miRNAs as Modern Biomarkers in Asthma Therapy
Abstract
:1. Introduction
2. miRNAs and Individual Groups of Drugs Used in Asthma (Glucocorticosteroids, Short-Acting Beta-Agonists, and Biological Therapy)
3. miRNAs and Pathways Responsible for the Development of Asthma: The Future Direction of Therapeutic Action
4. miRNAs and the Course of Viral Infections as a Factor of Asthma Exacerbation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The Immunology of Asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Virchow, J.C. Severe Asthma. Dtsch. Arztebl. Int. 2014, 111, 847–855. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.M.; McDonald, V.M.; Guo, M.; Reddel, H.K. “I Have Lost in Every Facet of My Life”: The Hidden Burden of Severe Asthma. Eur. Respir. J. 2017, 50, 1700765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.; Turner, S. Severe Asthma in Children—A Review of Definitions, Epidemiology, and Treatment Options in 2019. Pediatr. Pulmonol. 2019, 54, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Guilbert, T.W.; Bacharier, L.B.; Fitzpatrick, A.M. Severe Asthma in Children. J. Allergy Clin. Immunol. Pract. 2014, 2, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.G.; Simpson, J.L.; Hankin, R.; Powell, H.; Henry, R.L. Relationship between Induced Sputum Eosinophils and the Clinical Pattern of Childhood Asthma. Thorax 2003, 58, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.G.; Henry, R.L.; Thomas, P. Noninvasive Assessment of Airway Inflammation in Children: Induced Sputum, Exhaled Nitric Oxide, and Breath Condensate. Eur. Respir. J. 2000, 16, 1008–1015. [Google Scholar]
- Zhang, X.; Xu, Z.; Wen, X.; Huang, G.; Nian, S.; Li, L.; Guo, X.; Ye, Y.; Yuan, Q. The Onset, Development and Pathogenesis of Severe Neutrophilic Asthma. Immunol. Cell Biol. 2022, 100, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, A.; Okazaki, R.; Harada, T. Neutrophils and Asthma. Diagnostics 2022, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Nabe, T. Steroid-Resistant Asthma and Neutrophils. Biol. Pharm. Bull. 2020, 43, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Tiwari, A.; McGeachie, M.J. Recent MiRNA Research in Asthma. Curr. Allergy Asthma. Rep. 2022, 22, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, M.; Lorente-Sorolla, C.; Naharro, S.; Rodrigo-Muñoz, J.M.; del Pozo, V. Advances and Highlights of MiRNAs in Asthma: Biomarkers for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1628. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Dua, K.; Adcock, I.M.; Horvat, J.C.; Kim, R.Y.; Hansbro, P.M. Cellular Mechanisms Underlying Steroid-Resistant Asthma. Eur. Respir. Rev. 2019, 28, 190096. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Allegra, A.; Pioggia, G.; Picardi, G.; Musolino, C.; Gangemi, S. MicroRNA Profiling in Asthma: Potential Biomarkers and Therapeutic Targets. Am. J. Respir. Cell Mol. Biol. 2017, 57, 642–650. [Google Scholar] [CrossRef]
- Kierbiedź-Guzik, N.; Sozańska, B. The Potential Role of Serum and Exhaled Breath Condensate MiRNAs in Diagnosis and Predicting Exacerbations in Pediatric Asthma. Biomedicines 2023, 11, 763. [Google Scholar] [CrossRef]
- Cay, P.; Singer, C.A.; Ba, M.A. Gene Network Analysis for Identification of MicroRNA Biomarkers for Asthma. Respir. Res. 2022, 23, 378. [Google Scholar] [CrossRef]
- Wang, A.L.; Panganiban, R.; Qiu, W.; Kho, A.T.; Chupp, G.; Meyers, D.A.; Bleecker, E.R.; Weiss, S.T.; Lu, Q.; Tantisira, K.G. Drug Repurposing to Treat Glucocorticoid Resistance in Asthma. J. Pers. Med. 2021, 11, 175. [Google Scholar] [CrossRef]
- Grzanka, A.; Jarząb, J. Niegenomowy Mechanizm Działania Glikokortykosteroidów. Adv. Respir. Med. 2009, 77, 387–393. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Panganiban, R.; Kho, A.T.; McGeachie, M.J.; Farnam, L.; Chase, R.P.; Weiss, S.T.; Lu, Q.; Tantisira, K.G. Circulating MicroRNAs and Treatment Response in Childhood Asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, R.P.; Wang, Y.; Howrylak, J.; Chinchilli, V.M.; Craig, T.J.; August, A.; Ishmael, F.T. Circulating MicroRNAs as Biomarkers in Patients with Allergic Rhinitis and Asthma. J. Allergy Clin. Immunol. 2016, 137, 1423–1432. [Google Scholar] [CrossRef] [Green Version]
- Jardim, M.J.; Dailey, L.; Silbajoris, R.; Diaz-Sanchez, D. Distinct MicroRNA Expression in Human Airway Cells of Asthmatic Donors Identifies a Novel Asthma-Associated Gene. Am. J. Respir. Cell Mol. Biol. 2012, 47, 536–542. [Google Scholar] [CrossRef]
- ElKashef, S.M.M.A.E.; Ahmad, S.E.-A.; Soliman, Y.M.A.; Mostafa, M.S. Role of MicroRNA-21 and MicroRNA-155 as Biomarkers for Bronchial Asthma. Innate Immun. 2021, 27, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Elbehidy, R.M.; Youssef, D.M.; El-Shal, A.S.; Shalaby, S.M.; Sherbiny, H.S.; Sherief, L.M.; Akeel, N.E. MicroRNA–21 as a Novel Biomarker in Diagnosis and Response to Therapy in Asthmatic Children. Mol. Immunol. 2016, 71, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Horvat, J.C.; Pinkerton, J.W.; Starkey, M.R.; Essilfie, A.T.; Mayall, J.R.; Nair, P.M.; Hansbro, N.G.; Jones, B.; Haw, T.J.; et al. MicroRNA-21 Drives Severe, Steroid-Insensitive Experimental Asthma by Amplifying Phosphoinositide 3-Kinase–Mediated Suppression of Histone Deacetylase 2. J. Allergy Clin. Immunol. 2017, 139, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Kho, A.T.; McGeachie, M.J.; Moore, K.G.; Sylvia, J.M.; Weiss, S.T.; Tantisira, K.G. Circulating MicroRNAs and Prediction of Asthma Exacerbation in Childhood Asthma. Respir. Res. 2018, 19, 128. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo-Muñoz, J.M.; Gil-Martínez, M.; Lorente-Sorolla, C.; García-Latorre, R.; Valverde-Monge, M.; Quirce, S.; Sastre, J.; del Pozo, V. MiR-144-3p Is a Biomarker Related to Severe Corticosteroid-Dependent Asthma. Front. Immunol. 2022, 13, 858722. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Liew, M.F.; Lim, H.F.; Leung, B.P.; Wong, W.S.F. Promises and Challenges of Biologics for Severe Asthma. Biochem. Pharmacol. 2020, 179, 114012. [Google Scholar] [CrossRef] [PubMed]
- Nahid, M.A.; Satoh, M.; Chan, E.K.L. Mechanistic Role of MicroRNA-146a in Endotoxin-Induced Differential Cross-Regulation of TLR Signaling. J. Immunol. 2011, 186, 1723–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, K.A.; Roff, A.N.; Panganiban, R.P.; Douglas, S.; Ishmael, F.T. MicroRNA-146a Is Induced by Inflammatory Stimuli in Airway Epithelial Cells and Augments the Anti-Inflammatory Effects of Glucocorticoids. PLoS ONE 2018, 13, e0205434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidner, J.; Ekerljung, L.; Malmhäll, C.; Miron, N.; Rådinger, M. Circulating MicroRNAs Correlate to Clinical Parameters in Individuals with Allergic and Non-Allergic Asthma. Respir. Res. 2020, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Clayton, S.A.; Jones, S.W.; Kurowska-Stolarska, M.; Clark, A.R. The Role of MicroRNAs in Glucocorticoid Action. J. Biol. Chem. 2018, 293, 1865–1874. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Tay, H.L.; Maltby, S.; Xiang, Y.; Eyers, F.; Hatchwell, L.; Zhou, H.; Toop, H.D.; Morris, J.C.; Nair, P.; et al. MicroRNA-9 Regulates Steroid-Resistant Airway Hyperresponsiveness by Reducing Protein Phosphatase 2A Activity. J. Allergy Clin. Immunol. 2015, 136, 462–473. [Google Scholar] [CrossRef]
- Smith, S.S.; Dole, N.S.; Franceschetti, T.; Hrdlicka, H.C.; Delany, A.M. MicroRNA-433 Dampens Glucocorticoid Receptor Signaling, Impacting Circadian Rhythm and Osteoblastic Gene Expression. J. Biol. Chem. 2016, 291, 21717–21728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, S.; Nishida, A.; Hara, K.; Kamemoto, T.; Suetsugi, M.; Fujimoto, M.; Watanuki, T.; Wakabayashi, Y.; Otsuki, K.; McEwen, B.S.; et al. Characterization of the Vulnerability to Repeated Stress in Fischer 344 Rats: Possible Involvement of MicroRNA-Mediated down-Regulation of the Glucocorticoid Receptor. Eur. J. Neurosci. 2008, 27, 2250–2261. [Google Scholar] [CrossRef]
- Cataldo, D.; Louis, R.; Michils, A.; Peché, R.; Pilette, C.; Schleich, F.; Ninane, V.; Hanon, S. Severe Asthma: Oral Corticosteroid Alternatives and the Need for Optimal Referral Pathways. J. Asthma 2021, 58, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of Long-Term Oral Corticosteroid Therapy and Its Side-Effects in Severe Asthma in Adults: A Focused Review of the Impact Data in the Literature. Eur. Respir. J. 2018, 52, 1800703. [Google Scholar] [CrossRef]
- Imraish, A.; Abu-Thiab, T.; Zihlif, M. IL-13 and FOXO3 Genes Polymorphisms Regulate IgE Levels in Asthmatic Patients. Biomed Rep. 2021, 14, 55. [Google Scholar] [CrossRef]
- Alam, R.; Gorska, M.M. Mitogen-Activated Protein Kinase Signalling and ERK1/2 Bistability in Asthma. Clin. Exp. Allergy 2011, 41, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Gil-Martínez, M.; Lorente-Sorolla, C.; Rodrigo-Muñoz, J.M.; Lendínez, M.Á.; Núñez-Moreno, G.; de la Fuente, L.; Mínguez, P.; Mahíllo-Fernández, I.; Sastre, J.; Valverde-Monge, M.; et al. Analysis of Differentially Expressed MicroRNAs in Serum and Lung Tissues from Individuals with Severe Asthma Treated with Oral Glucocorticoids. Int. J. Mol. Sci. 2023, 24, 1611. [Google Scholar] [CrossRef]
- Adams, B.S.; Nguyen, H. Salmeterol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Billington, C.K.; Penn, R.B.; Hall, I.P. Β2 Agonists. Handb. Exp. Pharmacol. 2017, 237, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Yao, L.; Liu, C.; Tang, L.; Xing, T. Upregulation of MicroRNA-16 Alters the Response to Inhaled Β-agonists in Patients with Asthma Though Modulating Expression of ADRB2. Mol. Med. Rep. 2019, 19, 4027–4034. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Cho, S.; Woo, J.A.; Liggett, S.B. A CREB-mediated Increase in MiRNA Let-7f during Prolonged Β-agonist Exposure: A Novel Mechanism of β 2 -adrenergic Receptor Down-regulation in Airway Smooth Muscle. FASEB J. 2018, 32, 3680–3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Dragonieri, S.; Carpagnano, G.E. Biological Therapy for Severe Asthma. Asthma. Res. Pract. 2021, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Rial, M.J.; Cañas, J.A.; Rodrigo-Muñoz, J.M.; Valverde-Monge, M.; Sastre, B.; Sastre, J.; del Pozo, V. Changes in Serum MicroRNAs after Anti-IL-5 Biological Treatment of Severe Asthma. Int. J. Mol. Sci. 2021, 22, 3558. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; d’Alessandro, M.; Cameli, P.; Bianchi, F.; Sestini, P.; Bargagli, E.; Refini, R.M. Personalized Approach of Severe Eosinophilic Asthma Patients Treated with Mepolizumab and Benralizumab. Int. Arch. Allergy Immunol. 2020, 181, 746–753. [Google Scholar] [CrossRef]
- Cañas, J.A.; Valverde-Monge, M.; Rodrigo-Muñoz, J.M.; Sastre, B.; Gil-Martínez, M.; García-Latorre, R.; Rial, M.J.; Gómez-Cardeñosa, A.; Fernández-Nieto, M.; Pinillos-Robles, E.J.; et al. Serum MicroRNAs as Tool to Predict Early Response to Benralizumab in Severe Eosinophilic Asthma. J. Pers. Med. 2021, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Uehara, S.; Shirai, T.; Rachi, Y.; Kimura, T.; Akamatsu, T.; Itoh, K. Benralizumab Restores Gene and MicroRNA Expression Involved in Steroid Sensitivity in Severe Asthma. Allergy 2021, 76, 2589–2592. [Google Scholar] [CrossRef]
- Mimmi, S.; Lombardo, N.; Maisano, D.; Piazzetta, G.; Pelaia, C.; Pelaia, G.; Greco, M.; Foti, D.; Dattilo, V.; Iaccino, E. Spotlight on a Short-Time Treatment with the IL-4/IL-13 Receptor Blocker in Patients with CRSwNP: MicroRNAs Modulations and Preliminary Clinical Evidence. Genes 2022, 13, 2366. [Google Scholar] [CrossRef] [PubMed]
- Cherneva, R.V.; Kostadinov, D. Epigenetic Targets for Therapeutic Approaches in COPD and Asthma. Nutrigenomics &Ndash; Possible or Illusive. Folia. Med. 2019, 61, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, X.; Cheng, S.; Xu, Y.; Xuefei, Q.; Cao, Y.; Xie, J.; Wang, C.-Y.; Xu, Y.; Xiong, W. Small Interfering RNA Directed against MicroRNA-155 Delivered by a Lentiviral Vector Attenuates Asthmatic Features in a Mouse Model of Allergic Asthma. Exp. Ther. Med. 2017, 14, 4391–4396. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Zhang, F.; Peng, X.; Mao, X.; Lu, W.; Wu, R.; Huang, B.; Bao, Y.; Ma, L.; et al. Divergent Roles of MiR-3162-3p in Pulmonary Inflammation in Normal and Asthmatic Mice as Well as Antagonism of MiR-3162-3p in Asthma Treatment. Int. Arch. Allergy Immunol. 2020, 181, 594–605. [Google Scholar] [CrossRef]
- Fang, C.; Lu, W.; Li, C.; Peng, X.; Wang, Y.; Huang, X.; Yao, Z.; Cai, N.; Huang, Y.; Zhang, X.; et al. MiR-3162-3p Is a Novel MicroRNA That Exacerbates Asthma by Regulating β-Catenin. PLoS ONE 2016, 11, e0149257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Zhao, J.; Ye, Q.; Gu, X. Interference of MiR-943-3p with Secreted Frizzled-Related Proteins4 (SFRP4) in an Asthma Mouse Model. Cell Tissue Res. 2019, 378, 67–80. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Sun, H.; Yan, Y.; Huang, L.; Gu, W.; Jiang, W.; Wang, Y.; Zhu, C.; Ji, W.; et al. The Role of MiR-29c/B7-H3 Axis in Children with Allergic Asthma. J. Transl. Med. 2018, 16, 218. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, X.; Yan, Y.; Wang, Y.; Huang, L.; Wang, M.; Shao, X.; Chen, Z.; Ji, W. B7-H3 Participates in the Development of Asthma by Augmentation of the Inflammatory Response Independent of TLR2 Pathway. Sci. Rep. 2017, 7, 40398. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Zhang, S.; Wang, X.; Du, X.; Chen, J.; Zhou, G. MiR-106b-5p Targeting SIX1 Inhibits TGF-β1-induced Pulmonary Fibrosis and Epithelial-mesenchymal Transition in Asthma through Regulation of E2F1. Int. J. Mol. Med. 2021, 47, 4857. [Google Scholar] [CrossRef]
- Yang, Z.; Qu, Z.; Yi, M.; Lv, Z.; Wang, Y.; Shan, Y.; Ran, N.; Liu, X. MiR-204-5p Inhibits Transforming Growth Factor-Β1-Induced Proliferation and Extracellular Matrix Production of Airway Smooth Muscle Cells by Regulating Six1 in Asthma. Int. Arch. Allergy Immunol. 2020, 181, 239–248. [Google Scholar] [CrossRef]
- Xiong, T.; Du, Y.; Fu, Z.; Geng, G. MicroRNA-145-5p Promotes Asthma Pathogenesis by Inhibiting Kinesin Family Member 3A Expression in Mouse Airway Epithelial Cells. J. Int. Med. Res. 2019, 47, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Varahram, M.; Jamaati, H.; Garssen, J.; Mumby, S.E.; Adcock, I.M. Exosomes in Severe Asthma: Update in Their Roles and Potential in Therapy. Biomed Res. Int. 2018, 2018, 2862187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañas, J.A.; Sastre, B.; Rodrigo-Muñoz, J.M.; del Pozo, V. Exosomes: A New Approach to Asthma Pathology. Clin. Chim. Acta 2019, 495, 139–147. [Google Scholar] [CrossRef]
- Li, C.; Deng, C.; Zhou, T.; Hu, J.; Dai, B.; Yi, F.; Tian, N.; Jiang, L.; Dong, X.; Zhu, Q.; et al. MicroRNA-370 Carried by M2 Macrophage-Derived Exosomes Alleviates Asthma Progression through Inhibiting the FGF1/MAPK/STAT1 Axis. Int. J. Biol. Sci. 2021, 17, 1795–1807. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Huang, L.-H.; Zhu, Y.-K.; Cai, X.-J. LncRNA NEAT1 Promotes Airway Smooth Muscle Cell Inflammation by Activating the JAK3/STAT5 Pathway through Targeting of MiR-139. Exp. Lung Res. 2021, 47, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Huang, J.; Wasti, B.; Chen, Z.; Yuan, Y.; He, Y.; Li, D.; Jia, J.; Liu, S.; Liu, Y.; et al. MiR-146a-3p as a Potential Novel Therapeutic by Targeting MBD2 to Mediate Th17 Differentiation in Th17 Predominant Neutrophilic Severe Asthma. Clin. Exp. Med. 2023, 1–16. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, S.-G.; Xu, L.; Che, Y.-Y.; Jiang, S.-Y.; Zhu, L.; Zhao, M.; Teng, Y.-C. MiR-135b Alleviates Airway Inflammation in Asthmatic Children and Experimental Mice with Asthma via Regulating CXCL12. Immunol. Invest. 2022, 51, 496–510. [Google Scholar] [CrossRef]
- Chiba, Y.; Ando, Y.; Fujii, S.; Miyakawa, Y.; Suto, W.; Kamei, J.; Sakai, H.; Hanazaki, M. Downregulation of MiR-140-3p Is a Cause of Upregulation of RhoA Protein in Bronchial Smooth Muscle of Murine Experimental Asthma. Am. J. Respir. Cell Mol. Biol. 2021, 64, 138–140. [Google Scholar] [CrossRef]
- Chiba, Y.; Ando, Y.; Kato, Y.; Hanazaki, M.; Sakai, H. Down-Regulation of MiR-140-3p Is a Cause of the Interlukin-13-Induced up-Regulation of RhoA Protein in Bronchial Smooth Muscle Cells. Small GTPases 2022, 13, 1–6. [Google Scholar] [CrossRef]
- Chiba, Y.; Misawa, M. MicroRNAs and Their Therapeutic Potential for Human Diseases: MiR-133a and Bronchial Smooth Muscle Hyperresponsiveness in Asthma. J. Pharmacol. Sci. 2010, 114, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Li, C.; Gao, X. Overexpression of MiR-375 Reverses the Effects of Dexamethasone on the Viability, Migration, Invasion and Apoptosis of Human Airway Epithelial Cells by Targeting DUSP6. Int. J. Mol. Med. 2022, 49, 26. [Google Scholar] [CrossRef]
- Duan, X.-J.; Zhang, X.; Li, L.-R.; Zhang, J.-Y.; Chen, Y.-P. MiR-200a and MiR-200b Restrain Inflammation by Targeting ORMDL3 to Regulate the ERK/MMP-9 Pathway in Asthma. Exp. Lung Res. 2020, 46, 321–331. [Google Scholar] [CrossRef]
- He, N.; Liu, L.; Ding, J.; Sun, Y.; Xing, H.; Wang, S. MiR-222-3p Ameliorates Glucocorticoid-Induced Inhibition of Airway Epithelial Cell Repair through down-Regulating GILZ Expression. J. Recept. Signal Transduct. 2020, 40, 301–312. [Google Scholar] [CrossRef]
- Jartti, T.; Bønnelykke, K.; Elenius, V.; Feleszko, W. Role of Viruses in Asthma. Semin. Immunopathol. 2020, 42, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Leon-Icaza, S.A.; Zeng, M.; Rosas-Taraco, A.G. MicroRNAs in Viral Acute Respiratory Infections: Immune Regulation, Biomarkers, Therapy, and Vaccines. ExRNA 2019, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.S.; Lim, R.L.; Liu, J.; Ong, H.H.; Tan, V.J.; Lim, H.F.; Chung, K.F.; Adcock, I.M.; Chow, V.T.; Wang, D.Y. Respiratory Viral Infections in Exacerbation of Chronic Airway Inflammatory Diseases: Novel Mechanisms and Insights From the Upper Airway Epithelium. Front. Cell Dev. Biol. 2020, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Collison, A.M.; Sokulsky, L.A.; Kepreotes, E.; Pereira de Siqueira, A.; Morten, M.; Edwards, M.R.; Walton, R.P.; Bartlett, N.W.; Yang, M.; Nguyen, T.H.; et al. MiR-122 Promotes Virus-Induced Lung Disease by Targeting SOCS1. JCI Insight 2021, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, M.; Bochkov, Y.A.; Kreiner-Møller, E.; Bønnelykke, K.; Stein, M.M.; Du, G.; Bisgaard, H.; Jackson, D.J.; Gern, J.E.; Lemanske, R.F.; et al. Rhinovirus Wheezing Illness and Genetic Risk of Childhood-Onset Asthma. N. Engl. J. Med. 2013, 368, 1398–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCaskill, J.L.; Ressel, S.; Alber, A.; Redford, J.; Power, U.F.; Schwarze, J.; Dutia, B.M.; Buck, A.H. Broad-Spectrum Inhibition of Respiratory Virus Infection by MicroRNA Mimics Targeting P38 MAPK Signaling. Mol. Ther. Nucleic Acids 2017, 7, 256–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Sun, X.; Zhu, Y.; Qin, W. Downregulation of MiR-146a Inhibits Influenza A Virus Replication by Enhancing the Type I Interferon Response In Vitro and In Vivo. Biomed. Pharmacother. 2019, 111, 740–750. [Google Scholar] [CrossRef]
- Amini-Farsani, Z.; Yadollahi-Farsani, M.; Arab, S.; Forouzanfar, F.; Yadollahi, M.; Asgharzade, S. Prediction and Analysis of MicroRNAs Involved in COVID-19 Inflammatory Processes Associated with the NF-κB and JAK/STAT Signaling Pathways. Int. Immunopharmacol. 2021, 100, 108071. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ptashkin, R.N.; Wang, Q.; Liu, G.; Zhang, G.; Lee, I.; Lee, Y.S.; Bao, X. Human Metapneumovirus Infection Induces Significant Changes in Small Noncoding RNA Expression in Airway Epithelial Cells. Mol. Ther. Nucleic Acids 2014, 3, e163. [Google Scholar] [CrossRef] [PubMed]
- Tay, H.L.; Kaiko, G.E.; Plank, M.; Li, J.; Maltby, S.; Essilfie, A.-T.; Jarnicki, A.; Yang, M.; Mattes, J.; Hansbro, P.M.; et al. Antagonism of MiR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-Typeable Haemophilus Influenzae (NTHi) from Infected Lung. PLoS Pathog. 2015, 11, e1004549. [Google Scholar] [CrossRef]
- Payne, D. RNA Therapies. Nature 2019, 574, S1. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, C. Mist Begins to Clear for Lung Delivery of RNA. Nat. Biotechnol. 2020, 38, 1110–1112. [Google Scholar] [CrossRef]
- Siddiqui, S.; Johansson, K.; Joo, A.; Bonser, L.R.; Koh, K.D.; Le Tonqueze, O.; Bolourchi, S.; Bautista, R.A.; Zlock, L.; Roth, T.L.; et al. Epithelial MiR-141 Regulates IL-13–Induced Airway Mucus Production. JCI Insight 2021, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Sasso, J.M.; Ambrose, B.J.B.; Tenchov, R.; Datta, R.S.; Basel, M.T.; DeLong, R.K.; Zhou, Q.A. The Progress and Promise of RNA Medicine─An Arsenal of Targeted Treatments. J. Med. Chem. 2022, 65, 6975–7015. [Google Scholar] [CrossRef]
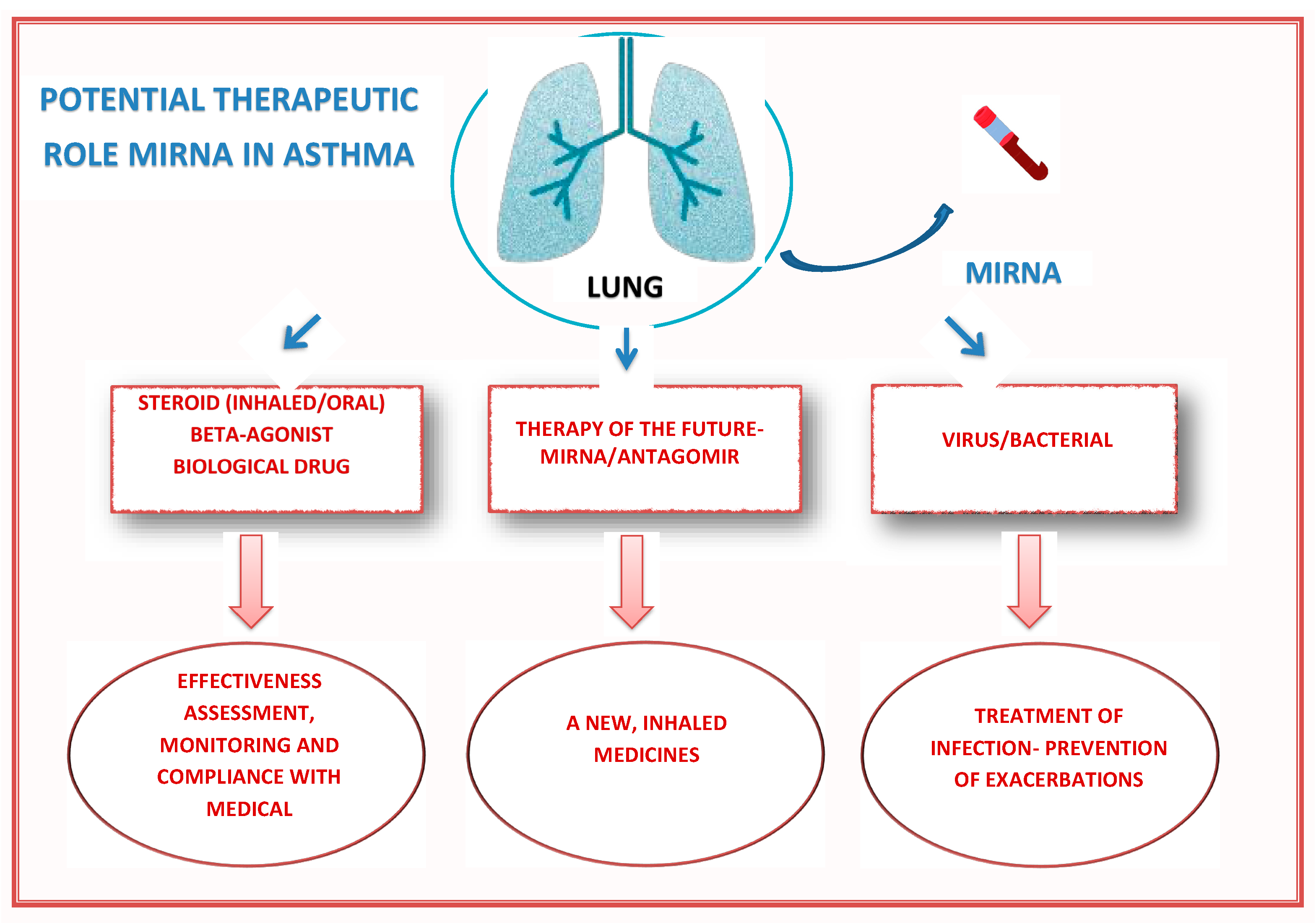
| Study, Year | Experiment/Population | miRNA | Molecular Effect | The Biological Effect Obtained After Using Antagomir or Mirna |
|---|---|---|---|---|
| C. Huilong et al. [1], 2017 | Induction of asthma by ovalbumin and analysis of cells in the bronchoalveolar lavage fluid and lung tissue/MICE | miRNA-155 | Increased production of IL-4, 5, 13, and hypersensitivity of airway | Antagomir miRNA-155; inhibited the inflammatory process |
| J. Liu et al. [2], 2020 | Induction of asthma by ovalbumin and analysis of cells in the bronchoalveolar lavage fluid/MICE | miRNA-3162-3p | Wnt/beta-catenin; increased production of the active form of beta-catenin, inflammatory response, and hypersensitivity of airway | Antagomir miRNA-3162-3p; anti-inflammatory effect such as GCs |
| J. Shen et al. [3], 2019 | Induction of asthma by ovalbumin and analysis of cells in the bronchoalveolar lavage fluid and lung tissue/MICE | miRNA-943-3p | Wnt/SFRP-4; increased production of the active form of beta-catenin, inflammatory response, and fibrosis in the airway | Antagomir miRNA-943-3p; anti-inflammatory effect such as GCs |
| X. Zhang et al. [4], 2018 | Analysis of blood sample/CHILDREN WITH ASTHMA EXCERBATIONS | miRNA-29c | B7-H3; increased eosinophilic infiltration, cytokine synthesis, mucus production, regulation of Th differentiation | Mimic miRNA-29c-inhibited the inflammatory process |
| S. Liu et al. [5], 2021 | Induction of asthma by ovalbumin in mice and analysis of cells in the lung; human bronchial epithelial cells/MICE; HUMAN | miRNA-106b-5p | TGF-B1/SIX1/E2F1; initiated remodeling, fibrosis of the airways | Mimic miRNA-106b-5p; inhibited remodeling of the respiratory tract and increased sensitivity to the GCs |
| Z. Yang et al. [6], 2020 | Smooth muscle cells collected during bronchoscopy/HUMAN | miRNA-204-5p | TGF-B1/SIX1; excessive smooth muscle cell proliferation and ECM production | Mimic miRNA-204-5p; suppressed the proliferation and ECM production of airway smooth muscle cells by directly regulating SIX1 |
| T. Xiong et al. [7], 2019 | Induction of allergic airways diseases by aeroallergen and analysis bronchial epithelial cells/MICE | miRNA-145-5p | KIF3-increased IL-4, 5, and 13 production, eosinophil infiltration, airway hyperresponsiveness | Antagomir miRNA-145; inhibited the inflammatory process |
| C. Li et al. [8], 2021 | Induction of asthma by ovalbumin and isolated exosomes from macrophage/MICE | miRNA-370 | MAPK/STAT; increased fibrosis and inflammatory process | Mimic miRNA-370; prevented airway remodeling and reduced inflammation |
| M. Zhu et al. [9], 2021 | Smooth muscle cells collected during bronchoscopy/HUMAN | miRNA-139 | lncRNA/JAK3/STAT5; increased production of TNF-a and IL-6, 8, and 1b | Mimic miRNA-139; reduced the production of cytokines |
| W. Duan et al. [10], 2023 | Analysis of blood sample in severe asthma; mouse model of Th17 predominant neutrophilic, severe asthma/HUMAN; MICE | miRNA-146a-3p | MBD2; initialized severe asthma, increased Th17 lymphocytes, production of mucus, airway hyperresponsiveness | Mimic miRNA-146a-3p; suppressed production of Th17 lymphocytes and inflammatory process, development of severe asthma |
| Y. Liu et al. [11], 2022 | Analysis of blood sample; induction of asthma by ovalbumin in mice and analysis bronchial epithelial cells/CHILDREN; MICE | miRNA-135b | CXCL-12; increased inflammatory process with Th17, airway hyperresponsiveness | Mimic miRNA-135b; decreased CXCL-12 production and inflammatory infiltration |
| Y. Chiba et al. [12], 2021 | Bronchial smooth muscle cell/HUMAN | miRNA-140-3p | RhoA; increased the strength contraction of smooth muscle | Mimic miRNA-140-3p; inhibited contraction of smooth muscle |
| X. Duan et al. [13], 2020 | Bronchial epithelial cells/HUMAN | miRNA-200a/200b | ORMDL3/ERK/MMP-9; increased production of TNF-a, IL-4, IL-5, IL-13, IL-1b, and the inflammatory process | Mimic miRNA-200a/200b; inhibited the inflammatory process |
| N. He et al. [14], 2020 | Bronchial epithelial cells treated by dexamethasone/HUMAN | miRNA-222-3p | GILZ; inhibited proliferation, migration, and cell differentiation around the damaged epithelium | Mimic 222-3p; promoted the repair of damaged epithelium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kierbiedź-Guzik, N.; Sozańska, B. miRNAs as Modern Biomarkers in Asthma Therapy. Int. J. Mol. Sci. 2023, 24, 11499. https://doi.org/10.3390/ijms241411499
Kierbiedź-Guzik N, Sozańska B. miRNAs as Modern Biomarkers in Asthma Therapy. International Journal of Molecular Sciences. 2023; 24(14):11499. https://doi.org/10.3390/ijms241411499
Chicago/Turabian StyleKierbiedź-Guzik, Natalia, and Barbara Sozańska. 2023. "miRNAs as Modern Biomarkers in Asthma Therapy" International Journal of Molecular Sciences 24, no. 14: 11499. https://doi.org/10.3390/ijms241411499
APA StyleKierbiedź-Guzik, N., & Sozańska, B. (2023). miRNAs as Modern Biomarkers in Asthma Therapy. International Journal of Molecular Sciences, 24(14), 11499. https://doi.org/10.3390/ijms241411499







