Unravelling the Therapeutic Potential of Antibiotics in Hypoxia in a Breast Cancer MCF-7 Cell Line Model
Abstract
:1. Introduction
2. Results
2.1. Antibiotics Do Not Affect Proliferation of Cancer Cells in Anchorage-Dependent Culture
2.2. Antibiotics Affect Mammosphere Formation under Hypoxia and Normoxia
2.3. Antibiotics Affect ALDH-Bright Cell Number under Hypoxia and Normoxia
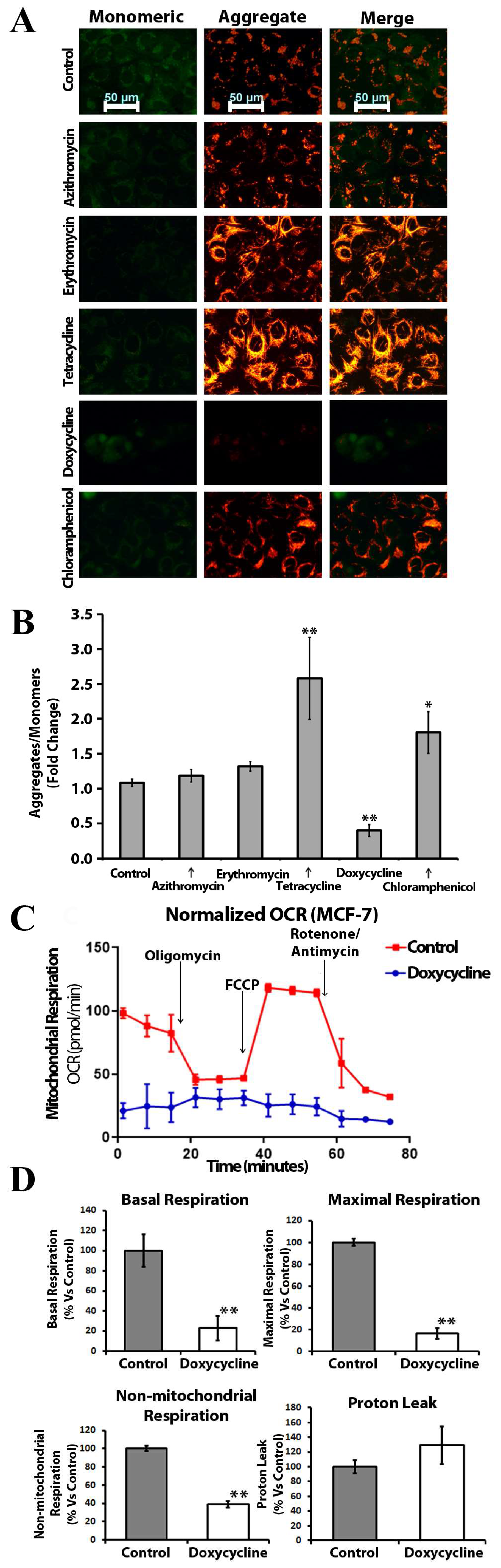
2.4. Doxycycline, Tetracycline, and Chloramphenicol Affect Mitochondrial Membrane Polarization and Inhibit Mitochondrial Metabolism
2.5. Anchorage-Independent Long-Term Culture with Tetracycline, Doxycycline, and Chloramphenicol Leads to an Increase in ABCG2 Protein Level in Normoxia
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Sphere Formation Assay
4.3. Western-Blot Analysis
4.4. Cell Proliferation Assay
4.5. Mitochondrial Membrane Potential Measurement
4.6. Evaluation of Mitochondrial Function
4.7. ALDEFLUOR Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.M.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [Green Version]
- Di Tomaso, T.; Mazzoleni, S.; Wang, E.; Sovena, G.; Clavenna, D.; Franzin, A.; Mortini, P.; Ferrone, S.; Doglioni, C.; Marincola, F.M.; et al. Immunobiological Characterization of Cancer Stem Cells Isolated from Glioblastoma Patients. Clin. Cancer Res. 2010, 16, 800–813. [Google Scholar] [CrossRef] [Green Version]
- Tirino, V.; Desiderio, V.; Paino, F.; De Rosa, A.; Papaccio, F.; La Noce, M.; Laino, L.; De Francesco, F.; Papaccio, G. Cancer stem cells in solid tumors: An overview and new approaches for their isolation and characterization. FASEB J. 2013, 27, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Choi, M.; Margineantu, D.; Margaretha, L.; Hesson, J.; Cavanaugh, C.; Blau, C.A.; Horwitz, M.S.; Hockenbery, D.; Ware, C.; et al. HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012, 31, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Prigione, A.; Fauler, B.; Lurz, R.; Lehrach, H.; Adjaye, J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells 2010, 28, 721–733. [Google Scholar] [CrossRef]
- Liu, P.P.; Liao, J.; Tang, Z.J.; Wu, W.J.; Yang, J.; Zeng, Z.L.; Hu, Y.; Wang, P.; Ju, H.Q.; Xu, R.H.; et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ. 2014, 21, 124–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciavardelli, D.; Rossi, C.; Barcaroli, D.; Volpe, S.; Consalvo, A.; Zucchelli, M.; De Cola, A.; Scavo, E.; Carollo, R.; D’Agostino, D.; et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014, 5, e1336. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian Cancer Spheroid Cells with Stem Cell-Like Properties Contribute to Tumor Generation, Metastasis and Chemotherapy Resistance through Hypoxia-Resistant Metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Fiorillo, M.; Peiris-Pagès, M.; Ozsvari, B.; Smith, D.L.; Sanchez-Alvarez, R.; Martinez-Outschoorn, U.E.; Cappello, A.R.; Pezzi, V.; Lisanti, M.P.; et al. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget 2015, 6, 14777–14795. [Google Scholar] [CrossRef] [Green Version]
- Grazia Cipolleschi, M.; Marzi, I.; Santini, R.; Fredducci, D.; Cristina Vinci, M.; D’Amico, M.; Rovida, E.; Stivarou, T.; Torre, E.; Dello Sbarba, P.; et al. Hypoxia-resistant profile implies vulnerability of cancer stem cells to physiological agents, which suggests new therapeutic targets. Cell Cycle 2014, 13, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, M.; Sotgia, F.; Lisanti, M.P. “Energetic” Cancer Stem Cells (e-CSCs): A New Hyper-Metabolic and Proliferative Tumor Cell Phenotype, Driven by Mitochondrial Energy. Front. Oncol. 2019, 8, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koc, E.C.; Burkhart, W.; Blackburn, K.; Moyer, M.B.; Schlatzer, D.M.; Moseley, A.; Spremulli, L.L. The large subunit of the mammalian mitochondrial ribosome. Analysis of the complement of ribosomal proteins present. J. Biol. Chem. 2001, 276, 43958–43969. [Google Scholar] [CrossRef] [Green Version]
- Stefano, G.B.; Samuel, J.; Kream, R.M. Antibiotics May Trigger Mitochondrial Dysfunction Inducing Psychiatric Disorders. Med. Sci. Monit. 2017, 23, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, A.J.; Ponzoni, M.; Guidoboni, M.; Resti, A.G.; Politi, L.S.; Cortelazzo, S.; Demeter, J.; Zallio, F.; Palmas, A.; Muti, G.; et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: A multicenter prospective trial. J. Natl. Cancer Inst. 2006, 98, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, R.; Harrison, H.; Hulit, J.; Smith, D.L.; Lisanti, M.P.; Sotgia, F. Mitochondria as new therapeutic targets for eradicating cancer stem cells: Quantitative proteomics and functional validation via MCT1/2 inhibition. Oncotarget 2014, 5, 11029–11037. [Google Scholar] [CrossRef] [Green Version]
- Lamb, R.; Ozsvari, B.; Lisanti, C.L.; Tanowitz, H.B.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget 2015, 6, 4569–4584. [Google Scholar] [CrossRef] [Green Version]
- Han, J.J.; Kim, T.M.; Jeon, Y.K.; Kim, M.K.; Khwarg, S.I.; Kim, C.W.; Kim, I.H.; Heo, D.S. Long-term outcomes of first-line treatment with doxycycline in patients with previously untreated ocular adnexal marginal zone B cell lymphoma. Ann. Hematol. 2015, 94, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Ponzoni, M.; Guidoboni, M.; De Conciliis, C.; Resti, A.G.; Mazzi, B.; Lettini, A.A.; Demeter, J.; Dell’Oro, S.; Doglioni, C.; et al. Regression of ocular adnexal lymphoma after Chlamydia psittaci-eradicating antibiotic therapy. J. Clin. Oncol. 2005, 23, 5067–5073. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.J.; Yao, D.E.; Zhuang, Y.F.; Hong, Y.; Zhu, X.C.; Fang, Z.R.; Yu, J.; Yu, Z.Y. Azithromycin enhances the favorable results of paclitaxel and cisplatin in patients with advanced non-small cell lung cancer. Genet. Mol. Res. 2014, 13, 2796–2805. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Ullah, A.; Leong, S.W.; Wang, J.; Wu, Q.; Ghauri, M.A.; Sarwar, A.; Su, Q.; Zhang, Y. Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1α interaction in lung cancer. Cell Death Dis. 2021, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anti-Cancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Ginouvès, A.; Ilc, K.; Macías, N.; Pouysségur, J.; Berra, E. PHDs overactivation during chronic hypoxia “desensitizes” HIFα and protects cells from necrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 4745–4750. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, C.; Scala, S.; Sozzi, G.; Roz, L.; Bertolini, G. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin. Cancer Biol. 2020, 60, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H. Chemotherapy-induced metastasis: Mechanisms and translational opportunities. Clin. Exp. Metastasis 2018, 35, 269–284. [Google Scholar] [CrossRef]
- Duivenvoorden, W.C.; Hirte, H.W.; Singh, G. Use of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cells. Invasion Metastasis 1997, 17, 312–322. [Google Scholar]
- Van den Bogert, C.; van Kernebeek, G.; de Leij, L.; Kroon, A.M. Inhibition of mitochondrial protein synthesis leads to proliferation arrest in the G1-phase of the cell cycle. Cancer Lett. 1986, 32, 41–51. [Google Scholar] [CrossRef]
- Iwasaki, H.; Inoue, H.; Mitsuke, Y.; Badran, A.; Ikegaya, S.; Ueda, T. Doxycycline induces apoptosis by way of caspase-3 activation with inhibition of matrix metalloproteinase in human T-lymphoblastic leukemia CCRF-CEM cells. J. Lab. Clin. Med. 2002, 140, 382–386. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, C.E.; Chen, W.L.; Tang, Y.Y.; Nie, J.Y.; Shen, L.D.; Ma, X.; Chen, D.D. Opposite response to hypoxia by breast cancer cells between cell proliferation and cell migration: A clue from microRNA expression profile. Oncol. Lett. 2018, 15, 2771–2780. [Google Scholar] [CrossRef] [Green Version]
- Hubbi, M.E.; Semenza, G.L. Regulation of cell proliferation by hypoxia-inducible factors. Am. J. Physiol. Cell Physiol. 2015, 309, C775–C782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, V.; Terzuoli, E.; Donnini, S.; Giachetti, A.; Morbidelli, L.; Ziche, M. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1alpha/VEGF signalling in MCF-7 breast cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 311. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 3904645. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Lo, M.C.; Moody, R.R.; Stevers, N.O.; Tinsley, S.L.; Sun, D. Doxycycline targets aldehyde dehydrogenase-positive breast cancer stem cells. Oncol. Rep. 2018, 39, 3041–3047. [Google Scholar] [CrossRef] [Green Version]
- Li, G.B.; Fu, R.Q.; Shen, H.M.; Zhou, J.; Hu, X.Y.; Liu, Y.X.; Li, Y.N.; Zhang, H.W.; Liu, X.; Zhang, Y.H.; et al. Polyphyllin I induces mitophagic and apoptotic cell death in human breast cancer cells by increasing mitochondrial PINK1 levels. Oncotarget 2017, 8, 10359–10374. [Google Scholar] [CrossRef] [Green Version]
- Mo, W.; Zhang, J.T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1–27. [Google Scholar]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Tahan, S.R.; Neuberg, D.S.; Dieffenbach, A.; Yacoub, L. Prediction of early relapse and shortened survival in patients with breast cancer by proliferating cell nuclear antigen score. Cancer 1993, 71, 3552–3559. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.W.; Wu, J.H.; Jiang, C.P. ABCG2: A potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010, 86, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bibi, M.; Min, P.; Deng, W.; Zhang, Y.; Du, J. SOX2 promotes hypoxia-induced breast cancer cell migration by inducing NEDD9 expression and subsequent activation of Rac1/HIF-1α signaling. Cell. Mol. Biol. Lett. 2019, 24, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, C.; Wang, C.; Wang, L.; Ma, M.; Niu, C.; Sun, X.; Du, J.; Dong, Z.; Zhu, S.; Lu, J.; et al. NEDD9 is a positive regulator of epithelial-mesenchymal transition and promotes invasion in aggressive breast cancer. PLoS ONE 2011, 6, e22666. [Google Scholar] [CrossRef] [Green Version]
- Russell, H.; Pranjol, M.Z.I. Transcription factors controlling E-cadherin down-regulation in ovarian cancer. Biosci. Horiz. 2018, 11, hzy010. [Google Scholar] [CrossRef] [Green Version]
- Leggett, S.E.; Hruska, A.M.; Guo, M.; Wong, I.Y. The epithelial-mesenchymal transition and the cytoskeleton in bioengineered systems. Cell Commun. Signal. 2021, 19, 32. [Google Scholar] [CrossRef]
- Scatena, C.; Roncella, M.; Di Paolo, A.; Aretini, P.; Menicagli, M.; Fanelli, G.; Marini, C.; Mazzanti, C.M.; Ghilli, M.; Sotgia, F.; et al. Doxycycline, an Inhibitor of Mitochondrial Biogenesis, Effectively Reduces Cancer Stem Cells (CSCs) in Early Breast Cancer Patients: A Clinical Pilot Study. Front. Oncol. 2018, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.P.; Brennan, K.; Brown, N.J.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013, 15, R92. [Google Scholar] [CrossRef]
- Goss, P.E.; Chambers, A.F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 2010, 10, 871–877. [Google Scholar] [CrossRef]
- Kuntz, E.M.; Baquero, P.; Michie, A.M.; Dunn, K.; Tardito, S.; Holyoake, T.L.; Helgason, G.V.; Gottlieb, E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 2017, 23, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, M.; Lamb, R.; Tanowitz, H.B.; Mutti, L.; Krstic-Demonacos, M.; Cappello, A.R.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Repurposing atovaquone: Targeting mitochondrial complex III and OXPHOS to eradicate cancer stem cells. Oncotarget 2016, 7, 34084–34099. [Google Scholar] [CrossRef] [Green Version]
- Soltysova, A.; Altanerova, V.; Altaner, C. Cancer stem cells. Neoplasma 2005, 52, 435–440. [Google Scholar] [PubMed]
- Alowaidi, F.; Hashimi, S.M.; Alqurashi, N.; Alhulais, R.; Ivanovski, S.; Bellette, B.; Meedenyia, A.; Lam, A.; Wood, S. Assessing stemness and proliferation properties of the newly established colon cancer ‘stem’ cell line, CSC480 and novel approaches to identify dormant cancer cells. Oncol. Rep. 2018, 39, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Veronese, S.M.; Gambacorta, M.; Gottardi, O.; Scanzi, F.; Ferrari, M.; Lampertico, P. Proliferation index as a prognostic marker in breast cancer. Cancer 1993, 71, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Sloan, B.; Scheinfeld, N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin. Drug Saf. 2008, 7, 571–577. [Google Scholar] [CrossRef]
- Chong, K.-H.; Chang, Y.-J.; Hsu, W.-H.; Tu, Y.-T.; Chen, Y.-R.; Lee, M.-C.; Tsai, K.-W. Breast Cancer with Increased Drug Resistance, Invasion Ability, and Cancer Stem Cell Properties through Metabolism Reprogramming. Int. J. Mol. Sci. 2022, 23, 12875. [Google Scholar] [CrossRef]
- Leimanis, M.L.; Georges, E. ABCG2 membrane transporter in mature human erythrocytes is exclusively homodimer. Biochem. Biophys. Res. Commun. 2007, 354, 345–350. [Google Scholar] [CrossRef] [PubMed]



| Antibiotic | Target | Tested Concentration | Concentration in This Study |
|---|---|---|---|
| Azithromycin | The 39S large mitoribosomal subunit | 1.85 µM–450 µM | 50 µM |
| Erythromycin | The 39S large mitoribosomal subunit | 1.85 µM–450 µM | 50 µM |
| Chloramphenicol | The 39S large mitoribosomal subunit | 9.26 µM–2.25 mM | 250 µM |
| Doxycycline | The 28S small mitoribosomal subunit | 930 nM–225 µM | 25 µM |
| Tetracycline | The 28S small mitoribosomal subunit | 1.85 µM–450 µM | 50 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhunzianov, A.A.; Nesterova, A.I.; Wanrooij, S.; Filina, Y.V.; Rizvanov, A.A.; Miftakhova, R.R. Unravelling the Therapeutic Potential of Antibiotics in Hypoxia in a Breast Cancer MCF-7 Cell Line Model. Int. J. Mol. Sci. 2023, 24, 11540. https://doi.org/10.3390/ijms241411540
Akhunzianov AA, Nesterova AI, Wanrooij S, Filina YV, Rizvanov AA, Miftakhova RR. Unravelling the Therapeutic Potential of Antibiotics in Hypoxia in a Breast Cancer MCF-7 Cell Line Model. International Journal of Molecular Sciences. 2023; 24(14):11540. https://doi.org/10.3390/ijms241411540
Chicago/Turabian StyleAkhunzianov, Almaz A., Alfiya I. Nesterova, Sjoerd Wanrooij, Yulia V. Filina, Albert A. Rizvanov, and Regina R. Miftakhova. 2023. "Unravelling the Therapeutic Potential of Antibiotics in Hypoxia in a Breast Cancer MCF-7 Cell Line Model" International Journal of Molecular Sciences 24, no. 14: 11540. https://doi.org/10.3390/ijms241411540
APA StyleAkhunzianov, A. A., Nesterova, A. I., Wanrooij, S., Filina, Y. V., Rizvanov, A. A., & Miftakhova, R. R. (2023). Unravelling the Therapeutic Potential of Antibiotics in Hypoxia in a Breast Cancer MCF-7 Cell Line Model. International Journal of Molecular Sciences, 24(14), 11540. https://doi.org/10.3390/ijms241411540







