Topical Treatment for Retinal Degenerative Pathologies: A Systematic Review
Abstract
:1. Introduction
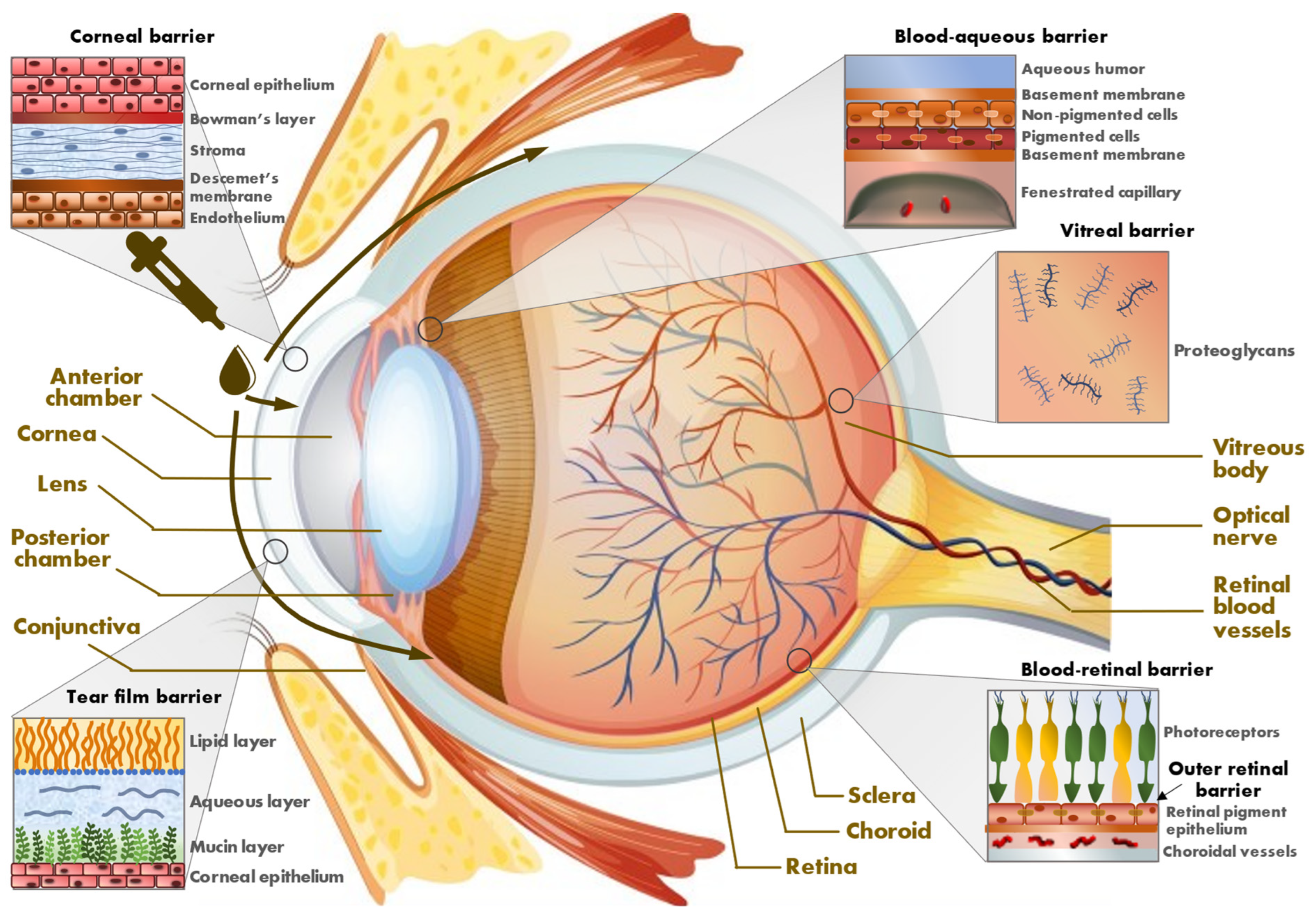
1.1. Eye Barriers
1.1.1. External Barriers
1.1.2. Internal Barriers
1.2. Drug Delivery for Retinal Pathology
2. Methods
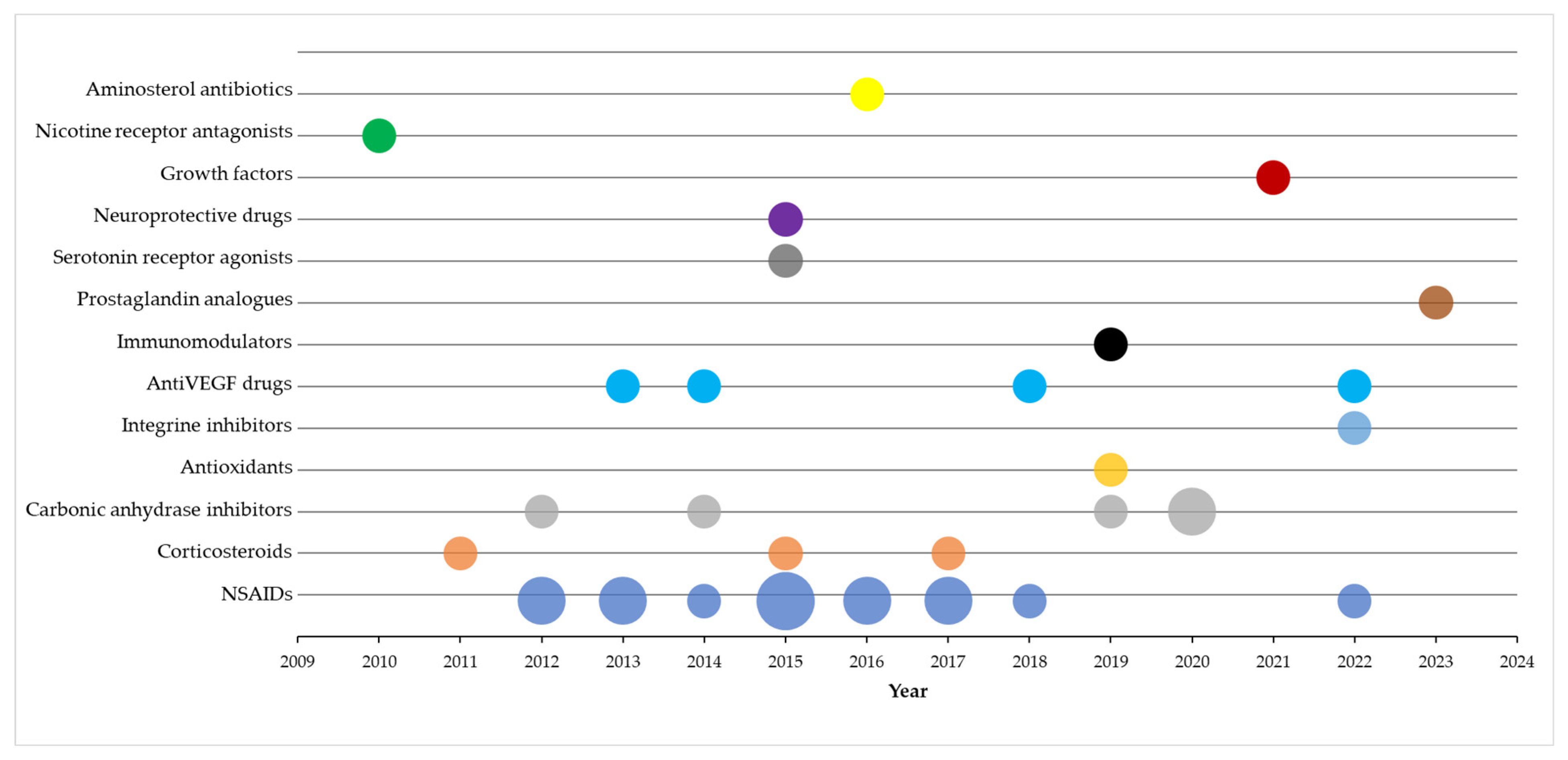
3. Results
4. Discussion
4.1. Anti-Inflammatory Drugs
4.2. Carbonic Anhydrase Inhibitors
4.3. Anti-VEGF
4.4. Integrin Inhibitors
4.5. Citicoline
4.6. Tandospirone
4.7. Recombinant Human Nerve Growth Factor
4.8. Prostaglandins
4.9. Coenzyme Q10
4.10. Mecamylamine
4.11. Squalamine
4.12. Interferons
4.13. Present Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Löscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ge, C.; Wang, D.; Xie, Q.; Wu, B.; Wang, J.; Nan, K.; Zheng, Q.; Chen, W. Overcoming the Anatomical and Physiological Barriers in Topical Eye Surface Medication Using a Peptide-Decorated Polymeric Micelle. ACS Appl. Mater. Interfaces 2019, 11, 39603–39612. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Van Santvliet, L.; Ludwig, A. Determinants of eye drop size. Surv. Ophthalmol. 2004, 49, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; Del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef]
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Liao, C.; Xu, J.; Chen, Y.; Ip, N.Y. Retinal Dysfunction in Alzheimer’s Disease and Implications for Biomarkers. Biomolecules 2021, 11, 1215. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agron, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; AREDS2 Research Group. Long-term Outcomes of Adding Lutein/Zeaxanthin and omega-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022, 140, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Straziota, C. CME after cataract surgery. The latest on what surgeons can do to crush cystoid macular edema. Ophthalmol. Manag. 2020, 24, 8. [Google Scholar]
- Del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Bodoki, A.E.; Iacob, B.-C.; Dinte, E.; Vostinaru, O.; Samoila, O.; Bodoki, E. Perspectives of Molecularly Imprinted Polymer-Based Drug Delivery Systems in Ocular Therapy. Polymers 2021, 13, 3649. [Google Scholar] [CrossRef] [PubMed]
- Karakahya, R.H.; Ozcan, T.S. Salvage of the retinal ganglion cells in transition phase in Alzheimer’s disease with topical coenzyme Q10, is it possible? Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 411–418. [Google Scholar] [CrossRef]
- Flaxel, C.; Schain, M.B.; Hamon, S.C.; Francis, P.J. Prospective randomized controlled trial of combination ranibizumab (Lucentis) and bromfenac (Xibrom) for neovascular age-related macular degeneration: A pilot study. Retina 2012, 32, 417–423. [Google Scholar] [CrossRef]
- Gomi, F.; Sawa, M.; Tsujikawa, M.; Nishida, K. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina 2012, 32, 1804–1810. [Google Scholar] [CrossRef]
- Turan-Vural, E.; Halili, E.; Serin, D. Assessing the effects of ketorolac and acetazolamide on macular thickness by optical coherence tomography following cataract surgery. Int. Ophthalmol. 2014, 34, 525–531. [Google Scholar] [CrossRef]
- Russo, A.; Costagliola, C.; Delcassi, L.; Romano, M.R.; Semeraro, F. A randomised controlled trial of ranibizumab with and without ketorolac eyedrops for exudative age-related macular degeneration. Br. J. Ophthalmol. 2013, 97, 1273–1276. [Google Scholar] [CrossRef]
- Wygledowska-Promienska, D.; Piotrowska-Gwozdz, A.; Piotrowska-Seweryn, A.; Mazur-Piotrowska, G.; Rokicki, W. Combination of bevacizumab and bromfenac therapy in age-related macular degeneration: A pilot study. Med. Sci. Monit. 2014, 20, 1168–1175. [Google Scholar]
- Friedman, S.M.; Almukhtar, T.H.; Baker, C.W.; Glassman, A.R.; Elman, M.J.; Bressler, N.M.; Maker, M.P.; Jampol, L.M.; Melia, M. Topical nepafenec in eyes with noncentral diabetic macular edema. Retina 2015, 35, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Tzelikis, P.F.; Vieira, M.; Hida, W.T.; Motta, A.F.; Nakano, C.T.; Nakano, E.M.; Alves, M.R. Comparison of ketorolac 0.4% and nepafenac 0.1% for the prevention of cystoid macular oedema after phacoemulsification: Prospective placebo-controlled randomised study. Br. J. Ophthalmol. 2015, 99, 654–658. [Google Scholar] [CrossRef]
- Yilmaz, U.; Kucuk, E.; Ulusoy, D.M.; Ozkose, A.; Atas, M.; Demircan, S.; Yuvaci, I. The assessment of changes in macular thickness in diabetic and non-diabetic patients: The effect of topical ketorolac on macular thickness change after ND:YAG laser capsulotomy. Cutan. Ocul. Toxicol. 2016, 35, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Ramezani, A.; Nikkhah, H.; Yaseri, M. The effect of topical sodium diclofenac on macular thickness in diabetic eyes after phacoemulsification: A randomized controlled trial. Int. Ophthalmol. 2017, 37, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, B.; Uzunel, U.D.; Kerci, S.G.; Sagban, L.; Kusbeci, T.; Orsel, T. Comparison of Subtenon Triamcinolone Acetonide Injection with Topical Nepafenac for the Treatment of Pseudophakic Cystoid Macular Edema. Ocul. Immunol. Inflamm. 2017, 25, 513–519. [Google Scholar] [CrossRef]
- McCafferty, S.; Harris, A.; Kew, C.; Kassm, T.; Lane, L.; Levine, J.; Raven, M. Pseudophakic cystoid macular edema prevention and risk factors; prospective study with adjunctive once daily topical nepafenac 0.3% versus placebo. BMC Ophthalmol. 2017, 17, 16. [Google Scholar] [CrossRef]
- Campa, C.; Salsini, G.; Perri, P. Comparison of the Efficacy of Dexamethasone, Nepafenac, and Bromfenac for Preventing Pseudophakic Cystoid Macular Edema: An Open-label, Prospective, Randomized Controlled Trial. Curr. Eye Res. 2018, 43, 362–367. [Google Scholar] [CrossRef]
- Tzelikis, P.F.; Morato, C.S.; Neves, N.T.; Hida, W.T.; Alves, M.R. Intraindividual comparison of nepafenac 0.3% for the prevention of macular edema after phacoemulsification. J. Cataract. Refract. Surg. 2018, 44, 440–446. [Google Scholar] [CrossRef]
- Howaidy, A.; Eldaly, Z.H.; Anis, M.; Othman, T.M. Prophylaxis of macular edema after cataract surgery in diabetic patients, topical Nepafenac versus intravitreal Ranibizumab. Eur. J. Ophthalmol. 2022, 32, 205–212. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Han, Y.S.; Mir, T.A.; Kherani, S.; Hafiz, G.; Krispel, C.; Alvin Liu, T.Y.; Wang, J.; Scott, A.W.; Zimmer-Galler, I. Increased Frequency of Topical Steroids Provides Benefit in Patients With Recalcitrant Postsurgical Macular Edema. Am. J. Ophthalmol. 2017, 178, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Hara, K.; Takai, Y.; Matsuoka, Y.; Nishimura, N.; Jansook, P.; Loftsson, T.; Stefansson, E.; Ohira, A. Topical dexamethasone-cyclodextrin microparticle eye drops for diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7944–7948. [Google Scholar] [CrossRef] [PubMed]
- Ohira, A.; Hara, K.; Johannesson, G.; Tanito, M.; Asgrimsdottir, G.M.; Lund, S.H.; Loftsson, T.; Stefansson, E. Topical dexamethasone gamma-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Hisatomi, T.; Yoshida, N.; Notomi, S.; Murakami, Y.; Enaida, H.; Ishibashi, T. The clinical efficacy of a topical dorzolamide in the management of cystoid macular edema in patients with retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Lemos Reis, R.F.; Moreira-Goncalves, N.; Estrela Silva, S.E.; Brandao, E.M.; Falcao-Reis, F.M. Comparison of topical dorzolamide and ketorolac treatment for cystoid macular edema in retinitis pigmentosa and Usher’s syndrome. Ophthalmologica 2015, 233, 43–50. [Google Scholar] [CrossRef]
- Tilma, K.K.; Bek, T. Dilatation of Retinal Arterioles Induced by Topical Dorzolamide for One Week Is Impaired in Patients with Type 1 Diabetes and Mild Retinopathy. Ophthalmologica 2020, 243, 236–242. [Google Scholar] [CrossRef]
- Liew, G.; Ho, I.V.; Ong, S.; Gopinath, B.; Mitchell, P. Efficacy of Topical Carbonic Anhydrase Inhibitors in Reducing Duration of Chronic Central Serous Chorioretinopathy. Transl. Vis. Sci. Technol. 2020, 9, 6. [Google Scholar] [CrossRef]
- Hsu, J.; Patel, S.N.; Wolfe, J.D.; Shah, C.P.; Chen, E.; Jenkins, T.L.; Wibbelsman, T.D.; Obeid, A.; Mikhail, M.; Garg, S.J.; et al. Effect of Adjuvant Topical Dorzolamide-Timolol vs Placebo in Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 560–567. [Google Scholar] [CrossRef]
- Boyer, D.S.; Kaiser, P.K.; Magrath, G.N.; Brady, K.; Edwards, S.; Tanzer, D.J.; Heier, J.S. The Safety and Biological Activity of OTT166, a Novel Topical Selective Integrin Inhibitor for the Treatment of Diabetic Eye Disease: A Phase 1b Study. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 553–560. [Google Scholar] [CrossRef]
- Danis, R.; McLaughlin, M.M.; Tolentino, M.; Staurenghi, G.; Ye, L.; Xu, C.F.; Kim, R.Y.; Johnson, M.W. Pazopanib eye drops: A randomised trial in neovascular age-related macular degeneration. Br. J. Ophthalmol. 2014, 98, 172–178. [Google Scholar] [CrossRef]
- Singh, R.; Wurzelmann, J.I.; Ye, L.; Henderson, L.; Hossain, M.; Trivedi, T.; Kelly, D.S. Clinical evaluation of pazopanib eye drops in healthy subjects and in subjects with neovascular age-related macular degeneration. Retina 2014, 34, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Wolf, S.; Kaiser, P.K.; Boyer, D.; Schmelter, T.; Sandbrink, R.; Zeitz, O.; Deeg, G.; Richter, A.; Zimmermann, T.; et al. The Developing Regorafenib Eye drops for neovascular Age-related Macular degeneration (DREAM) study: An open-label phase II trial. Br. J. Clin. Pharmacol. 2019, 85, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Poor, S.H.; Weissgerber, G.; Adams, C.M.; Bhatt, H.; Browning, D.J.; Chastain, J.; Ciulla, T.A.; Ferriere, M.; Gedif, K.; Glazer, L.C.; et al. A Randomized, Double-Masked, Multicenter Trial of Topical Acrizanib (LHA510), a Tyrosine Kinase VEGF-Receptor Inhibitor, in Treatment-Experienced Subjects With Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2022, 239, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Tawada, A.; Sugawara, T.; Ogata, K.; Hagiwara, A.; Yamamoto, S. Improvement of central retinal sensitivity six months after topical isopropyl unoprostone in patients with retinitis pigmentosa. Indian J. Ophthalmol. 2013, 61, 95–99. [Google Scholar]
- Jaffe, G.J.; Schmitz-Valckenberg, S.; Boyer, D.; Heier, J.; Wolf-Schnurrbusch, U.; Staurenghi, G.; Schmidt-Erfurth, U.; Holz, F.G. Randomized Trial to Evaluate Tandospirone in Geographic Atrophy Secondary to Age-Related Macular Degeneration: The GATE Study. Am. J. Ophthalmol. 2015, 160, 1226–1234. [Google Scholar] [CrossRef]
- Parisi, V.; Centofanti, M.; Ziccardi, L.; Tanga, L.; Michelessi, M.; Roberti, G.; Manni, G. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1327–1340. [Google Scholar] [CrossRef]
- Beykin, G.; Stell, L.; Halim, M.S.; Nunez, M.; Popova, L.; Nguyen, B.T.; Growth, S.L.; Dennis, A.; Li, Z.; Atkins, M.; et al. Corrigendum to Phase 1b Randomized Controlled Study of Short Course Topical Recombinant Human Nerve Growth Factor (rhNGF) for Neuroenhancement in Glaucoma: Safety, Tolerability, and Efficacy Measure Outcomes. Am. J. Ophthalmol. 2022, 234, 223–234. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Shah, S.M.; Hafiz, G.; Heier, J.S.; Lit, E.S.; Zimmer-Galler, I.; Channa, R.; Nguyen, Q.D.; Syed, B.; Do, D.V.; et al. Topical mecamylamine for diabetic macular edema. Am. J. Ophthalmol. 2010, 149, 839–851.e1. [Google Scholar] [CrossRef]
- Afarid, M.; Meshksar, A.; Salehi, A.; Safarpour, M.M. Evaluation of the Effect of Topical Interferon Alpha2b as a Complementary Treatment of Macular Edema of Patients with Diabetic Retinopathy: A Double-Blind Placebo-Controlled Randomized Clinical Trial Study. Retina 2020, 40, 936–942. [Google Scholar] [CrossRef]
- Wroblewski, J.J.; Hu, A.Y. Topical Squalamine 0.2% and Intravitreal Ranibizumab 0.5 mg as Combination Therapy for Macular Edema Due to Branch and Central Retinal Vein Occlusion: An Open-Label, Randomized Study. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 914–923. [Google Scholar] [CrossRef]
- Ross, A.E.; Bengani, L.C.; Tulsan, R.; Maidana, D.E.; Salvador-Culla, B.; Kobashi, H.; Kolovou, P.E.; Zhai, H.; Taghizadeh, K.; Kuang, L.; et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials 2019, 217, 119285. [Google Scholar] [CrossRef] [PubMed]
- Shikamura, Y.; Yamazaki, Y.; Matsunaga, T.; Sato, T.; Ohtori, A.; Tojo, K. Hydrogel Ring for Topical Drug Delivery to the Ocular Posterior Segment. Curr. Eye Res. 2016, 41, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A.; Pipkin, J.D.; Rork, G.; Sendo, T.; Finne, U.; Repta, A.J. Controlled drug delivery devices for experimental ocular studies with timolol 2. Ocular and systemic absorption in rabbits. Int. J. Pharm. 1990, 61, 241–249. [Google Scholar] [CrossRef]
- Acheampong, A.A.; Shackleton, M.; John, B.; Burke, J.; Wheeler, L.; Tang-Liu, D. Distribution of brimonidine into anterior and posterior tissues of monkey, rabbit, and rat eyes. Drug Metab. Dispos. 2002, 30, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Tomomatsu, T.; Matsumura, T.; Takihara, Y.; Kozai, S.; Arimura, S.; Yokota, S.; Inatani, M. Vitreous and aqueous concentrations of brimonidine following topical application of brimonidine tartrate 0.1% ophthalmic solution in humans. J. Ocul. Pharmacol. Ther. 2015, 31, 282–285. [Google Scholar] [CrossRef]
- Hu, S.; Koevary, S. Efficacy of Antibody Delivery to the Retina and Optic Nerve by Topical Administration. J. Ocul. Pharmacol. Ther. 2016, 32, 203–210. [Google Scholar] [CrossRef]
- Genead, M.A.; Fishman, G.A. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with retinitis pigmentosa and usher syndrome. Arch. Ophthalmol. 2010, 128, 1146–1150. [Google Scholar] [CrossRef]
- Chastain, J.E.; Sanders, M.E.; Curtis, M.A.; Chemuturi, N.V.; Gadd, M.E.; Kapin, M.A.; Markwardt, K.L.; Dahlin, D.C. Distribution of topical ocular nepafenac and its active metabolite amfenac to the posterior segment of the eye. Exp. Eye Res. 2016, 145, 58–67. [Google Scholar] [CrossRef]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef]
- Gaynes, B.I.; Onyekwuluje, A. Topical ophthalmic NSAIDs: A discussion with focus on nepafenac ophthalmic suspension. Clin. Ophthalmol. 2008, 2, 355–368. [Google Scholar] [CrossRef]
- Lindstrom, R.; Kim, T. Ocular permeation and inhibition of retinal inflammation: An examination of data and expert opinion on the clinical utility of nepafenac. Curr. Med. Res. Opin. 2006, 22, 397–404. [Google Scholar] [CrossRef]
- O’Brien, T.P. Emerging guidelines for use of NSAID therapy to optimize cataract surgery patient care. Curr. Med. Res. Opin. 2005, 21, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv. Ophthalmol. 2010, 55, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.; Raizman, M.; Ernest, P.; Gayton, J.; Lehmann, R. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J. Cataract Refract. Surg. 2007, 33, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.L.; Graff, G.; Spellman, J.M.; Yanni, J.M. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation 2000, 24, 371–384. [Google Scholar] [CrossRef]
- Kida, T.; Kozai, S.; Takahashi, H.; Isaka, M.; Tokushige, H.; Sakamoto, T. Pharmacokinetics and efficacy of topically applied nonsteroidal anti-inflammatory drugs in retinochoroidal tissues in rabbits. PLoS ONE. 2014, 9, e96481. [Google Scholar] [CrossRef]
- Gamache, D.A.; Graff, G.; Brady, M.T.; Spellman, J.M.; Yanni, J.M. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation 2000, 24, 357–370. [Google Scholar] [CrossRef]
- Bucci, F.A., Jr.; Waterbury, L.D.; Amico, L.M. Prostaglandin E2 inhibition and aqueous concentration of ketorolac 0.4% (acular LS) and nepafenac 0.1% (nevanac) in patients undergoing phacoemulsification. Am. J. Ophthalmol. 2007, 144, 146–147. [Google Scholar] [CrossRef]
- Nardi, M. Nepafenac in the Prevention and Treatment of Ocular Inflammation and Pain Following Cataract Surgery and in the Prevention of Post-operative Macular Oedema in Diabetic Patients. Eur. Ophthalmic Rev. 2012, 6, 169. [Google Scholar] [CrossRef]
- Kessel, L.; Tendal, B.; Jorgensen, K.J.; Erngaard, D.; Flesner, P.; Andresen, J.L.; Hjortdal, J. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: A systematic review. Ophthalmology 2014, 121, 1915–1924. [Google Scholar] [CrossRef]
- McGhee, C.N. Pharmacokinetics of ophthalmic corticosteroids. Br. J. Ophthalmol. 1992, 76, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.T.; Tran, T.; Lim, L.L.; Samarawickrama, C.; Arnold, J.; Gillies, M.; Catt, C.; Mitchell, L.; Symons, A.; Buttery, R.; et al. Local delivery of corticosteroids in clinical ophthalmology: A review. Clin. Exp. Ophthalmol. 2020, 48, 366–401. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Stefansson, E. Cyclodextrins in eye drop formulations: Enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol. Scand. 2002, 80, 144–150. [Google Scholar] [CrossRef]
- Kristinsson, J.K.; Fridriksdottir, H.; Thorisdottir, S.; Sigurdardottir, A.M.; Stefansson, E.; Loftsson, T. Dexamethasone-cyclodextrin-polymer co-complexes in aqueous eye drops. Aqueous humor pharmacokinetics in humans. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1199–1203. [Google Scholar]
- Loftsson, T.; Hreinsdottir, D.; Stefansson, E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007, 59, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.H.; Konraethsdottir, F.; Loftsson, T.; Stefansson, E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007, 85, 598–602. [Google Scholar] [CrossRef]
- Johannesson, G.; Moya-Ortega, M.D.; Asgrimsdottir, G.M.; Lund, S.H.; Thorsteinsdottir, M.; Loftsson, T.; Stefansson, E. Kinetics of gamma-cyclodextrin nanoparticle suspension eye drops in tear fluid. Acta Ophthalmol. 2014, 92, 550–556. [Google Scholar] [CrossRef]
- Jansook, P.; Ritthidej, G.C.; Ueda, H.; Stefansson, E.; Loftsson, T. yCD/HPyCD mixtures as solubilizer: Solid-state characterization and sample dexamethasone eye drop suspension. J. Pharm. Pharm. Sci. 2010, 13, 336–350. [Google Scholar] [CrossRef]
- Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnurch, A. Thiolated Hydroxypropyl-beta-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2612. [Google Scholar] [CrossRef]
- Niffenegger, J.H.; Fong, D.S.; Wong, K.L.; Modjtahedi, B.S. Treatment of Secondary Full-Thickness Macular Holes with Topical Therapy. Ophthalmol. Retin. 2020, 4, 695–699. [Google Scholar] [CrossRef]
- Mandelcorn, E.D.; Al-Falah, M.; Zhao, L.D.; Kertes, P.; Devenyi, R.; Lam, W.C. A prospective randomized clinical trial comparing nepafenac, intravitreal triamcinolone and no adjuvant therapy for epiretinal membrane. Acta Ophthalmol. 2022, 100, e297–e303. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.S.; Park, T.S.; Moon, Y.S.; Oh, J.H. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 2005, 25, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Edington, M.; Connolly, J.; Chong, N.V. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Naithani, P.; Puranik, S.; Vashisht, N.; Khanduja, S.; Kumar, S.; Garg, S. Role of topical nepafenac in prevention and treatment of macular edema after vitreoretinal surgery. Retina 2012, 32, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jansook, P.; Stefansson, E. Topical drug delivery to the eye: Dorzolamide. Acta Ophthalmol. 2012, 90, 603–608. [Google Scholar] [CrossRef]
- Sugrue, M.F. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog. Retin. Eye Res. 2000, 19, 87–112. [Google Scholar] [CrossRef]
- Kadam, R.S.; Jadhav, G.; Ogidigben, M.; Kompella, U.B. Ocular pharmacokinetics of dorzolamide and brinzolamide after single and multiple topical dosing: Implications for effects on ocular blood flow. Drug Metab. Dispos. 2011, 39, 1529–1537. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Banditt, P. Clinical Pharmacokinetics of Dorzolamide. Clin. Pharmacokinet. 2002, 41, 197–205. [Google Scholar] [CrossRef]
- Horita, S.; Watanabe, M.; Katagiri, M.; Nakamura, H.; Haniuda, H.; Nakazato, T.; Kagawa, Y. Species differences in ocular pharmacokinetics and pharmacological activities of regorafenib and pazopanib eye-drops among rats, rabbits and monkeys. Pharmacol. Res. Perspect. 2019, 7, e00545. [Google Scholar] [CrossRef]
- Butler, N.J.; Suhler, E.B.; Rosenbaum, J.T. Interferon alpha 2b in the treatment of uveitic cystoid macular edema. Ocul. Immunol. Inflamm. 2012, 20, 86–90. [Google Scholar] [CrossRef]
- Onodera, H.; Sasaki, S.; Otake, S.; Tomohiro, M.; Shibuya, K.; Nomura, M. Scientific viewpoints in ocular toxicity assessment: Departure from conventional practice. Anim. Eye Res. 2013, 32, 3–13. [Google Scholar]
- Sadeghi, A.; Puranen, J.; Ruponen, M.; Valtari, A.; Subrizi, A.; Ranta, V.P.; Toropainen, E.; Urtti, A. Pharmacokinetics of intravitreal macromolecules: Scaling between rats and rabbits. Eur. J. Pharm. Sci. 2021, 159, 105720. [Google Scholar] [CrossRef] [PubMed]
- Ladd, M.E.; Bachert, P.; Meyerspeer, M.; Moser, E.; Nagel, A.M.; Norris, D.G.; Schmitter, S.; Speck, O.; Straub, S.; Zaiss, M. Pros and cons of ultra-high-field MRI/MRS for human application. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 109, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Andronesi, O.; Bogner, W.; Choi, I.Y.; Coello, E.; Cudalbu, C.; Juchem, C.; Kemp, G.J.; Kreis, R.; Krssak, M.; et al. Minimum Reporting Standards for in vivo Magnetic Resonance Spectroscopy (MRSinMRS): Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4484. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef]
- Bodoki, E.; Vostinaru, O.; Samoila, O.; Dinte, E.; Bodoki, A.E.; Swetledge, S.; Astete, C.E.; Sabliov, C.M. Topical nanodelivery system of lutein for the prevention of selenite-induced cataract. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 188–197. [Google Scholar] [CrossRef]


| Drug Class | Representatives | Routes of Administration | Main Barrier to Target | Main Disadvantages |
|---|---|---|---|---|
| Antioxidants | Lutein, Zeaxanthin (& derivatives), Vitamins C and E | Systemic—oral | Blood-retinal | Low bioavailability, low intestinal absorption |
| Anti-inflammatory: NSAIDs | Bromfenac, Nepafenac | Topical | Corneal | Low bioavailability |
| Anti-inflammatory: Corticosteroids | Triamcinolone | IVT | Internal limiting membrane | Surgical procedure (invasive) |
| Metilprednisolone Dexamethasone | Oral or i.v. Subtenon injection i.v. | Blood-retinal Scleral Blood-retinal | Systemic adverse reactions, low bioavailability Invasive, low bioavailability, systemic adverse reactions Systemic adverse reactions, low bioavailability | |
| Anti-VEGF | Bevacizumab, Aflibercept, Brolucizumab, etc. | IVT | Internal limiting membrane | Surgical procedure (invasive) |
| Drug and Regimen | Proposed Mechanism | Pathology Studied | No. of Eyes Treated | Duration | Main Outcomes | Declared Success | Ref. |
|---|---|---|---|---|---|---|---|
| Bromfenac 2×/day, 12 months (plus Ranibizumab IVT, 1×/month—4 months, then as needed) | NSAID, Prostaglandin synthesis inhibitor | nAMD | 20 | 12 months | BCVA similar; Need of intravitreal injection similar; OCT-CRT lower than in control group ** | Partial | [17] |
| Bromfenac 2×/day, 6 months, (plus at least 1 dozen Ranibizumab IVT), versus sham (Ranibizumab, only) | NSAID Prostaglandin synthesis inhibitor | nAMD | 15 | 6 months | Need of intravitreal injection 2.2 versus 3.2; BCVA similar; OCT-CRT similar | Partial | [18] |
| Ketorolac 0.5%, versus Acetazolamide 250 mg/day, and control | NSAID Prostaglandin synthesis inhibitor | CME after cataract surgery | 27 | 1 month | BCVA, OCT-CRT better in Ketorolac and Acetazolamide group, versus control **; No difference Ketorolac versus Acetazolamide | Yes | [19] |
| Ketorolac 0.45%, 3×/day, 6 months plus Ranibizumab 0.5 mg IVT (1×/month—3 month, then on demand), versus Ranibizumab alone | NSAID Prostaglandin synthesis inhibitor | nAMD | 28 | 6 months | BCVA similar in the 2 groups; Need for IVT, similar; OCT-CRT—Ketorolac combination with greater reduction ** | Yes | [20] |
| Bromfenac 0.09% 2 × 1 (plus Bevacizumab 1.25 mg, IVT); control Bevacizumab 1.25 mg, IVT | NSAID Prostaglandin synthesis inhibitor | nAMD | 26 | 6 months | BCVA, OCT-CRT better than control (Bevacizumab only) ** | Yes | [21] |
| Nepafenac, 0.1%, 3×/day | NSAID Prostaglandin synthesis inhibitor | DME (noncentral) | 61 | 12 months | BCVA, OCT-CRT—no difference from baseline and versus placebo | No | [22] |
| Ketorolac 0.4% or Nepafenac 0.1% versus placebo | NSAID Prostaglandin synthesis inhibitor | CME after uncomplicated cataract surgery | 84 | 3 months | BCVA, OCT-CRT not different between groups; 2.1%, 2.4% and 2.9% of CME also at postoperative 4 weeks in the placebo, ketorolac and nepafenac groups, respectively | no | [23] * |
| Ketorolac 0.5% 4 × 1 (1 week) | NSAID Prostaglandin synthesis inhibitor | Macular edema after Nd:YAG laser capsulotomy | 44 | 6 months | OCT—CRT lower versus control ** (Fluorometholone 0.1%) | Yes | [24] |
| Diclofenac 0.1%, 4×/day, preop and 6 weeks after phacoemulsification | NSAID, Prostaglandin synthesis inhibitor | Diabetic cataract—profilaxy of CME after cataract surgery | 54 | 3 months | BCVA similar in treated versus not treated; OCT-CRT lower in treated ** | Partial | [25] |
| Nepafenac 0.1%, 2 × 1, versus Subtenon Triamcinolone. | NSAID Prostaglandin synthesis inhibitor; Decrease VEGF mRNA | CME after cataract surgery | 24 | 6 months | BCVA increase **; OCT -CRT decreased **; Effect better then control | Yes | [26] |
| Nepafenac 0.3%, 1×/day, 5 weeks, versus placebo | NSAID Prostaglandin synthesis inhibitor | CME after uncomplicated cataract surgery | 503 | 1.5 months | CME significantly reduced in patients with preoperative risk factors ** | Yes | [27] * |
| Nepafenac 0.1%, 3x/day, or Bromfenac 0.09%, 2×/day, Control Dexamethasone | NSAID Prostaglandin synthesis inhibitor | CME after uncomplicated cataract surgery | 96 | 1 month | No CME in treated groups (both Nepafenac and Bromfenac) BCVA, OCT-CRT similar in treated and controls | Partial | [28] |
| Nepafenac 0.3%, bilateral surgery (one eye treaded, the other eye placebo); 30 days between surgeries of the 2 eyes | NSAID Prostaglandin synthesis inhibitor | CME after uncomplicated cataract surgery | 112 | 3 months | OCT-CRT improved in treated, at 5 weeks **; No CME in treated, at 5 weeks (versus 3.57%) BCVA similar | Yes | [29] |
| Nepafenac 0.1%, 3 × 1/day, 4 weeks, versus 1 dose Ranibizumab 0.5 mg, IVT at surgery | NSAID Prostaglandin synthesis inhibitor | Diabetic cataract—prophylaxis of CME after cataract surgery | 38 | 3 months | OCT-CRT—preserved, same effect as Ranibizumab | Yes | [30] |
| Prednisolone acetonide high dose—every hour, versus low dose—4×/day (plus ketorolac) | Corticosteroid-Phospholipase A2 inhibitor. | CME after cataract surgery | 22 | 4 months | BCVA similar in high and low dose; OCT-CRT—similar in high and low dose | No | [31] * |
| Dexamethasone-Cyclodextrin Microparticles 1.5%, 3 × 1/day, or 6 × 1/day, 4 weeks | Corticosteroid-Phospholipase A2 inhibitor | DME | 19 | 2 months | BCVA, OCT-CRT improvement at week 4 **; BCVA returned to baseline at week 8, OCT-CRT remained decreased ** | Yes | [32] * |
| Dexamethasone-cyclodextrin nanoparticles 1.5%, 1×/day, or 2 × 1/day, or 3 × 1/day (1 month) | Corticosteroid-Phospholipase A2 inhibitor | DME | 12 | 3 months | BCVA improvement; OCT-CRT decreased. ** Effect comparable to Triamcinolone (subtenon injection) | Yes | [33] |
| Dorzolamide, 3×/day | Carbonic anhydrase inhibition | CME in retinitis pigmentosa | 16 | 6 months | OCT-CRT decrease in 81% **; Visual field improvement ** | Yes | [34] |
| Dorzolamide 2×/day; Ketorolac 0.5% 4×/day | Carbonic anhydrase inhibition; NSAID | CME in retinitis pigmentosa | 13 Dorzolamide 15 Ketorolac | 12 months | BCVA increase (both’s treatment; unclear result at 12 months for Dorzolamide); OCT-CRT unchanged | Partial | [35] |
| Dorzolamide (Trusopt), 2×/day, 7 days | Carbonic anhydrase inhibition | Normals; DR | 41 (20 with diabetic retinopathy) | 7 days | Dynamic vessel analyzer -Dilated vessels in normal subjects ** -No effect in diabetic | Yes | [36] |
| Dorzolamide (Trusopt), 4×/day, 3 months | Carbonic anhydrase inhibition; may improve subretinal fluid absorption through the RPE | Chronic Central Serous Chorioretinopathy | 18 | 3 months | BCVA similar to controls; OCT-CRT decreased **; Subretinal fluid resolution (77.8% versus 40% in controls) ** | Yes | [37] |
| Dorzolamide-Timolol, 2×/day, versus placebo (in parallel to antiVEGF IVT regimen, as needed) | Carbonic anhydrase inhibition; beta-blocker | nAMD | 27 | 3 months | OCT-CRT lower in treated **; BCVA similar | Yes | [38] * |
| Coenzyme Q10 0.1%, 2×/day, 6 months | Reduction of mitochondrial disfunction, oxidative stress, chronic neuro-inflammation | Alzheimer’s disease with visual function deteriorations | 30 | 6 months | OCT RNFL increase ** | Yes | [16] |
| Integrin inhibitor (OTT166, 2.5/5%), 2×/day, 28 days | Decrease angiogenesis, exudation, inflammation, and fibrosis (RGD binding integrins including αvβ3, αvβ6, and αvβ8) | Diabetic retinopathy; DME | 44 | 2 months | OCT-CRT reduction in 37% (responders) ** | Partial | [39] * |
| Pazopanib, 5 mg/mL, 3 × 1/day, or 1 × 1/day, or 2 mg/mL 3 × 1/day, 28 days1 | Multitarget tyrosine kinase inhibitor—all VEGF receptor subtypes, and platelet-derived growth factor | nAMD | 68 | 1 months | BCVA improvement in 5 mg/mL 3 × 1/day group **; OCT-CRT improvement in CFH T allele genotype subset of AMD ** | Yes | [40] * |
| Pazopanib 10 mg/mL, 4 × 2/day, or 4 × 1/day, 2 weeks (study 1), 4 × 1/day, 12 weeks (study 2), versus placebo | Multitarget tyrosine kinase inhibitor—all VEGF receptor subtypes, and platelet-derived growth factor | nAMD | 34 (study 1) 19 (study 2) | 3 months | Well tolerated; BCVA, OCT-CRT not changed; Study 2–9 patients with rescue therapy (IVT) | No | [41] |
| Regorafenib, 25 μL, 30 mg/mL, 3×/day, 3 months | Multikinase inhibitor—VEGF receptor 2/3, and platelet-derived growth factor receptor β | nAMD | 51 | 3 months | BCVA decreased **; rescue IVT in 20 patients | No | [42] * |
| Acrizanib (LHA510) 2%, 2 × 1/day—8 weeks, then 3 × 1/day—4 weeks | Tyrosine kinase-VEGF receptor inhibitor | nAMD | 33 | 3 months | OCT- macular fluid accumulation; Ranibizumab IVT (rescue) needed same as in placebo group | No | [43] |
| Isopropyl Unoprostone (Rescula), 2×/day | Increased retinal and choroidal circulation; Neuroprotection | Retinitis pigmentosa | 30 | 6 months | Microperimetry improved; ** BCVA improved ** | Yes | [44] |
| Tandospirone 1%, or 1.75%, 2×/day, versus vehicle | Neuroprotection (agonist on 5-HT1A receptor) | GA-AMD | 508 | 30 months | Lesion growths similar—1.73, 1.76, and 1.71 mm2 for 1.0%, 1.75% and vehicle | No | [45] * |
| Citicoline, 3×/day, versus beta-blockers | Neuroenhancement (stabilizes cell membranes by increasing phosphatidylcholine and sphingomyelin synthesis). | Glaucoma | 24 | 4 months | VEP, ERG—increased amplitudes, shortened latency **; Values returned to baseline 2 months after Citicoline washout | Yes | [46] |
| Recombinant Human Nerve Growth Factor (rhNGF), 180 μg/mL, 3×/day—8 weeks, versus vehicle control | Neuroenhancement, retinal ganglion cell survival. | Glaucoma | 40 | 8 months | No adverse reactions; OCT-RNFL and visual field, similar to control | No | [47] * |
| Mecamylamine 1%, 2×/day | Nonspecific nicotine receptor antagonist | DME | 21 | 4 months | 8 eyes—improvement (BCVA, OCT-CRT) 4 eyes—worse (BCVA, OCT-CRT) | Mixed | [48] * |
| Interferon α2b 1 million U/mL, 4×/day, 4 weeks | Antiproliferative antiangiogenic and immunomodulatory properties. | DME | 25 | 2 months | BCVA increased; OCT-CRT decreased (statistically non-significant, versus placebo—artificial tears) | Partial (objective criteria not met) | [49] |
| Squalamine 2×/day, 10 weeks, plus Ranibizumab 0.5 mg IVT as needed. After week 10, 2 groups were formed (continued Squalamine 2×/day, or not) | Angiostatic aminosterol; Inhibition of VEGF, PDGF, basic fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF)—impact on endothelial cell, angiogenesis | CME in Retinal vein occlusion | 20 | 6 months | Combination therapy improved BCVA outcome; Squalamine alone did not improved CME | Partial | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoilă, L.; Voștinaru, O.; Dinte, E.; Bodoki, A.E.; Iacob, B.-C.; Bodoki, E.; Samoilă, O. Topical Treatment for Retinal Degenerative Pathologies: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 8045. https://doi.org/10.3390/ijms24098045
Samoilă L, Voștinaru O, Dinte E, Bodoki AE, Iacob B-C, Bodoki E, Samoilă O. Topical Treatment for Retinal Degenerative Pathologies: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(9):8045. https://doi.org/10.3390/ijms24098045
Chicago/Turabian StyleSamoilă, Lăcrămioara, Oliviu Voștinaru, Elena Dinte, Andreea Elena Bodoki, Bogdan-Cezar Iacob, Ede Bodoki, and Ovidiu Samoilă. 2023. "Topical Treatment for Retinal Degenerative Pathologies: A Systematic Review" International Journal of Molecular Sciences 24, no. 9: 8045. https://doi.org/10.3390/ijms24098045





