Comparison between Cultivated Oral Mucosa and Ocular Surface Epithelia for COMET Patients Follow-Up
Abstract
:1. Introduction
2. Results
2.1. Gene Expression Profiling of Holoclones from Oral Mucosa, Limbus and Conjunctiva
2.2. PITX2 mRNA Is Overexpressed in Oral Mucosa Compared to Ocular Surface Tissues
2.3. Analysis of PITX2 Isoforms
2.4. Validation of the Results by In Situ Hybridization (ISH)
2.5. Validation of the Results at Protein Level
2.6. Phenotypic Characterization of the Patient after COMET
2.7. Angiogenic and Antiangiogenic Comparison between Oral Mucosa, Limbus and Conjunctiva
2.7.1. Proangiogenic Factors
2.7.2. Antiangiogenic Factors
3. Discussion
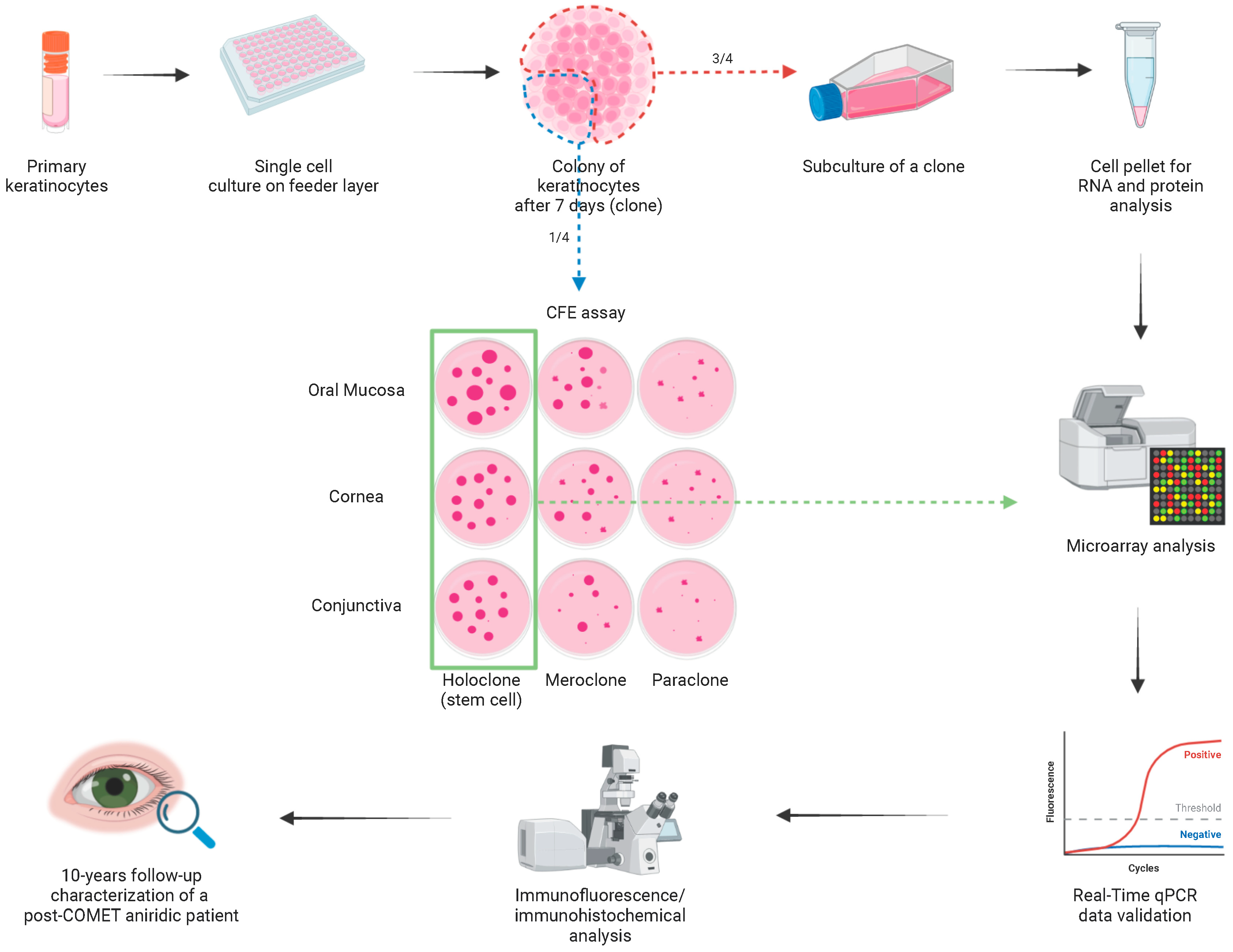
4. Materials and Methods
4.1. Patients and Specimens
4.2. COMET Transplantation
4.3. Cell Cultures
4.4. Clonal Analysis and Colony-Forming Efficiency Assay
4.5. Microarray Analyses
4.6. Real-Time PCR
4.7. In Situ Hybridization (ISH)
4.8. Immunofluorescence and Immunohistochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiavelli, C.; Attico, E.; Sceberras, V.; Fantacci, M.; Melonari, M.; Pellegrini, G. Stem cells and ocular regeneration. In Encyclopedia of Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 169–179. [Google Scholar]
- Sejpal, K.; Bakhtiari, P.; Deng, S.X. Presentation, Diagnosis and Management of Limbal Stem Cell Deficiency. Middle East Afr. J. Ophthalmol. 2013, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Adamo, D.; Attico, E.; Pellegrini, G. Education for the translation of Advanced Therapy Medicinal Products. Front. Med. 2023, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Ardigò, D.; Milazzo, G.; Iotti, G.; Guatelli, P.; Pelosi, D.; De Luca, M. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl. Med. 2018, 7, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Attico, E.; Sceberras, V.; Pellegrini, G. Approaches for Effective Clinical Application of Stem Cell Transplantation. Curr. Transplant. Rep. 2018, 5, 244–250. [Google Scholar] [CrossRef]
- Daya, S.M. Conjunctival-limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Kenyon, K.R.; Tseng, S.C.G. Limbal Autograft Transplantation for Ocular Surface Disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Maurizi, E.; Adamo, D.; Magrelli, F.M.; Galaverni, G.; Attico, E.; Merra, A.; Maffezzoni, M.B.R.; Losi, L.; Genna, V.G.; Sceberras, V.; et al. Regenerative Medicine of Epithelia: Lessons from the Past and Future Goals. Front. Bioeng. Biotechnol. 2021, 9, 652214. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; de Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Rama, P.; Matuska, S.; Lambiase, A.; Bonini, S.; Pocobelli, A.; Colabelli, R.G.; Spadea, L.; Fasciani, R.; Balestrazzi, E.; et al. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen. Med. 2013, 8, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Attico, E.; Galaverni, G.; Pellegrini, G. Clinical Studies of COMET for Total LSCD: A Review of the Methods and Molecular Markers for Follow-Up Characterizations. Curr. Ophthalmol. Rep. 2021, 9, 25–37. [Google Scholar] [CrossRef]
- Pellegrini, G. Changing the Cell Source in Cell Therapy? N. Engl. J. Med. 2004, 351, 1170–1172. [Google Scholar] [CrossRef]
- Soma, T.; Hayashi, R.; Sugiyama, H.; Tsujikawa, M.; Kanayama, S.; Oie, Y.; Nishida, K. Maintenance and Distribution of Epithelial Stem/Progenitor Cells after Corneal Reconstruction Using Oral Mucosal Epithelial Cell Sheets. PLoS ONE 2014, 9, e110987. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Yamato, M.; Nishida, K.; Okano, T. Evidence of the survival of ectopically transplanted oral mucosal epithelial stem cells after repeated wounding of cornea. Mol. Ther. 2014, 22, 1544–1555. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L.J. Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Attico, E.; Galaverni, G.; Bianchi, E.; Losi, L.; Manfredini, R.; Lambiase, A.; Rama, P.; Pellegrini, G. SOX2 Is a Univocal Marker for Human Oral Mucosa Epithelium Useful in Post-COMET Patient Characterization. Int. J. Mol. Sci. 2022, 23, 5785. [Google Scholar] [CrossRef]
- Kalabusheva, E.P.; Shtompel, A.S.; Rippa, A.L.; Ulianov, S.V.; Razin, S.V.; Vorotelyak, E.A. A Kaleidoscope of Keratin Gene Expression and the Mosaic of Its Regulatory Mechanisms. Int. J. Mol. Sci. 2023, 24, 5603. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Barrandon, Y.; Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 1987, 84, 2302–2306. [Google Scholar] [CrossRef]
- Enzo, E.; Cattaneo, C.; Consiglio, F.; Polito, M.P.; Bondanza, S.; De Luca, M. Clonal analysis of human clonogenic keratinocytes. Methods Cell Biol. 2022, 170, 101–116. [Google Scholar] [CrossRef]
- Vela, I.; Morrissey, C.; Zhang, X.; Chen, S.; Corey, E.; Strutton, G.M.; Nelson, C.C.; Nicol, D.L.; Clements, J.A.; Gardiner, E.M. PITX2 and non-canonical Wnt pathway interaction in metastatic prostate cancer. Clin. Exp. Metastasis 2013, 31, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, M.; Yam, G.H.-F.; Peh, G.S.; Colman, A.; Dunn, N.R.; Mehta, J.S. Directed differentiation of periocular mesenchyme from human embryonic stem cells. Differentiation 2018, 99, 62–69. [Google Scholar] [CrossRef]
- Yam, G.H.-F.; Seah, X.; Yusoff, N.Z.B.M.; Setiawan, M.; Wahlig, S.; Htoon, H.M.; Peh, G.S.L.; Kocaba, V.; Mehta, J.S. Characterization of Human Transition Zone Reveals a Putative Progenitor-Enriched Niche of Corneal Endothelium. Cells 2019, 8, 1244. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, Q.; Yu, X.; Merugu, S.B.; Mangukiya, H.B.; Smith, N.; Li, Z.; Zhang, B.; Negi, H.; Rong, R.; et al. Tumor-secreted anterior gradient-2 binds to VEGF and FGF2 and enhances their activities by promoting their homodimerization. Oncogene 2017, 36, 5098–5109. [Google Scholar] [CrossRef]
- Zhu, Q.; Mangukiya, H.B.; Mashausi, D.S.; Guo, H.; Negi, H.; Merugu, S.B.; Wu, Z.; Li, D. Anterior gradient 2 is induced in cutaneous wound and promotes wound healing through its adhesion domain. FEBS J. 2017, 284, 2856–2869. [Google Scholar] [CrossRef]
- Kase, S.; He, S.; Sonoda, S.; Kitamura, M.; Spee, C.; Wawrousek, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. αB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 2010, 115, 3398–3406. [Google Scholar] [CrossRef]
- Liu, L.; Qi, X.; Chen, Z.; Shaw, L.; Cai, J.; Smith, L.H.; Grant, M.B.; Boulton, M.E. Targeting the IRE1α/XBP1 and ATF6 arms of the unfolded protein response enhances VEGF blockade to prevent retinal and choroidal neovascularization. Am. J. Pathol. 2013, 182, 1412–1424. [Google Scholar] [CrossRef]
- Yang, W.-W.; Yang, L.-Q.; Zhao, F.; Chen, C.-W.; Xu, L.-H.; Fu, J.; Li, S.-L.; Ge, X.-Y. Epiregulin promotes lung metastasis of salivary adenoid cystic carcinoma. Theranostics 2017, 7, 3700–3714. [Google Scholar] [CrossRef] [PubMed]
- Semov, A.; Moreno, M.J.; Onichtchenko, A.; Abulrob, A.; Ball, M.; Ekiel, I.; Pietrzynski, G.; Stanimirovic, D.; Alakhov, V. Metastasis-associated protein S100A4 induces angiogenesis through interaction with annexin II and accelerated plasmin formation. J. Biol. Chem. 2005, 280, 20833–20841. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumian, N.; Klingelhöfer, J.; Grigorian, M.; Christensen, C.; Kriajevska, M.; Tulchinsky, E.; Georgiev, G.; Berezin, V.; Bock, E.; Rygaard, J.; et al. The metastasis-associated Mts1(S100A4) protein could act as an angiogenic factor. Oncogene 2001, 20, 4685–4695. [Google Scholar] [CrossRef]
- Lamagna, C.; Hodivala-Dilke, K.M.; Imhof, B.A.; Aurrand-Lions, M. Antibody against Junctional Adhesion Molecule-C Inhibits Angiogenesis and Tumor Growth. Cancer Res. 2005, 65, 5703–5710. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, H.; Chen, P.; Xie, L.; Wang, Y. Inhibitory Effect of Canstatin in Alkali Burn-Induced Corneal Neovascularization. Ophthalmic Res. 2011, 46, 66–72. [Google Scholar] [CrossRef]
- Okada, M.; Yamawaki, H. A current perspective of canstatin, a fragment of type IV collagen alpha 2 chain. J. Pharmacol. Sci. 2019, 139, 59–64. [Google Scholar] [CrossRef]
- Moore, J.E.; McMullen, T.C.B.; Campbell, I.L.; Rohan, R.; Kaji, Y.; Afshari, N.A.; Usui, T.; Archer, D.B.; Adamis, A.P. The Inflammatory Milieu Associated with Conjunctivalized Cornea and Its Alteration with IL-1 RA Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2905–2915. [Google Scholar]
- Ma, X.; Li, J. Corneal neovascularization suppressed by TIMP2 released from human amniotic membranes. Yan Ke Xue Bao Eye Sci. Yan Ke Xue Bao Bian Ji Bu 2005, 21, 56–61. [Google Scholar]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Sekiyama, E.; Nakamura, T.; Cooper, L.J.; Kawasaki, S.; Hamuro, J.; Fullwood, N.J.; Kinoshita, S. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1352–1358. [Google Scholar] [CrossRef]
- Ferrari, G.; Giacomini, C.; Bignami, F.; Moi, D.; Ranghetti, A.; Doglioni, C.; Naldini, L.; Rama, P.; Mazzieri, R. Angiopoietin 2 expression in the cornea and its control of corneal neovascularisation. Br. J. Ophthalmol. 2016, 100, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Van Grasdorff, S.; Wouters, K.; Rozema, J.; Koppen, C.; Lion, E.; Cools, N.; Berneman, Z.; Tassignon, M.-J. Human Tears Reveal Insights into Corneal Neovascularization. PLoS ONE 2012, 7, e36451. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.J.; Yeh, L.-K.; Tsai, Y.-J.; Lai, C.-H.; Chen, C.-C.; Lai, J.-Y.; Sun, C.-C.; Chang, G.; Hwang, T.-L.; Chen, J.-K.; et al. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5615–5623. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, S.; Nishida, K.; Yamato, M.; Hayashi, R.; Sugiyama, H.; Soma, T.; Maeda, N.; Okano, T.; Tano, Y. Analysis of angiogenesis induced by cultured corneal and oral mucosal epithelial cell sheets in vitro. Exp. Eye Res. 2007, 85, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Gaddipati, S.; Muralidhar, R.; Sangwan, V.S.; Mariappan, I.; Vemuganti, G.K.; Balasubramanian, D. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J. Ophthalmol. 2014, 62, 644–648. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.J.; Ryu, J.S.; Kim, Y.H.; Jeon, S.; Oh, J.Y.; Choung, H.K.; Khwarg, S.I.; Wee, W.R.; Kim, M.K. Prospective Clinical Trial of Corneal Reconstruction with Biomaterial-Free Cultured Oral Mucosal Epithelial Cell Sheets. Cornea 2018, 37, 76–83. [Google Scholar] [CrossRef]
- Henderson, T.R.M.; Coster, D.J.; Williams, K.A. The long term outcome of limbal allografts: The search for surviving cells. Br. J. Ophthalmol. 2001, 85, 604–609. [Google Scholar] [CrossRef]
- Williams, K.A.; Brereton, H.M.; Aggarwal, R.; Sykes, P.J.; Turner, D.R.; Russ, G.R.; Coster, D.J. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am. J. Ophthalmol. 1995, 120, 342–350. [Google Scholar] [CrossRef]
- Sjoqvist, S.; Kasai, Y.; Shimura, D.; Ishikawa, T.; Ali, N.; Iwata, T.; Kanai, N. Oral keratinocyte-derived exosomes regulate proliferation of fibroblasts and epithelial cells. Biochem. Biophys. Res. Commun. 2019, 514, 706–712. [Google Scholar] [CrossRef]
- Sjöqvist, S.; Ishikawa, T.; Shimura, D.; Kasai, Y.; Imafuku, A.; Bou-Ghannam, S.; Iwata, T.; Kanai, N. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J. Extracell. Vesicles 2019, 8, 1565264. [Google Scholar] [CrossRef]
- Xiao, Y.-T.; Xie, H.-T.; Liu, X.; Duan, C.-Y.; Qu, J.-Y.; Zhang, M.-C.; Zhao, X.-Y. Subconjunctival Injection of Transdifferentiated Oral Mucosal Epithelial Cells for Limbal Stem Cell Deficiency in Rats. J. Histochem. Cytochem. 2021, 69, 177–190. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, L.; Carulli, S.; Cocchiarella, F.; Quaglino, D.; Enzo, E.; Franchini, E.; Giannetti, A.; De Santis, G.; Recchia, A.; Pellegrini, G.; et al. Long-term stability and safety of transgenic cultured epidermal stem cells in gene therapy of junctional epidermolysis bullosa. Stem Cell Rep. 2014, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.-Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- DE Luca, M.; Albanese, E.; Megna, M.; Cancedda, R.I.; Mangiante, P.E.; Cadoni, A.; Franzi, A.T. Evidence that human oral epithelium reconstituted in vitro and transplanted onto patients with defects in the oral mucosa retains properties of the original donor site. Transplantation 1990, 50, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, T.; Nakamura, T.; Kojyo, M.; Koizumi, N.; Sotozono, C.; Kinoshita, S. Ocular Surface Reconstruction with Combination of Cultivated Autologous Oral Mucosal Epithelial Transplantation and Penetrating Keratoplasty. Am. J. Ophthalmol. 2006, 142, 757–764.e1. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Cooper, L.J.; Rigby, H.; Fullwood, N.J.; Kinoshita, S. Phenotypic Investigation of Human Eyes with Transplanted Autologous Cultivated Oral Mucosal Epithelial Sheets for Severe Ocular Surface Diseases. Ophthalmology 2007, 114, 1080–1088. [Google Scholar] [CrossRef]
- Chen, H.-C.J.; Chen, H.-L.; Lai, J.-Y.; Chen, C.-C.; Tsai, Y.-J.; Kuo, M.-T.; Chu, P.-H.; Sun, C.-C.; Chen, J.-K.; Ma, D.H.-K. Persistence of transplanted oral mucosal epithelial cells in human cornea. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4660–4668. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Lagali, N.; Wowra, B.; Fries, F.N.; Latta, L.; Moslemani, K.; Utheim, T.P.; Wylegala, E.; Seitz, B.; Käsmann-Kellner, B. Early phenotypic features of aniridia-associated keratopathy and association with PAX6 coding mutations. Ocul. Surf. 2020, 18, 130–140. [Google Scholar] [CrossRef]
- Latta, L.; Figueiredo, F.C.; Ashery-Padan, R.; Collinson, J.M.; Daniels, J.; Ferrari, S.; Szentmáry, N.; Solá, S.; Shalom-Feuerstein, R.; Lako, M.; et al. Pathophysiology of aniridia-associated keratopathy: Developmental aspects and unanswered questions. Ocul. Surf. 2021, 22, 245–266. [Google Scholar] [CrossRef]
- Gage, P.J.; Kuang, C.; Zacharias, A.L. The homeodomain transcription factor PITX2 is required for specifying correct cell fates and establishing angiogenic privilege in the developing cornea. Dev. Dyn. 2014, 243, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sun, Z.; Sweat, Y.; Sweat, M.; Venugopalan, S.R.; Eliason, S.; Cao, H.; Paine, M.L.; Amendt, B.A. Pitx2-Sox2-Lef-1 interactions specify progenitor oral/dental epithelial cell signaling centers. Development 2020, 147, dev186023. [Google Scholar] [CrossRef] [PubMed]
- Essner, J.J.; Branford, W.W.; Zhang, J.; Yost, H.J. Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development 2000, 127, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Gage, P.J.; Drouin, J.; A Camper, S. Pitx2 is required at multiple stages of pituitary organogenesis: Pituitary primordium formation and cell specification. Development 2002, 129, 329–337. [Google Scholar] [CrossRef]
- Liu, W.; Selever, J.; Lu, M.F.; Martin, J.F. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development 2003, 130, 6375–6385. [Google Scholar] [CrossRef]
- Sofyanti, E.; Boel, T.; Pratamawati, T.; Auerkari, E.I. Prediction of Regulatory Networks of PITX2 Gene Expression in Mandibular Asymmetry Related to Oral Muscle Function. In Proceedings of the International Dental Conference of Sumatera Utara 2017 (IDCSU 2017), Medan, Indonesia, 7–9 December 2017; Volume 8, pp. 88–92. [Google Scholar] [CrossRef]
- Franco, D.; Campione, M. The role of Pitx2 during Cardiac Development. Trends Cardiovasc. Med. 2003, 13, 157–163. [Google Scholar] [CrossRef]
- Martin, D.M.; Skidmore, J.M.; Philips, S.T.; Vieira, C.; Gage, P.J.; Condie, B.G.; Raphael, Y.; Martinez, S.; Camper, S. PITX2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev. Biol. 2004, 267, 93–108. [Google Scholar] [CrossRef]
- Chen, L.; Martino, V.; Dombkowski, A.; Williams, T.; West-Mays, J.; Gage, P.J. AP-2β is a downstream effector of PITX2 required to specify endothelium and establish angiogenic privilege during corneal development. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1072–1081. [Google Scholar] [CrossRef]
- Perveen, R.; Lloyd, I.C.; Clayton-Smith, J.; Churchill, A.; van Heyningen, V.; Hanson, I.; Taylor, D.; McKeown, C.; Super, M.; Kerr, B.; et al. Phenotypic Variability and Asymmetry of Rieger Syndrome Associated with PITX2 Mutations. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2456–2460. [Google Scholar]
- Zhang, J.-X.; Tong, Z.-T.; Yang, L.; Wang, F.; Chai, H.-P.; Zhang, F.; Xie, M.-R.; Zhang, A.-L.; Wu, L.-M.; Hong, H.; et al. PITX2: A promising predictive biomarker of patients’ prognosis and chemoradioresistance in esophageal squamous cell carcinoma. Int. J. Cancer 2013, 132, 2567–2577. [Google Scholar] [CrossRef]
- Semaan, A.; Uhl, B.; Branchi, V.; Lingohr, P.; Bootz, F.; Kristiansen, G.; Kalff, J.C.; Matthaei, H.; Pantelis, D.; Dietrich, D. Significance of PITX2 Promoter Methylation in Colorectal Carcinoma Prognosis. Clin. Color. Cancer 2018, 17, e385–e393. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.K.C.; Chan, D.W.; Liu, V.W.S.; Leung, T.H.Y.; Cheung, A.N.Y.; Ngan, H.Y.S. Increased expression of PITX2 transcription factor contributes to ovarian cancer progression. PLoS ONE 2012, 7, e37076. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guigon, C.J.; Fan, J.; Cheng, S.-Y.; Zhu, G.-Z. Pituitary homeobox 2 (PITX2) promotes thyroid carcinogenesis by activation of cyclin D2. Cell Cycle 2010, 9, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.J.; Espinoza, H.M.; McWilliams, B.; Chappell, K.; Morton, L.; Hjalt, T.A.; Semina, E.V.; Amendt, B.A. Differential regulation of gene expression by PITX2 isoforms. J. Biol. Chem. 2002, 277, 25001–25010. [Google Scholar] [CrossRef] [PubMed]
- Lamba, P.; Hjalt, T.A.; Bernard, D.J. Novel forms of Paired-like homeodomain transcription factor 2 (PITX2): Generation by alternative translation initiation and mRNA splicing. BMC Mol. Biol. 2008, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Sceberras, V.; Maria Magrelli, F.; Adamo, D.; Maurizi, E.; Attico, E.; Giuseppe Genna, V.; Lazzeri, M.; Barbagli, G.; Pellegrini, G. The cell as a tool to understand and repair urethra. In Scientific Advances in Reconstructive Urology and Tissue Engineering, 1st ed.; Hofer, M.D., Ed.; Elsevier: London, UK, 2022; Volume 1, pp. 1–24. [Google Scholar]
- Sceberras, V.; Attico, E.; Bianchi, E.; Galaverni, G.; Melonari, M.; Corradini, F.; Fantacci, M.; Ribbene, A.; Losi, L.; Balò, S.; et al. Preclinical study for treatment of hypospadias by advanced therapy medicinal products. World J. Urol. 2020, 38, 2115–2122. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]





| Epithelium | Strain | N. of Holoclones |
|---|---|---|
| Conjunctiva | CON-89 | 6 |
| CON-90 | 3 | |
| Limbus | LE-51 | 2 |
| LE-113 | 6 | |
| Oral Mucosa | MO-14 | 6 |
| MO-34 | 9 |
| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| PITX2 tot | CAGCCTGAGACTGAAAGCA | GCCCACGACCTTCTAGCAT |
| PITX2A | GCGTGTGTGCAATTAGAGAAAG | CCGAAGCCATTCTTGCATAG |
| PITX2B | GCCGTTGAATGTCTCTTCTC | CCTTTGCCGCTTCTTCTTAG |
| PITX2C | ACTTTCCGTCTCCGGACTTT | CGCGACGCTCTACTAGTC |
| GAPDH | GACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attico, E.; Galaverni, G.; Torello, A.; Bianchi, E.; Bonacorsi, S.; Losi, L.; Manfredini, R.; Lambiase, A.; Rama, P.; Pellegrini, G. Comparison between Cultivated Oral Mucosa and Ocular Surface Epithelia for COMET Patients Follow-Up. Int. J. Mol. Sci. 2023, 24, 11522. https://doi.org/10.3390/ijms241411522
Attico E, Galaverni G, Torello A, Bianchi E, Bonacorsi S, Losi L, Manfredini R, Lambiase A, Rama P, Pellegrini G. Comparison between Cultivated Oral Mucosa and Ocular Surface Epithelia for COMET Patients Follow-Up. International Journal of Molecular Sciences. 2023; 24(14):11522. https://doi.org/10.3390/ijms241411522
Chicago/Turabian StyleAttico, Eustachio, Giulia Galaverni, Andrea Torello, Elisa Bianchi, Susanna Bonacorsi, Lorena Losi, Rossella Manfredini, Alessandro Lambiase, Paolo Rama, and Graziella Pellegrini. 2023. "Comparison between Cultivated Oral Mucosa and Ocular Surface Epithelia for COMET Patients Follow-Up" International Journal of Molecular Sciences 24, no. 14: 11522. https://doi.org/10.3390/ijms241411522
APA StyleAttico, E., Galaverni, G., Torello, A., Bianchi, E., Bonacorsi, S., Losi, L., Manfredini, R., Lambiase, A., Rama, P., & Pellegrini, G. (2023). Comparison between Cultivated Oral Mucosa and Ocular Surface Epithelia for COMET Patients Follow-Up. International Journal of Molecular Sciences, 24(14), 11522. https://doi.org/10.3390/ijms241411522










