Carbamoylated Erythropoietin-Induced Cerebral Blood Perfusion and Vascular Gene Regulation
Abstract
1. Introduction
2. Results
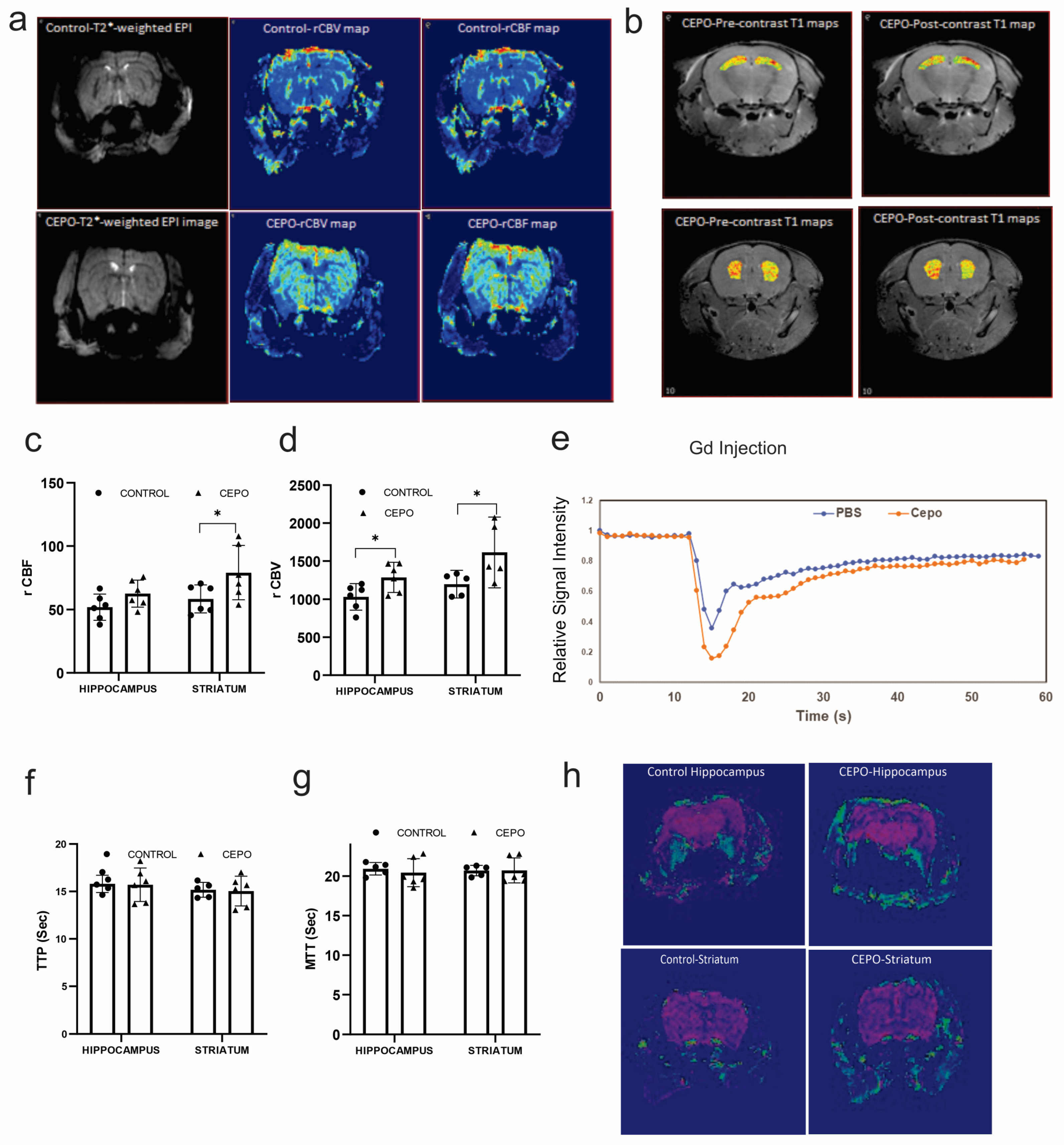
2.1. Chronic CEPO Administration Increased Hippocampal and Striatal Blood Perfusion without Affecting BBB Integrity
2.2. CEPO Elevated HIF1A Gene Expression without Elevating Angiogenic Factors in HUVECs
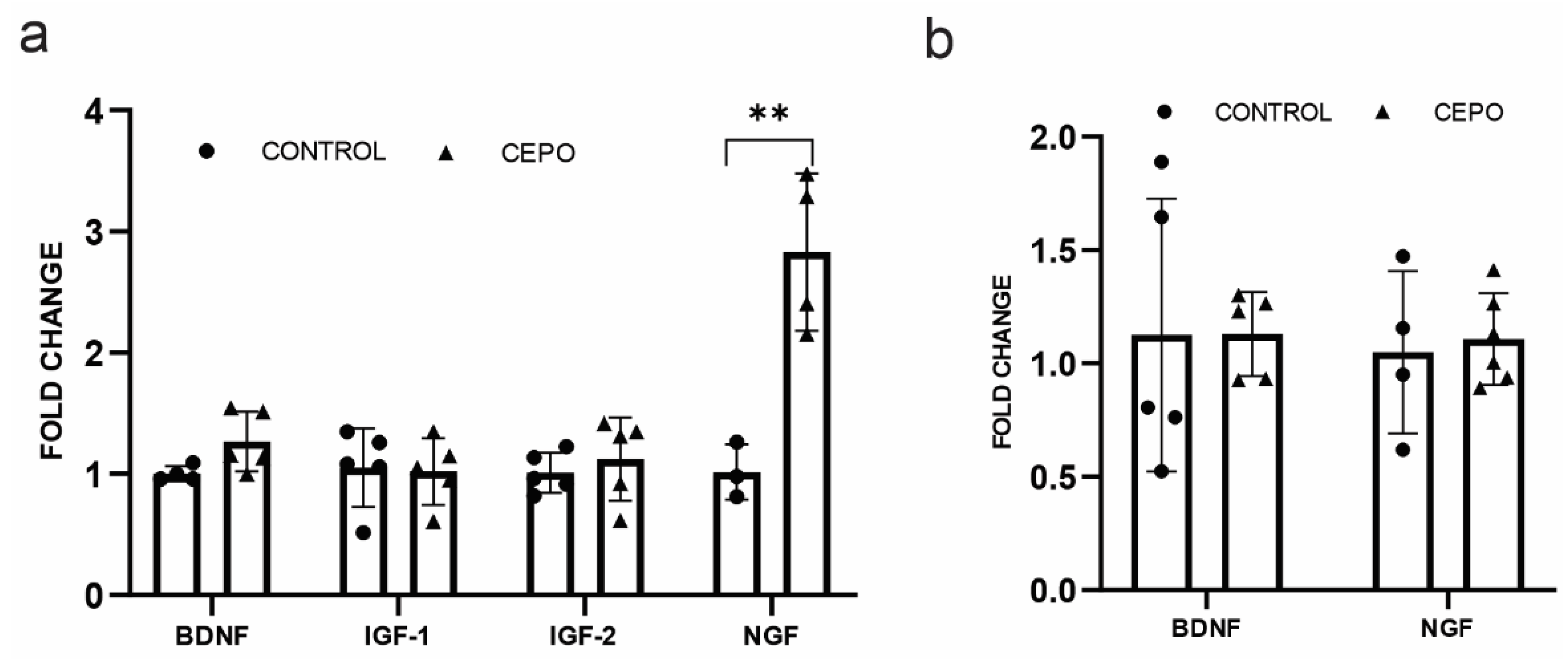
2.3. CEPO Differentially Regulates the Transcription of Neurotrophic Factors in the HUVECs
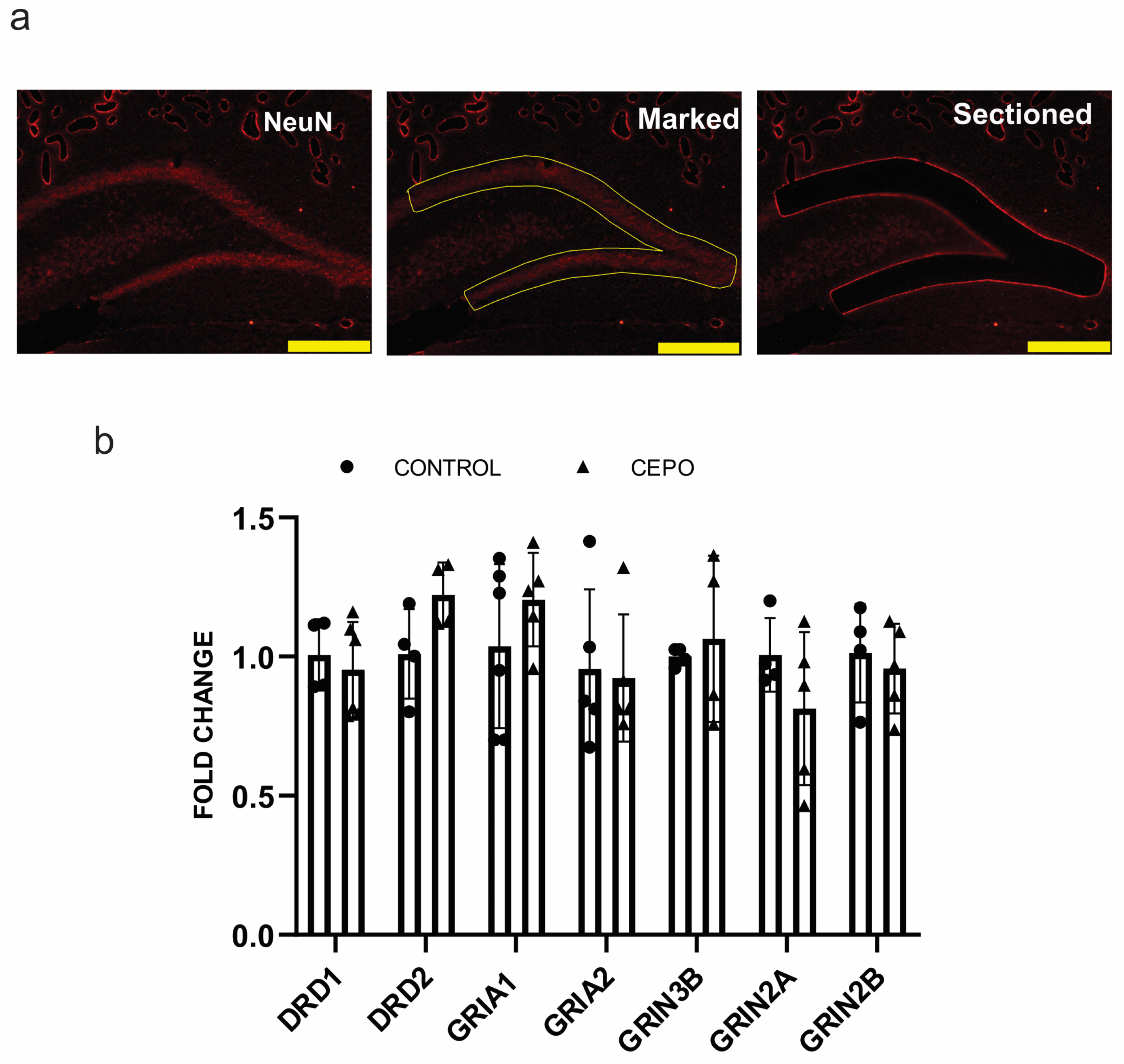
2.4. CD131 and EPO Receptor Gene Expression Is Elevated in CEPO-Treated HUVECs and Unchanged in the Dentate Gyrus
2.5. Glutamatergic and Dopaminergic Receptor Expression Was Intact in CEPO-Treated Rats
2.6. CEPO Promotes Vasodilation by Transcriptionally Regulating EDRF and CGRP Genes
3. Discussion
4. Materials and Methods
4.1. Carbamoylation of EPO
4.2. Cell Culture
4.3. RNA Extraction and Quantitative PCR Analyses in HUVEC
4.4. Animals
4.5. Laser Microdissection and RNA Preparation
4.6. cDNA Preparation and Gene Expression
4.7. Brain Blood Flow Analysis Using Magnetic Resonance Imaging (MRI) and Data Analysis
4.8. Tube Formation Assay
4.9. Migration Assay
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miskowiak, K.W.; Vinberg, M.; Christensen, E.M.; Bukh, J.D.; Harmer, C.J.; Ehrenreich, H.; Kessing, L.V. Recombinant human erythropoietin for treating treatment-resistant depression: A double-blind, randomized, placebo-controlled phase 2 trial. Neuropsychopharmacology 2013, 39, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Ehrenreich, H.; Christensen, E.M.; Kessing, L.V.; Vinberg, M. Recombinant human erythropoietin to target cognitive dysfunction in bipolar disorder. J. Clin. Psychiatry 2014, 75, 1347–1355. [Google Scholar] [CrossRef]
- Miskowiak, K.; O’Sullivan, U.; Harmer, C.J. Erythropoietin reduces neural and cognitive processing of fear in human models of antidepressant drug action. Biol. Psychiatry 2007, 62, 1244–1250. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Favaron, E.; Hafizi, S.; Inkster, B.; Goodwin, G.M.; Cowen, P.J.; Harmer, C.J. Erythropoietin modulates neural and cognitive processing of emotional information in biomarker models of antidepressant drug action in depressed patients. Psychopharmacology 2010, 210, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Keogh, C.L.; Yu, S.P.; Wei, L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J. Cereb. Blood Flow. Metab. 2007, 27, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Trincavelli, M.L.; Da Pozzo, E.; Ciampi, O.; Cuboni, S.; Daniele, S.; Abbracchio, M.P.; Martini, C. Regulation of erythropoietin receptor activity in endothelial cells by different erythropoietin (EPO) derivatives: An in vitro study. Int. J. Mol. Sci. 2013, 14, 2258–2281. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Jiang, Y.; Hartnett, M.E. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am. J. Pathol. 2014, 184, 1230–1239. [Google Scholar] [CrossRef]
- Tonia, T.; Mettler, A.; Robert, N.; Schwarzer, G.; Seidenfeld, J.; Weingart, O.; Hyde, C.; Engert, A.; Bohlius, J. Erythropoietin or Darbepoetin for patients with cancer. Cochrane Database Syst. Rev. 2012, 12, CD003407. [Google Scholar] [CrossRef]
- Ding, J.; Wang, J.; Li, Q.Y.; Yu, J.Z.; Ma, C.G.; Wang, X.; Lu, C.Z.; Xiao, B.G. Neuroprotection and CD131/GDNF/Akt pathway of carbamylated erythropoietin in hypoxic neurons. Mol. Neurobiol. 2017, 54, 5051–5060. [Google Scholar] [CrossRef]
- Bohr, S.; Patel, S.J.; Vasko, R.; Shen, K.; Iracheta-Vellve, A.; Lee, J.; Bale, S.S.; Chakraborty, N.; Brines, M.; Cerami, A.; et al. Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells. J. Mol. Med. 2015, 93, 199–210. [Google Scholar] [CrossRef]
- Roher, A.E.; Debbins, J.P.; Malek-Ahmadi, M.; Chen, K.; Pipe, J.G.; Maze, S.; Belden, C.; Maarouf, C.L.; Thiyyagura, P.; Mo, H.; et al. Cerebral blood flow in Alzheimer’s disease. Vasc. Health Risk Manag. 2012, 8, 599–611. [Google Scholar] [CrossRef]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wang, Z.; Zhang, X.; Shu, H.; Wang, Z.; Liu, D.; Zhang, Z. Cerebral blood flow changes in remitted early- and late-onset depression patients. Oncotarget 2017, 8, 76214–76222. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging Animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F.; Fariñas, I. Molecular and Cellular Approaches to Neural Development. In Neurotrophic Factors and Their Receptors. Roles in Neuronal Development and Function; Cowan, W.M., Jessell, T.M., Zipursky, S.L., Eds.; Oxford University Press: New York, NY, USA, 1997; pp. 220–263. [Google Scholar]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999, 22, 295–318. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Tiwari, N.K.; Sathyanesan, M.; Schweinle, W.; Newton, S.S. Carbamoylated erythropoietin induces a neurotrophic gene profile in neuronal cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 132–141. [Google Scholar] [CrossRef]
- Adembri, C.; Massagrande, A.; Tani, A.; Miranda, M.; Margheri, M.; De Gaudio, R.; Pellegrini-Giampietro, D.E. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit. Care Med. 2008, 36, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.; Brines, M.; Lauria, G.; Savino, C.; Gilardini, A.; Nicolini, G.; Rodriguez-Menendez, V.; Oggioni, N.; Canta, A.; Penza, P.; et al. Protective effect of erythropoietin and its carbamylated derivative in experimental cisplatin peripheral neurotoxicity. Clin. Cancer Res. 2006, 12, 2607–2612. [Google Scholar] [CrossRef]
- Fiordaliso, F.; Chimenti, S.; Staszewsky, L.; Bai, A.; Carlo, E.; Cuccovillo, I.; Doni, M.; Mengozzi, M.; Tonelli, R.; Ghezzi, P.; et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia–reperfusion injury. Proc. Natl. Acad. Sci. USA 2005, 102, 2046–2051. [Google Scholar] [CrossRef]
- Imamura, R.; Okumi, M.; Isaka, Y.; Ichimaru, N.; Moriyama, T.; Imai, E.; Nonomura, N.; Takahara, S.; Okuyama, A. Carbamylated erythropoietin improves angiogenesis and protects the kidneys from ischemia-reperfusion injury. Cell Transplant. 2008, 17, 135–141. [Google Scholar] [CrossRef]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Plate, K.H.; Breier, G.; Weich, H.A.; Risau, W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992, 359, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Z.; Li, Y.; Wang, K.; Dai, C.F.; Huang, S.A.; Chen, D.B.; Zheng, J. Distinct roles of hif1a in endothelial adaptations to physiological and ambient oxygen. Mol. Cell. Endocrinol. 2014, 391, 60–67. [Google Scholar] [CrossRef]
- Funamoto, M.; Fujio, Y.; Kunisada, K.; Negoro, S.; Tone, E.; Osugi, T.; Hirota, H.; Izumi, M.; Yoshizaki, K.; Walsh, K.; et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J. Biol. Chem. 2000, 275, 10561–10566. [Google Scholar] [CrossRef]
- Osugi, T.; Oshima, Y.; Fujio, Y.; Funamoto, M.; Yamashita, A.; Negoro, S.; Kunisada, K.; Izumi, M.; Nakaoka, Y.; Hirota, H.; et al. Cardiac-specific activation of signal transducer and activator of transcription 3 promotes vascular formation in the heart. J. Biol. Chem. 2002, 277, 6676–6681. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G.; Vacca, A.; Ribatti, D.; Bussolino, F. In vivo activation of jak2/stat-3 pathway during angiogenesis induced by GM-CSF. FASEB J. 2002, 16, 1–19. [Google Scholar] [CrossRef]
- Dey, S.; Li, X.; Teng, R.; Alnaeeli, M.; Chen, Z.; Rogers, H.; Noguchi, C.T. Erythropoietin regulates POMC expression via STAT3 and potentiates leptin response. J. Mol. Endocrinol. 2016, 56, 55–67. [Google Scholar] [CrossRef]
- Cao, Y.; Lathia, J.D.; Eyler, C.E.; Wu, Q.; Li, Z.; Wang, H.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. Erythropoietin Receptor Signaling Through STAT3 Is Required For Glioma Stem Cell Maintenance. Genes Cancer 2010, 1, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernàndez, J.; Holland, P.W. Archetypal organization of the amphioxus hox gene cluster. Nature 1992, 370, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Krumlauf, R. Hox genes in vertebrate development. Cell 1994, 78, 191–201. [Google Scholar] [CrossRef]
- Contaldo, C.; Elsherbiny, A.; Lindenblatt, N.; Plock, J.A.; Trentz, O.; Giovanoli, P.; Menger, M.D.; Wanner, G.A. Erythropoietin enhances oxygenation in critically perfused tissue through modulation of nitric oxide synthase. Shock 2009, 31, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Ford, H. MINI-REVIEW homeobox genes: A link between development, cell cycle, and cancer? Cell Biol. Int. 1999, 22, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, H.; Chow, N.; Sallstrom, J.; Bell, R.D.; Deane, R.; Brooks, A.I.; Kanagala, S.; Rubio, A.; Sagare, A.; et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat. Med. 2005, 11, 959–965. [Google Scholar] [CrossRef]
- Tolins, J.P.; Shultz, P.J.; Raij, L. Role of endothelium-derived relaxing factor in regulation of vascular tone and remodeling. update on humoral regulation of vascular tone. Hypertension 1991, 17, 909–916. [Google Scholar] [CrossRef]
- Shepherd, J.T.; Katusić, Z.S. Endothelium-derived vasoactive factors: I. Endothelium-dependent relaxation. Hypertension 1991, 18, III76. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Marsidi, J.L.; Brown, K.N. Physiology, Endothelial Derived Relaxation Factor. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Husain, M.; Moss, J. Endothelium-dependent vascular smooth muscle control. J. Clin. Anesth. 1988, 1, 135–145. [Google Scholar] [CrossRef]
- Pohl, U.; Busse, R. Hypoxia stimulates release of endothelium-derived relaxant factor. Am. J. Physiol. 1989, 256, H1595–H1600. [Google Scholar] [CrossRef]
- Ozaka, T.; Doi, Y.; Kayashima, K.; Fujimoto, S. Weibel-Palade bodies as a storage site of calcitonin gene-related peptide and endothelin-1 in blood vessels of the rat carotid body. Anat. Rec. 1997, 247, 388–394. [Google Scholar] [CrossRef]
- Visočnik, D.; Zaletel, M.; Žvan, B.; Zupan, M. Enhanced hemodynamic and clinical response to αCGRP in migraine patients—A TCD study. Front. Neurol. 2021, 12, 638903. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed]
- Meens, M.J.; Mattheij, N.J.; van Loenen, P.B.; Spijkers, L.J.; Lemkens, P.; Nelissen, J.; Compeer, M.G.; Alewijnse, A.E.; De Mey, J.G. G-protein βγ subunits in vasorelaxing and anti-endothelinergic effects of calcitonin gene-related peptide. Br. J. Pharmacol. 2012, 166, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Bracko, O.; Cruz Hernández, J.C.; Park, L.; Nishimura, N.; Schaffer, C.B. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2021, 41, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Pinkham, A.; Loughead, J.; Ruparel, K.; Wu, W.C.; Overton, E.; Gur, R.; Gur, R. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. Neuroimaging 2011, 194, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Marano, G.; Traversi, G.; Bria, P.; Mazza, S. Primary cerebral blood flow deficiency and Alzheimer’s disease: Shadows and lights. J. Alzheimers Dis. 2011, 23, 375–389. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Watt, M.J.; Haiar, J.M.; Scholl, J.L.; Davies, S.R.; Paulsen, R.T.; Wiederin, J.; Ciborowski, P.; Newton, S.S. Carbamoylated erythropoietin modulates cognitive outcomes of social defeat and differentially regulates gene expression in the dorsal and ventral hippocampus. Transl. Psychiatry 2018, 8, 113. [Google Scholar] [CrossRef]
- Sampath, D.; McWhirt, J.; Sathyanesan, M.; Newton, S.S. Carbamoylated erythropoietin produces antidepressant-like effects in male and female mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 96, 109754. [Google Scholar] [CrossRef]
- Pekas, N.J.; Petersen, J.L.; Sathyanesan, M.; Newton, S.S. Design and development of a behaviorally active recombinant neurotrophic factor. Drug Des. Dev. Ther. 2020, 14, 5393–5403. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Schmainda, K.M.; Weisskoff, R.M. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am. J. Neuroradiol. 2006, 27, 859–867. [Google Scholar] [PubMed]
- Bade, A.N.; Gendelman, H.E.; Boska, M.D.; Liu, Y. MEMRI is a biomarker defining nicotine-specific neuronal responses in subregions of the rodent brain. Am. J. Transl. Res. 2017, 9, 601–610. [Google Scholar] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadanandan, J.; Sathyanesan, M.; Liu, Y.; Tiwari, N.K.; Newton, S.S. Carbamoylated Erythropoietin-Induced Cerebral Blood Perfusion and Vascular Gene Regulation. Int. J. Mol. Sci. 2023, 24, 11507. https://doi.org/10.3390/ijms241411507
Sadanandan J, Sathyanesan M, Liu Y, Tiwari NK, Newton SS. Carbamoylated Erythropoietin-Induced Cerebral Blood Perfusion and Vascular Gene Regulation. International Journal of Molecular Sciences. 2023; 24(14):11507. https://doi.org/10.3390/ijms241411507
Chicago/Turabian StyleSadanandan, Jayanarayanan, Monica Sathyanesan, Yutong Liu, Neeraj K. Tiwari, and Samuel S. Newton. 2023. "Carbamoylated Erythropoietin-Induced Cerebral Blood Perfusion and Vascular Gene Regulation" International Journal of Molecular Sciences 24, no. 14: 11507. https://doi.org/10.3390/ijms241411507
APA StyleSadanandan, J., Sathyanesan, M., Liu, Y., Tiwari, N. K., & Newton, S. S. (2023). Carbamoylated Erythropoietin-Induced Cerebral Blood Perfusion and Vascular Gene Regulation. International Journal of Molecular Sciences, 24(14), 11507. https://doi.org/10.3390/ijms241411507







