2 blockade augments the effects of tyrosine kinase inhibitors by inhibiting angiogenesis and oncogenic signaling in oncogene-driven non-small-cell lung cancers. Cancer Sci. 2021, 112, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trial | Phase | ORR (n) | Median DoR | Median PFS | Median OS |
|---|---|---|---|---|---|
| PROFILE 1001 [27,28] | 1b | 72% (38/53) | 24.7 months | 19.3 months | 51.4 months |
| EUCROSS [29] | 2 | 70% (21/30) | 19 months | 15.9 months | 20 months |
| METROS [30] | 2 | 65% (17/26) | 21.4 months | 22.8 months | - |
| AcSè [31] | 2 | 69% (25/36) | - | 5.5 months | 17 months |
| Clinical Trial | Phase | ORR | Median TTR | Median TTP | Median PFS | Median OS |
|---|---|---|---|---|---|---|
| STARTRK-1 STARTRK-2 ALKA-372-001 [52] | 1/2 | 67.1% | 0.95 months | 2.8 months | 15.7 months | Not reached |
| STARTRK-NG [53] | 1/2 | 57.7% | - | - | Not reached | - |
| Clinical Trial | Phase | ORR | Median PFS | Median OS |
|---|---|---|---|---|
| PROFILE 1001 [27,28] | 1b | 72% | 19.3 months | 51.4 months |
| EUCROSS [29] | 2 | 70% | 15.9 months | 20 months |
| METROS [30] | 2 | 65% | 22.8 months | - |
| AcSè [31] | 2 | 69% | 5.5 months | 17 months |
| STARTRK-1 STARTRK-2 ALKA-372-001 [52] | 1/2 | 67.1% | 15.7 months | Not reached |
| STARTRK-NG [53] | 1/2 | 57.7% | Not reached | - |
| Clinical Trial | Drug | Phase | ORR | Median PFS | Median OS |
|---|---|---|---|---|---|
| PROFILE 1001 [27,28] | Crizotinib | 1b | 72% | 19.3 months | 51.4 months |
| EUCROSS [29] | Crizotinib | 2 | 70% | 15.9 months | 20 months |
| METROS [30] | Crizotinib | 2 | 65% | 22.8 months | NR |
| AcSè [31] | Crizotinib | 2 | 69% | 5.5 months | 17 months |
| STARTRK-1 STARTRK-2 ALKA-372-001 [52] | Entrectinib | 1/2 | 67.1% | 15.7 months | NR |
| ASCEND-5 [59] | Ceritinib | 2 | 62% | 9.3 months (19.3 in crizotinib-naïve pts) | 24 months |
| NCT 01970865 [64,65] | Lorlatinib | 1/2 | 62% in TKI-naïve pts 35% in crizotinib-pretreated pts | 21 months in TKI-naïve pts 8.5 months in crizotinib-pretreated pts | - |
| Dudnik et al. [69] | Brigatinib | 1 | 37% (29% in crizotinib-pretreated pts) | - | - |
| TRIDENT-1 [73] | Repotrectinib | 1/2 | 78.9% in TKI-naïve pts 37.5% in pretreated pts | - | - |
| United States and Japan [75,76] | Taletrectinib | 1 | 58.3% (66.7% in TKI-naïve pts) | 4 months | - |
| NCT03608007 [77,78] | Ensartinib | 2 | 27% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanzione, B.; Del Conte, A.; Bertoli, E.; De Carlo, E.; Revelant, A.; Spina, M.; Bearz, A. Therapeutical Options in ROS1—Rearranged Advanced Non Small Cell Lung Cancer. Int. J. Mol. Sci. 2023, 24, 11495. https://doi.org/10.3390/ijms241411495
Stanzione B, Del Conte A, Bertoli E, De Carlo E, Revelant A, Spina M, Bearz A. Therapeutical Options in ROS1—Rearranged Advanced Non Small Cell Lung Cancer. International Journal of Molecular Sciences. 2023; 24(14):11495. https://doi.org/10.3390/ijms241411495
Chicago/Turabian StyleStanzione, Brigida, Alessandro Del Conte, Elisa Bertoli, Elisa De Carlo, Alberto Revelant, Michele Spina, and Alessandra Bearz. 2023. "Therapeutical Options in ROS1—Rearranged Advanced Non Small Cell Lung Cancer" International Journal of Molecular Sciences 24, no. 14: 11495. https://doi.org/10.3390/ijms241411495
APA StyleStanzione, B., Del Conte, A., Bertoli, E., De Carlo, E., Revelant, A., Spina, M., & Bearz, A. (2023). Therapeutical Options in ROS1—Rearranged Advanced Non Small Cell Lung Cancer. International Journal of Molecular Sciences, 24(14), 11495. https://doi.org/10.3390/ijms241411495






