ω-3 Polyunsaturated Fatty Acids Improve the Blood–Brain-Barrier Integrity in Contrast-Induced Blood–Brain-Barrier Injury in Uremic Mice
Abstract
1. Introduction
2. Results
2.1. Ischemia–Reperfusion-Induced Renal Damage Induces Uremic Production
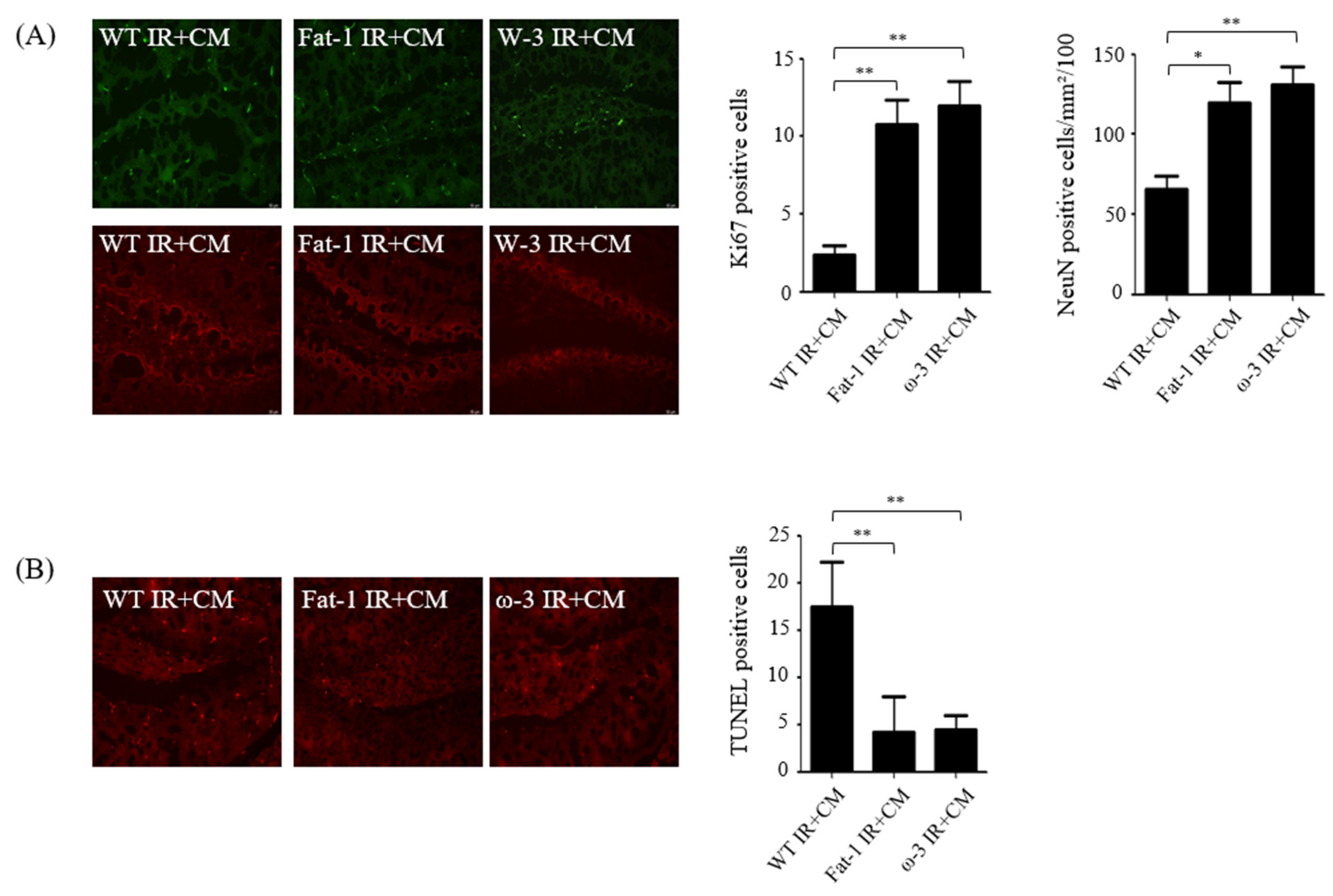
2.2. Effects of CM Administration on Hippocampal Nerve Damage after Renal IR
2.3. In Non-Uremic Mouse Brain, CM Administration Has No Effect
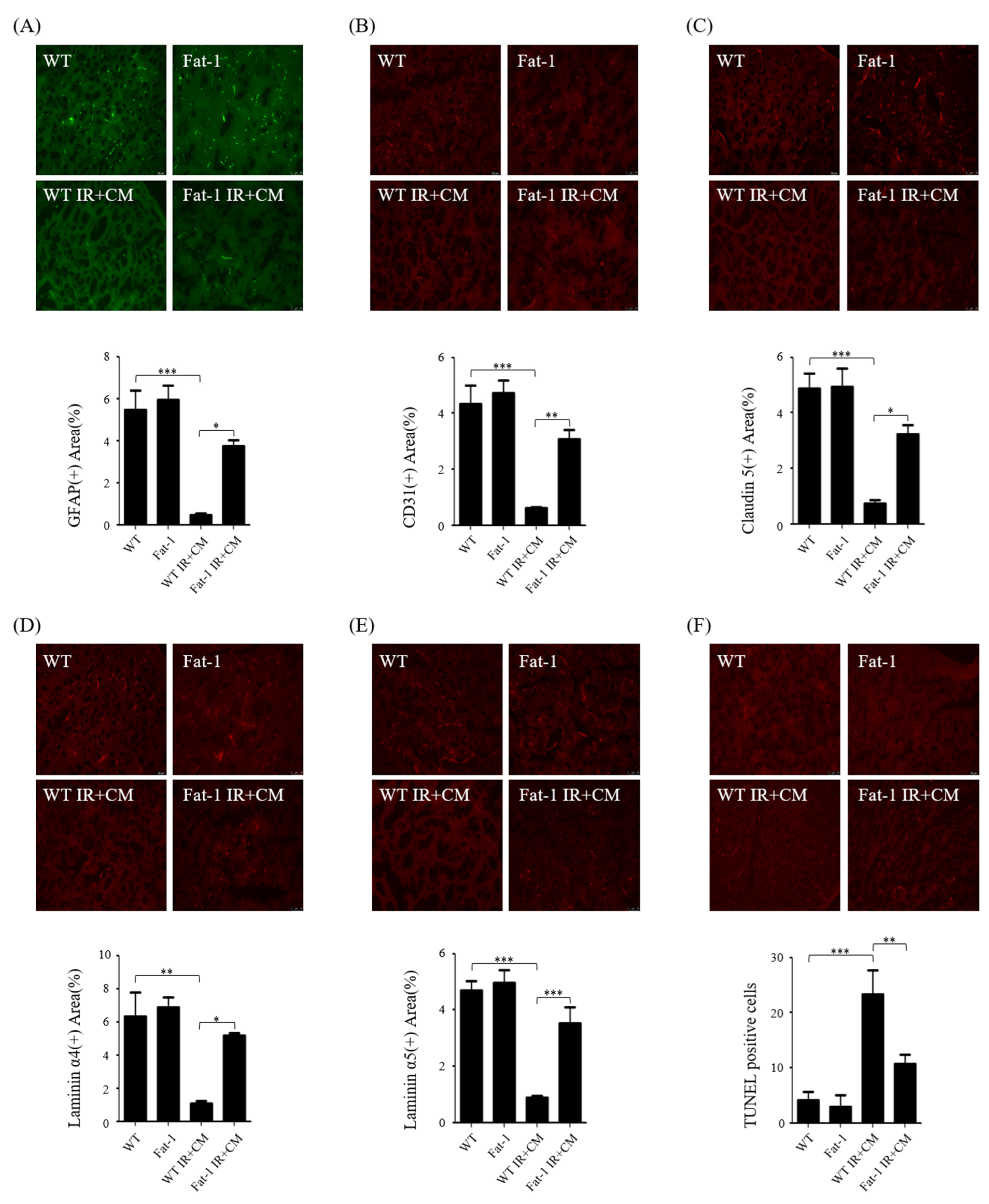
2.4. CM-Induced BBB Injuries Are Attenuated in Fat-1 Uremic Mice
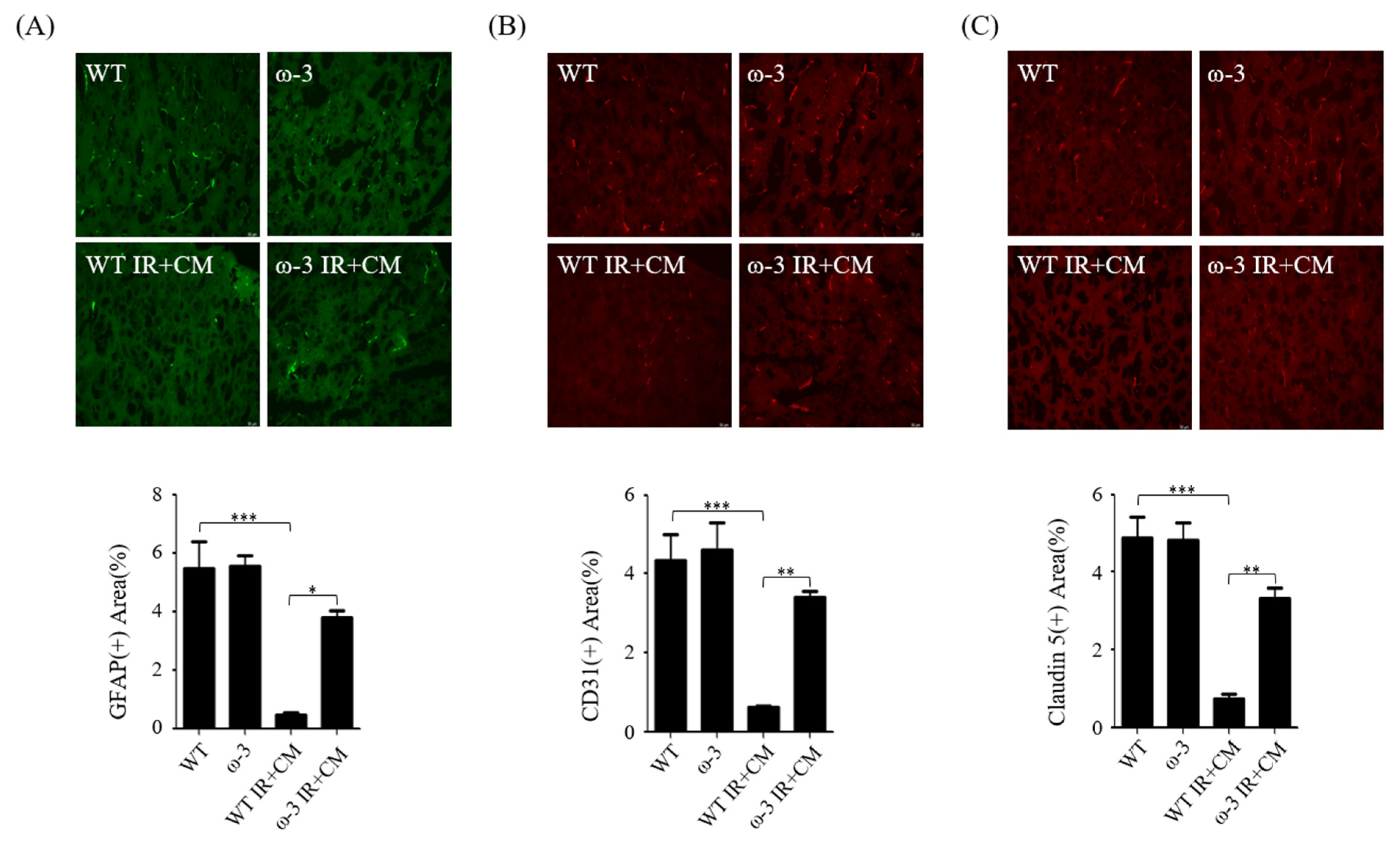
2.5. ω-3 PUFAs Attenuate BBB Injury via Contrast in Uremic Mice
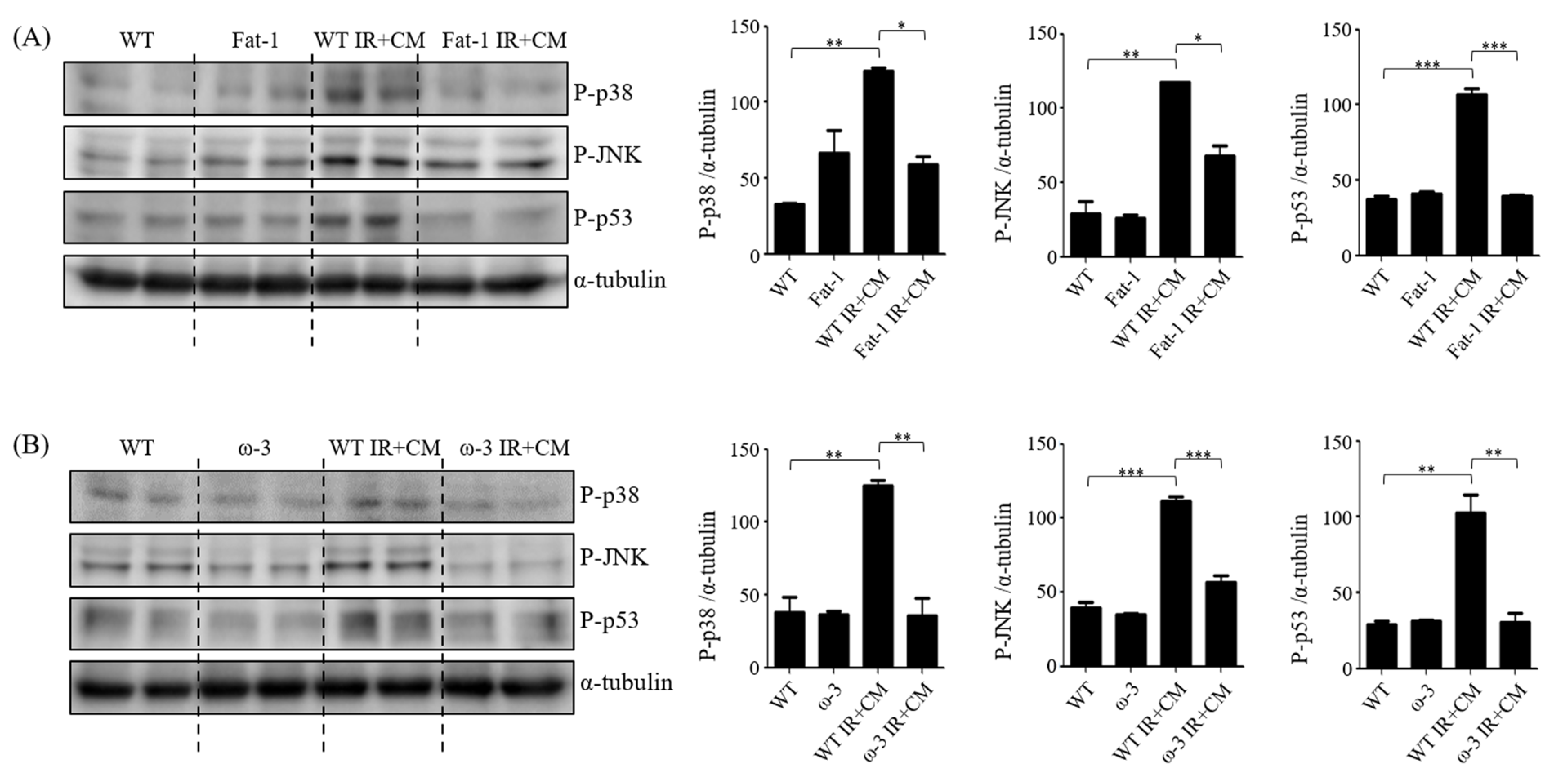
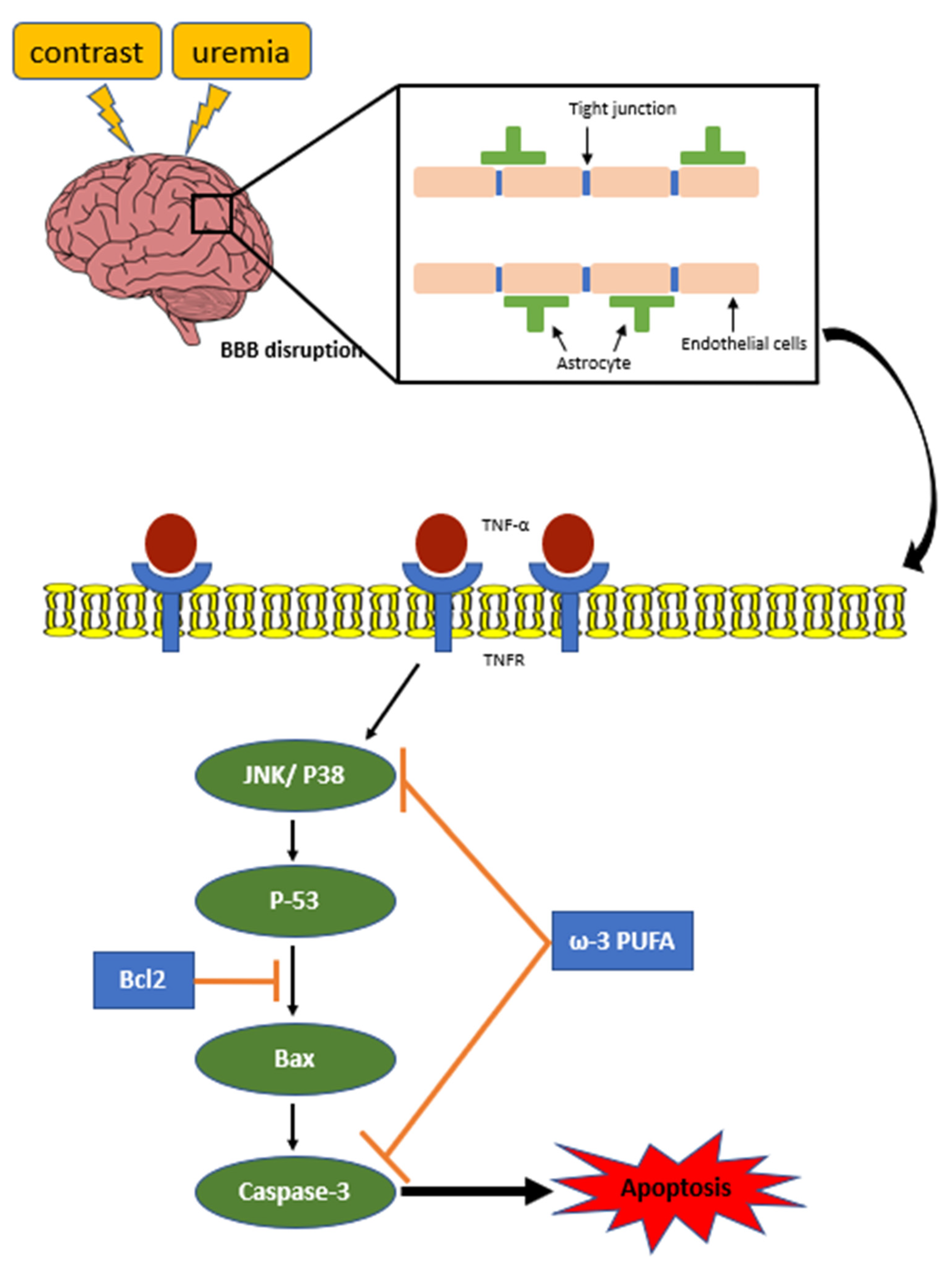
2.6. ω-3/Fat-1 Reduces JNK/p-38 Signaling in the BBB of Uremic Mice
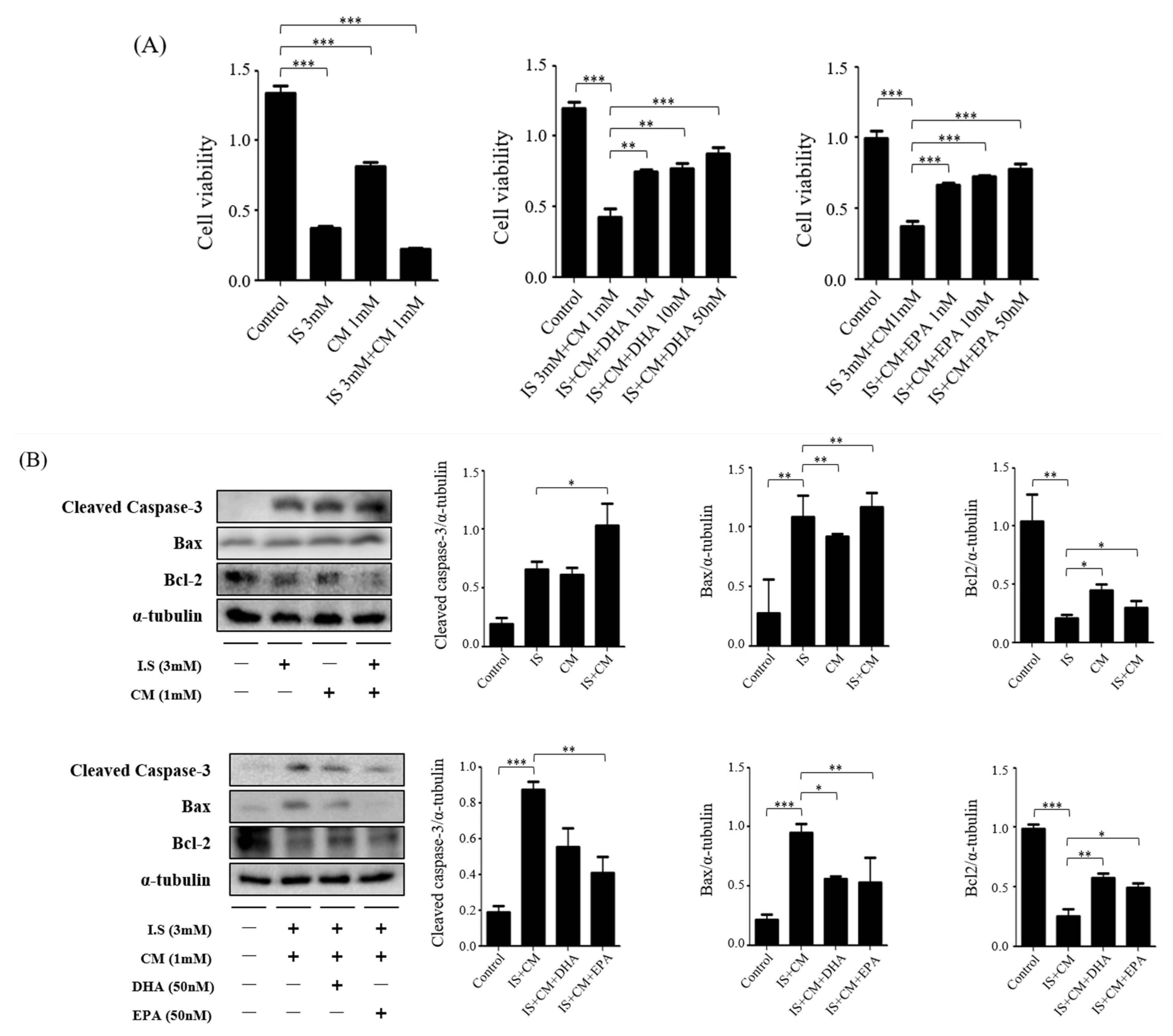
2.7. Survival Effect of ω-3 PUFAs in HBEC-5i Cells Treated with Indoxyl Sulfate (IS) and CM
2.8. Survival Effect of ω-3 PUFAs in HT22 Cells Treated with IS and CM
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Drug Treatment
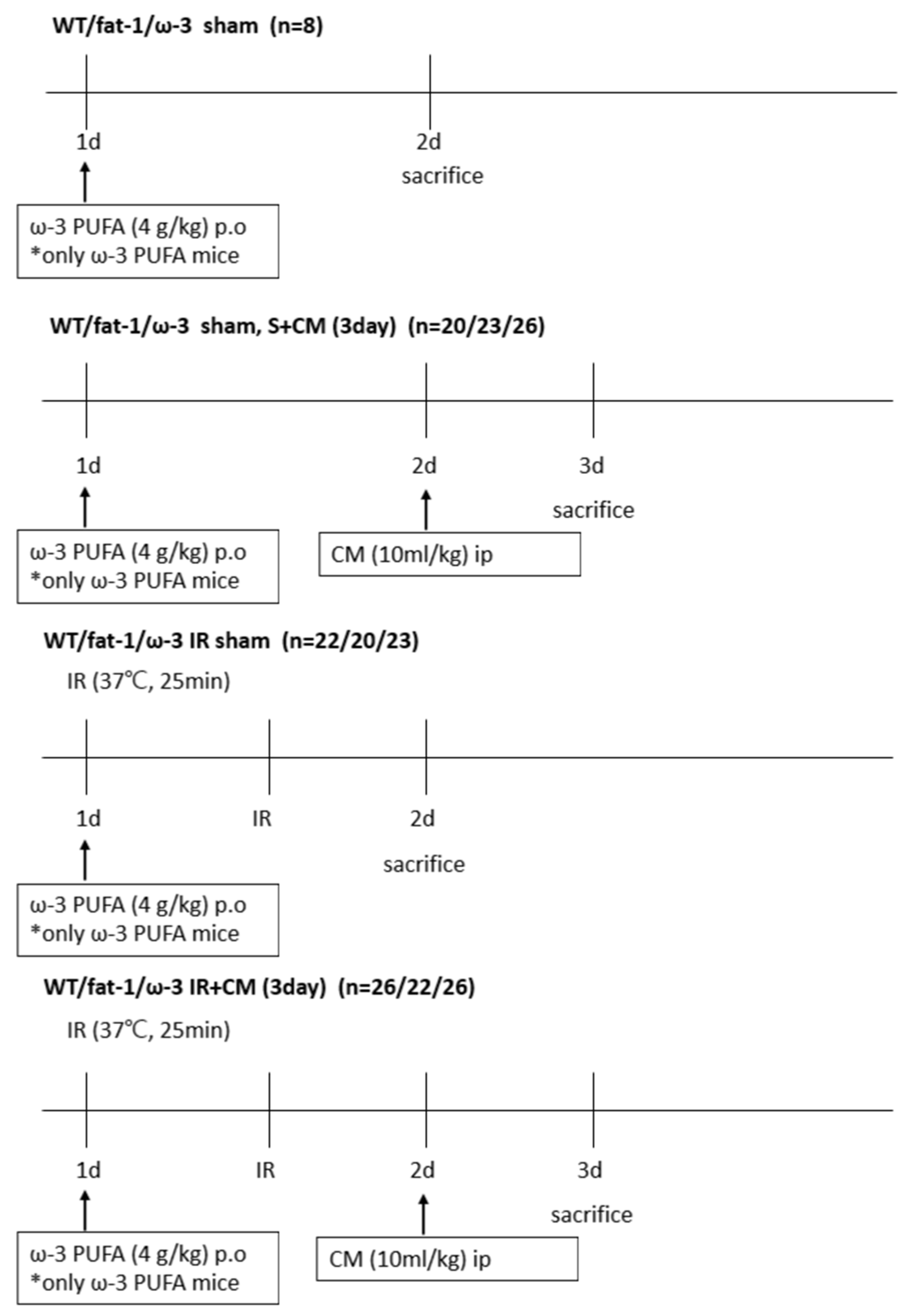
4.2. Animal Model
4.3. Blood and Tissue Preparation
4.4. Tissue-Injury Score
4.5. Western Blot Analysis
4.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Staining
4.7. Immunofluorescence Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Modi, K.; Padala, S.A.; Gupta, M. Contrast-Induced Nephropathy; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Junck, L.; Marshall, W.H. Neurotoxicity of radiological contrast agents. Ann. Neurol. 1983, 13, 469–484. [Google Scholar] [CrossRef]
- Stacul, F.; on behalf of the Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR); van der Molen, A.J.; Reimer, P.; Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.-F.; et al. Contrast induced nephropathy: Updated ESUR Contrast Media Safety Committee guidelines. Eur. Radiol. 2011, 21, 2527–2541. [Google Scholar] [CrossRef]
- Avsenik, J.; Bisdas, S.; Popovic, K.S. Blood-brain barrier permeability imaging using perfusion computed tomography. Radiol. Oncol. 2015, 49, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bobot, M.; Thomas, L.; Moyon, A.; Fernandez, S.; McKay, N.; Balasse, L.; Garrigue, P.; Brige, P.; Chopinet, S.; Poitevin, S.; et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020, 31, 1509–1521. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T. Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat. Rev. Nephrol. 2019, 15, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Ward, L.J.; Arefin, S.; Ebert, T.; Laucyte-Cibulskiene, A.; Pilote, L.; Norris, C.M.; Raparelli, V.; Kautzky-Willer, A.; Herrero, M.T.; et al. Blood–brain barrier and gut barrier dysfunction in chronic kidney disease with a focus on circulating biomarkers and tight junction proteins. Sci. Rep. 2022, 12, 4414. [Google Scholar] [CrossRef] [PubMed]
- Andone, S.; Balasa, R.; Barcutean, L.; Bajko, Z.; Ion, V.; Motataianu, A.; Stoian, A.; Maier, S. Contrast Medium-Induced Encephalopathy after Coronary Angiography–Case Report. J. Crit. Care Med. 2021, 7, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yan, L.; Zeng, H.; Chen, W.; Lu, J.-H.; Wan, J.-B.; Su, H.; Yao, X. Fish oil protects the blood–brain barrier integrity in a mouse model of Alzheimer’s disease. Chin. Med. 2020, 15, 29. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Neurological Diseases in Relation to the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef]
- Barnes, S.; Chowdhury, S.; Gatto, N.M.; Fraser, G.E.; Lee, G.J. Omega-3 fatty acids are associated with blood–brain barrier integrity in a healthy aging population. Brain Behav. 2021, 11, e2273. [Google Scholar] [CrossRef] [PubMed]
- Asih, P.R.; Prikas, E.; Stefanoska, K.; Tan, A.R.P.; Ahel, H.I.; Ittner, A. Functions of p38 MAP Kinases in the Central Nervous System. Front. Mol. Neurosci. 2020, 13, 570586. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-W.; Tu, Y.-F.; Huang, C.-C.; Ho, C.-J. JNK signaling is the shared pathway linking neuroinflammation, blood–brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflamm. 2012, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- BIN Park, G.; Choi, Y.; Kim, Y.S.; Lee, H.-K.; Kim, D.; Hur, D.Y. ROS-mediated JNK/p38-MAPK activation regulates Bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. Int. J. Oncol. 2014, 44, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 61. [Google Scholar] [CrossRef]
- Xu, F.; Song, Y.; Guo, A. Anti-Apoptotic Effects of Docosahexaenoic Acid in IL-1beta-Induced Human Chondrosarcoma Cell Death through Involvement of the MAPK Signaling Pathway. Cytogenet. Genome Res. 2019, 158, 17–24. [Google Scholar] [CrossRef]
- Lonergan, P.E.; Martin, D.S.; Horrobin, D.F.; Lynch, M.A. Neuroprotective effect of eicosapentaenoic acid in hippocampus of rats exposed to gamma-irradiation. J. Biol. Chem. 2002, 277, 20804–20811. [Google Scholar] [CrossRef]
- Xue, H.; Wan, M.; Song, D.; Li, Y.; Li, J. Eicosapentaenoic acid and docosahexaenoic acid modulate mitogen-activated protein kinase activity in endothelium. Vasc. Pharmacol. 2006, 44, 434–439. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, A.; Turczyn, P.; Frasuńska, J.; Paradowska-Gorycka, A.; Tarnacka, B. Significance of Omega-3 Fatty Acids in the Prophylaxis and Treatment after Spinal Cord Injury in Rodent Models. Mediat. Inflamm. 2020, 2020, 3164260. [Google Scholar] [CrossRef]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 polyunsaturated fatty acids suppress the inflammatory responses of lipopolysaccharide-stimulated mouse microglia by activating SIRT1 pathways. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Li, Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-kappaB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef]
- Pua, L.J.W.; Mai, C.-W.; Chung, F.F.-L.; Khoo, A.S.-B.; Leong, C.-O.; Lim, W.-M.; Hii, L.-W. Functional Roles of JNK and p38 MAPK Signaling in Nasopharyngeal Carcinoma. Int. J. Mol. Sci. 2022, 23, 1108. [Google Scholar] [CrossRef]
- Natale, G.; Calabrese, V.; Marino, G.; Campanelli, F.; Urciuolo, F.; de Iure, A.; Ghiglieri, V.; Calabresi, P.; Bossola, M.; Picconi, B. Effects of uremic toxins on hippocampal synaptic transmission: Implication for neurodegeneration in chronic kidney disease. Cell Death Discov. 2021, 7, 295. [Google Scholar] [CrossRef] [PubMed]
- Malek, M. Brain consequences of acute kidney injury: Focusing on the hippocampus. Kidney Res. Clin. Pract. 2018, 37, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sato, E.; Mishima, E.; Watanabe, M.; Abe, T.; Takahashi, N.; Nakayama, M. Effect of uremic toxins on hippocampal cell damage: Analysis in vitro and in rat model of chronic kidney disease. Heliyon 2021, 7, e06221. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-M.; Chuang, Y.-W.; Huang, S.-T.; Huang, J.-A.; Chen, C.-H.; Chung, M.-C.; Wu, C.-Y.; Chang, P.-Y.; Hsu, C.-C.; Wu, M.-J. Risk of Dementia after Exposure to Contrast Media: A Nationwide, Population-Based Cohort Study. Biomedicines 2022, 10, 2015. [Google Scholar] [CrossRef]
- Wilcox, J.; Sage, M.R.; Evill, C.A. Effect of intravenous contrast material on the integrity of the blood-brain barrier: Experimental study. Am. J. Neuroradiol. 1984, 5, 41–43. [Google Scholar]
- Liu, X.; Madhankumar, A.B.; Miller, P.A.; Duck, K.A.; Hafenstein, S.; Rizk, E.; Slagle-Webb, B.; Sheehan, J.M.; Connor, J.R.; Yang, Q.X. MRI contrast agent for targeting glioma: Interleukin-13 labeled liposome encapsulating gadolinium-DTPA. Neuro-Oncol. 2016, 18, 691–699. [Google Scholar] [CrossRef]
- Correale, J.; Villa, A. Cellular Elements of the Blood-Brain Barrier. Neurochem. Res. 2009, 34, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, J.; Chen, S.; Liu, G.; Wu, C.; Lv, Q.; Liao, H. Astrocytic p75[1] expression provoked by ischemic stroke exacerbates the blood-brain barrier disruption. Glia 2022, 70, 892–912. [Google Scholar] [CrossRef] [PubMed]
- Brunner, N.; Stein, L.; Cornelius, V.; Knittel, R.; Fallier-Becker, P.; Amasheh, S. Blood-Brain Barrier Protein Claudin-5 Expressed in Paired Xenopus laevis Oocytes Mediates Cell-Cell Interaction. Front. Physiol. 2020, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Nirwane, A.; Yao, Y. Laminins and their receptors in the CNS. Biol. Rev. Camb. Philos. Soc. 2019, 94, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, H.; Li, T.; Liu, J.; Zhao, L.; Sun, M.; Jian, Y. The Role of Blood-Brain Barrier Damage in the Pathogenesis of Contrast-Induced Encephalopathy. Arch. Gen. Intern. Med. 2018, 2, 34–40. [Google Scholar]
- Xie, Z.; Tong, S.; Chu, X.; Feng, T.; Geng, M. Chronic Kidney Disease and Cognitive Impairment: The Kidney-Brain Axis. Kidney Dis. 2022, 8, 275–285. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-kappaB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2017, 14, 143. [Google Scholar] [CrossRef]
- Kim, E.-J.; Ham, Y.R.; Shin, J.A.; Jeong, J.Y.; Na, K.R.; Lee, K.W.; Kim, J.-J.; Choi, D.E. Omega-3 Polyunsaturated Fatty Acid Attenuates Uremia-Induced Brain Damage in Mice. Int. J. Mol. Sci. 2021, 22, 11802. [Google Scholar] [CrossRef]
- Yatsushige, H.; Ostrowski, R.P.; Tsubokawa, T.; Colohan, A.; Zhang, J.H. Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J. Neurosci. Res. 2007, 85, 1436–1448. [Google Scholar] [CrossRef]
- Nito, C.; Kamada, H.; Endo, H.; Niizuma, K.; Myer, D.J.; Chan, P.H. Role of the p38 Mitogen-Activated Protein Kinase/Cytosolic Phospholipase A2 Signaling Pathway in Blood—Brain Barrier Disruption after Focal Cerebral Ischemia and Reperfusion. J. Cereb. Blood Flow Metab. 2008, 28, 1686–1696. [Google Scholar] [CrossRef]
- Stinghen, A.E.M.; Chillon, J.-M.; Massy, Z.A.; Boullier, A. Differential Effects of Indoxyl Sulfate and Inorganic Phosphate in a Murine Cerebral Endothelial Cell Line (bEnd.3). Toxins 2014, 6, 1742–1760. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Nakagawa, S.; Morofuji, Y.; Dohgu, S.; Watanabe, D.; Horie, N.; Izumo, T.; Niwa, M.; Walter, F.R.; Santa-Maria, A.R.; et al. MAP Kinase Pathways in Brain Endothelial Cells and Crosstalk with Pericytes and Astrocytes Mediate Contrast-Induced Blood–Brain Barrier Disruption. Pharmaceutics 2021, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- Versteilen, A.M.; Di Maggio, F.; Leemreis, J.R.; Groeneveld, A.B.; Musters, R.J.; Sipkema, P. Molecular mechanisms of acute renal failure following ischemia/reperfusion. Int. J. Artif. Organs 2004, 27, 1019–1029. [Google Scholar] [PubMed]
- Chang, Y.-K.; Choi, D.E.; Na, K.-R.; Lee, S.-J.; Suh, K.-S.; Kim, S.Y.; Shin, Y.-T.; Lee, K.W. Erythropoietin Attenuates Renal Injury in an Experimental Model of Rat Unilateral Ureteral Obstruction via Anti-Inflammatory and Anti-Apoptotic Effects. J. Urol. 2009, 181, 1434–1443. [Google Scholar] [CrossRef]
- Nazari, S.E.; Naimi, H.; Sayyed-Hosseinian, S.-H.; Vahedi, E.; Daghiani, M.; Asgharzadeh, F.; Askarnia-Faal, M.-M.; Avan, A.; Khazaei, M.; Hassanian, S.M. Effect of angiotensin II pathway inhibitors on post-surgical adhesion band formation: A potential repurposing of old drugs. Injury 2022, 53, 3642–3649. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.A.; Park, H.; Choi, H.; Chang, Y.-K.; Kim, J.-J.; Ham, Y.R.; Na, K.R.; Lee, K.W.; Choi, D.E. ω-3 Polyunsaturated Fatty Acids Improve the Blood–Brain-Barrier Integrity in Contrast-Induced Blood–Brain-Barrier Injury in Uremic Mice. Int. J. Mol. Sci. 2023, 24, 12168. https://doi.org/10.3390/ijms241512168
Shin JA, Park H, Choi H, Chang Y-K, Kim J-J, Ham YR, Na KR, Lee KW, Choi DE. ω-3 Polyunsaturated Fatty Acids Improve the Blood–Brain-Barrier Integrity in Contrast-Induced Blood–Brain-Barrier Injury in Uremic Mice. International Journal of Molecular Sciences. 2023; 24(15):12168. https://doi.org/10.3390/ijms241512168
Chicago/Turabian StyleShin, Jin Ah, Hyerim Park, Hyunsu Choi, Yoon-Kyung Chang, Jwa-Jin Kim, Young Rok Ham, Ki Ryang Na, Kang Wook Lee, and Dae Eun Choi. 2023. "ω-3 Polyunsaturated Fatty Acids Improve the Blood–Brain-Barrier Integrity in Contrast-Induced Blood–Brain-Barrier Injury in Uremic Mice" International Journal of Molecular Sciences 24, no. 15: 12168. https://doi.org/10.3390/ijms241512168
APA StyleShin, J. A., Park, H., Choi, H., Chang, Y.-K., Kim, J.-J., Ham, Y. R., Na, K. R., Lee, K. W., & Choi, D. E. (2023). ω-3 Polyunsaturated Fatty Acids Improve the Blood–Brain-Barrier Integrity in Contrast-Induced Blood–Brain-Barrier Injury in Uremic Mice. International Journal of Molecular Sciences, 24(15), 12168. https://doi.org/10.3390/ijms241512168






