Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance
Abstract
:1. Introduction
2. Plant Defense and Terpene Volatiles
2.1. Fatty Acid Derivatives
2.2. Benzene Ring/Phenylpropane Compounds
2.3. Terpenoids
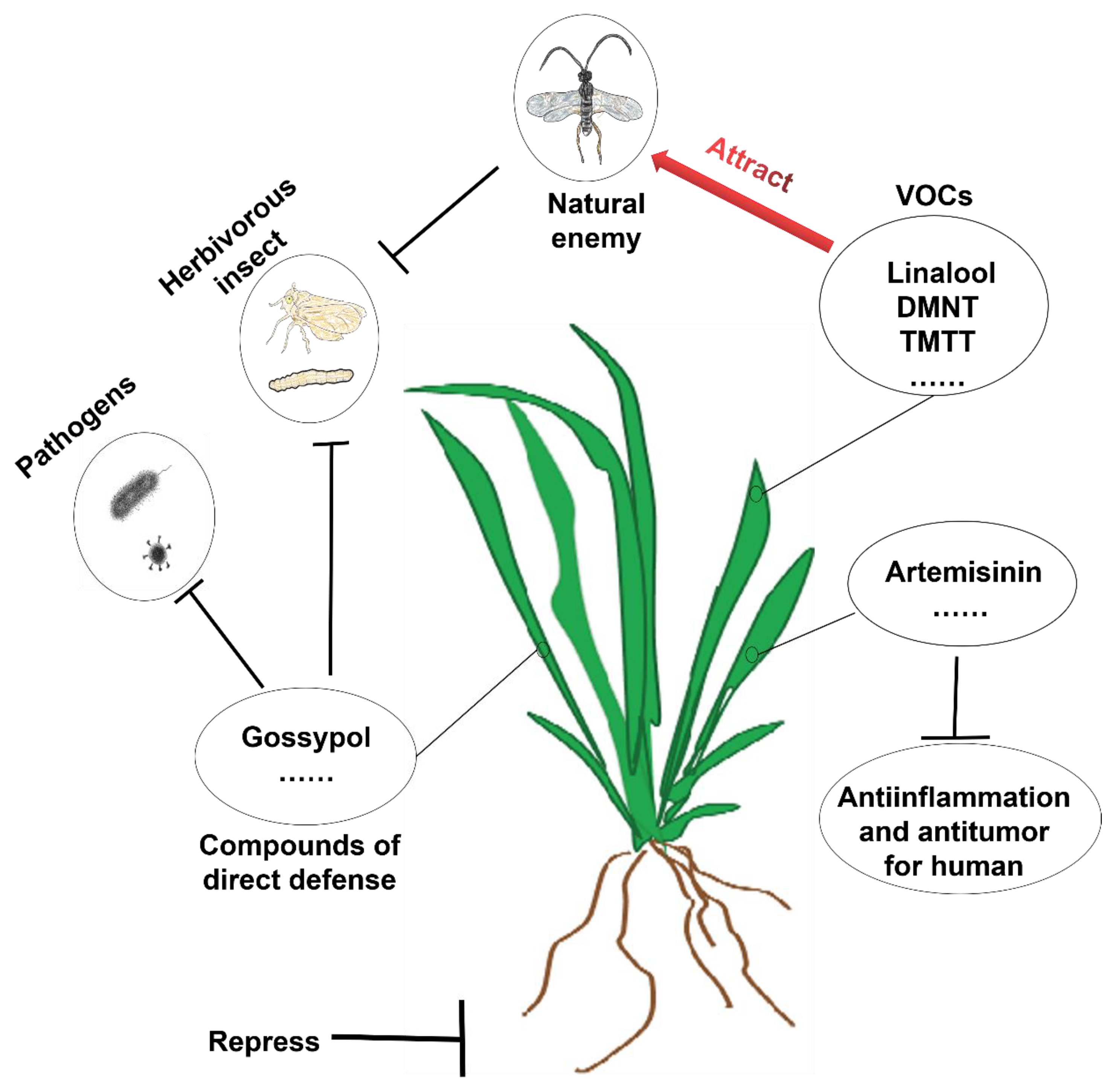
3. Ecological Functions of Terpenoids
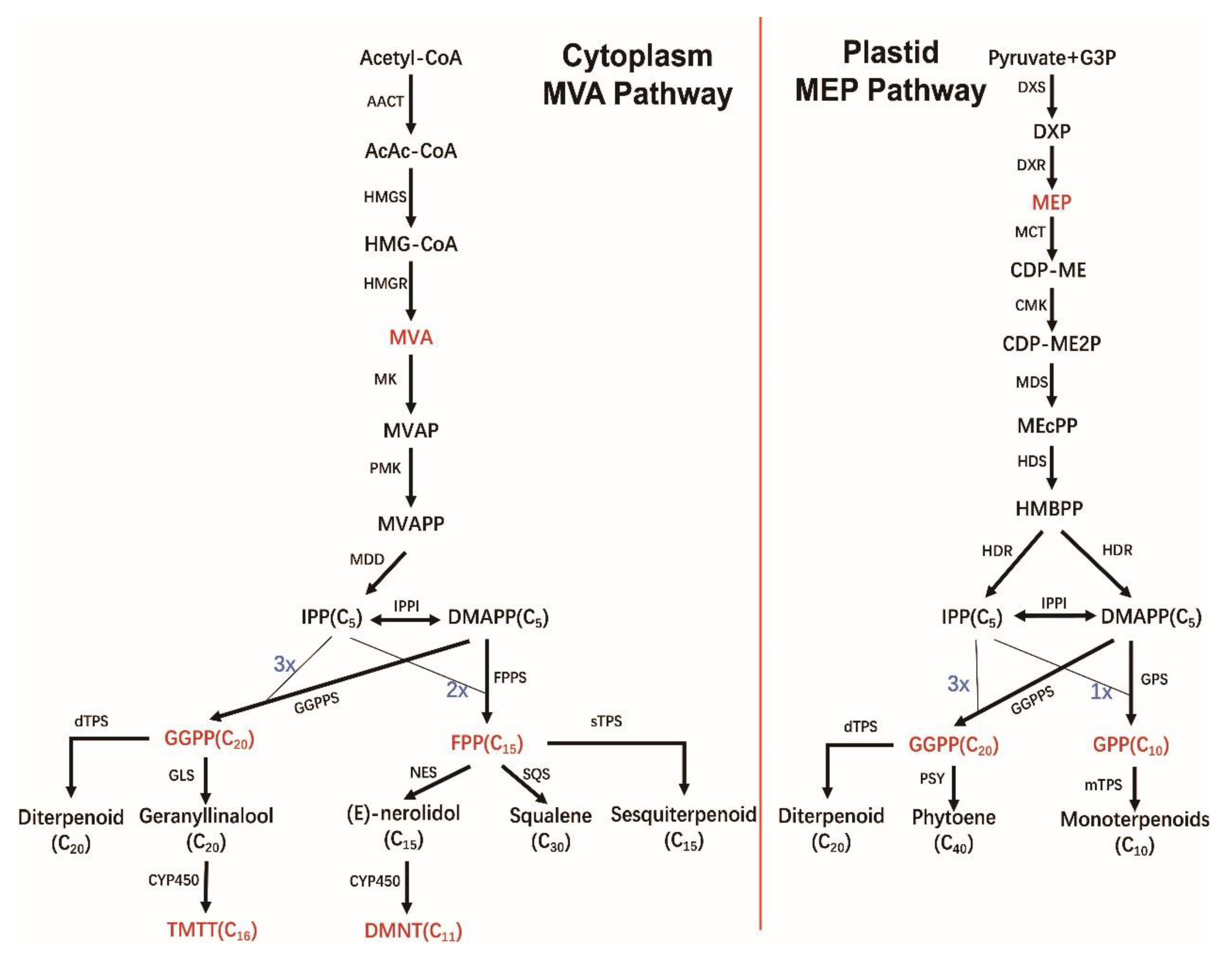
4. Biosynthesis of Terpenoids
4.1. C5 Precursor IPP and DMAPP Formation Phase
4.2. Direct Precursor Formation Stage
4.3. Terpene Formation and Modification Stage
4.4. Transport of Terpenoids
5. Synthetic Genes Related to Terpenoids and Their Transcriptional Regulation
5.1. Terpene Synthase (TPS)
5.2. Transcriptional Regulation of Terpenoids
6. Progress of Terpene Homologue Research
6.1. Discovery and Ecological Function of Terpene Homologues DMNT and TMTT
6.1.1. Attracting Pollination Insects
6.1.2. Attracting the Natural Enemies of Insects
6.1.3. Pest Avoidance
6.1.4. Inducing Defense Responses in Neighboring Plants
6.2. Biosynthesis of DMNT and TMTT
7. Research and Application Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Lin, Y.; Zhang, Q. Review and prospect of transgenic rice research. Chin. Sci. Bull. 2009, 54, 4049–4068. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Ling, F.; You, A. Application and Development of Bt Insect Resistance Genes in Rice Breeding. Sustainability 2023, 15, 9779. [Google Scholar] [CrossRef]
- Tang, W.; Chen, H.; Xu, C.; Li, X.; Lin, Y.; Zhang, Q. Development of insect-resistant transgenic indica rice with a synthetic cry1C* gene. Mol. Breed. 2006, 18, 1. [Google Scholar] [CrossRef]
- Ye, R.; Huang, H.; Yang, Z.; Chen, T.; Liu, L.; Li, X.; Chen, H.; Lin, Y. Development of insect-resistant transgenic rice with Cry1C*-free endosperm. Pest. Manag. Sci. 2009, 65, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, H.; Tang, W.; Hua, H.; Lin, Y. Development and characterisation of transgenic rice expressing two Bacillus thuringiensis genes. Pest. Manag. Sci. 2011, 67, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Zhou, F.; Chen, H.; Lin, Y. Development of marker-free insect-resistant indica rice by Agrobacterium tumefaciens-mediated co-transformation. Front. Plant Sci. 2016, 7, 1608. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Jing, S.; Song, X.; Zhu, L.; Zheng, F.; Sun, B. Reconstitution of the Flavor Signature of Laobaigan-Type Baijiu Based on the Natural Concentrations of Its Odor-Active Compounds and Nonvolatile Organic Acids. J. Agric. Food Chem. 2022, 70, 837–846. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, I.T.; Preston, C.A. The eco-physiological complexity of plant responses to insect herbivores. Planta 1990, 208, 137–145. [Google Scholar] [CrossRef]
- Sabelis, M.W.; Janssen, A.; Kant, M.R. The enemy of my enemy is my ally. Science 2001, 291, 2104–2105. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.S. Inducible direct plant defense against insect herbivores: A review. Insect Sci. 2008, 15, 101–114. [Google Scholar] [CrossRef]
- Su, J.; Zhu, S.; Zhang, Z.; Ge, F. Effect of synthetic aphid alarm pheromone (E)-β-farnesene on development and reproduction of Aphis gossypii (Homoptera: Aphididae). J. Econ. Entomol. 2006, 99, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W. Indigestion is a plant’s best defense. Proc. Natl. Acad. Sci. USA 2005, 102, 18771–18772. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Torres, K.; Herrera, J.M.; Brito, R.F.; Wink, M.; Legal, L. Activity of quinolizidine alkaloids from three Mexican Lupinus against the lepidopteran crop pest Spodoptera frugiperda. BioControl 2009, 54, 459–466. [Google Scholar] [CrossRef]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Lou, Y.; Hua, X.; Turlings, T.C.; Cheng, J.; Chen, X.; Ye, G. Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. J. Chem. Ecol. 2006, 32, 2375. [Google Scholar] [CrossRef] [Green Version]
- Tamiru, A.; Bruce, T.J.A.; Richter, A.; Woodcock, C.M.; Midega, C.A.O.; Degenhardt, J.; Kelemu, S.; Pickett, J.A.; Khan, Z.R. A maize landrace that emits defense volatiles in response to herbivore eggs possesses a strongly inducible terpene synthase gene. Ecol. Evol. 2017, 7, 2835–2845. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- McMullen, M.D.; Kresovich, S.; Villeda, H.S.; Bradbury, P.; Li, H.; Sun, Q.; Flint-Garcia, S.; Thornsberry, J.; Acharya, C.; Bottoms, C.; et al. Genetic properties of the maize nested association mapping population. Science 2009, 325, 737–740. [Google Scholar] [CrossRef] [Green Version]
- Nagegowda, D.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J. Green Leaf Volatiles: Airborne Signals That Protect against Biotic and Abiotic Stresses. Biol. Life Sci. Forum 2020, 11, 526. [Google Scholar] [CrossRef]
- Pierik, R.; Ballaré, C.; Dicke, M. Ecology of plant volatiles: Taking a plant community perspective. Plant Cell Environ. 2014, 37, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Shulaev, V.; Silverman, P.; Raskin, I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 1997, 385, 718–721, Correction at Nature 1997, 385, 718–721. [Google Scholar] [CrossRef]
- Kolosova, N.; Gorenstein, N.; Kish, C.M.; Dudareva, N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 2001, 13, 2333–2347. [Google Scholar] [CrossRef] [Green Version]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648, Correction at Chem. Rev. 2018, 118, 11795. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Dicke, M.; Schnitzler, J.; Turlings, T. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, J.; Jin, J.; Zhang, N.; Lei, L.; Gao, T.; Jing, T.; Zhang, S.; Wu, Y.; et al. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. J. Integr. Plant Biol. 2020, 62, 1461–1468. [Google Scholar] [CrossRef]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Li, H.; Wang, X.; Zhang, R.; Yang, X.; Jiang, Q.; Shi, Q. Revealing the mechanisms for linalool antifungal activity against Fusarium oxysporum and its efficient control of Fusarium Wilt in tomato plants. Int. J. Mol. Sci. 2022, 24, 458. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Chung, M.-S.; Kang, M.; Chung, B.Y.; Lee, S. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma 2016, 253, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kiryu, M.; Hamanaka, M.; Yoshitomi, K.; Mochizuki, S.; Akimitsu, K.; Gomi, K. Rice terpene synthase 18 (OsTPS18) encodes a sesquiterpene synthase that produces an antibacterial (E)-nerolidol against a bacterial pathogen of rice. J. Gen. Plant Pathol. 2018, 84, 221–229. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Akimitsu, K.; Gomi, K. Rice terpene synthase 24 (OsTPS24) encodes a jasmonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Yuan, J.S.; Köllner, T.G.; Chen, Y.; Guo, Y.; Zhuang, X.; Chen, X.; Zhang, Y.-J.; Fu, J.; et al. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol. J. 2018, 16, 1778–1787. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, S.; Miyoshi, S.; Tamaoki, D.; Yamada, S.; Tanaka, K.; Uji, Y.; Tanaka, S.; Akimitsu, K.; Gomi, K. Isolation of jasmonate-induced sesquiterpene synthase of rice: Product of which has an antifungal activity against Magnaporthe oryzae. J. Plant Physiol. 2014, 171, 625–632. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, X.; Zhou, F.; Chen, H.; Lin, Y. Expression of a Peppermint (E)-β-Farnesene Synthase Gene in Rice Has Significant Repelling Effect on Bird Cherry-Oat Aphid (Rhopalosiphum padi). Plant Mol. Biol. Rep. 2015, 33, 1967–1974. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Zhang, W.; Jiang, B.; Tao, S.-M.; Dai, H.-Y.; Xu, X.-T.; Sun, Y.-X.; Yang, L.; Zhang, Y.-J. The main component of the aphid alarm pheromone (E)-β-farnesene affects the growth and development of Spodoptera exigua by mediating juvenile hormone-related genes. Front. Plant Sci. 2022, 13, 863626. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Chen, Z.; Li, J.; Huang, J.; Cao, J.; Cui, M.; Ban, L. (E)-β-farnesene synthase gene affects aphid behavior in transgenic Medicago sativa. Pest. Manag. Sci. 2019, 75, 622–631. [Google Scholar] [CrossRef]
- Aartsma, Y.; Pappagallo, S.; Van Der Werf, W.; Dicke, M.; Bianchi, F.J.J.A.; Poelman, E.H. Spatial scale, neighbouring plants and variation in plant volatiles interactively determine the strength of host-parasitoid relationships. Oikos 2020, 129, 1429–1439. [Google Scholar] [CrossRef]
- Hausch, B.J.; Lorjaroenphon, Y.; Cadwallader, K.R. Flavor chemistry of lemon-lime carbonated beverages. J. Agric. Food Chem. 2015, 63, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pragadheesh, V.S.; Chanotiya, C.S.; Rastogi, S.; Shasany, A.K. Scent from Jasminum grandiflorum flowers: Investigation of the change in linalool enantiomers at various developmental stages using chemical and molecular methods. Hytochemistry 2017, 140, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kokkiripati, P.K.; Bhakshu, L.M.; Marri, S.; Padmasree, K.; Row, A.T.; Raghavendra, A.S.; Tetali, S.D. Gum resin of Boswellia serrata inhibited human monocytic (THP-1) cell activation and platelet aggregation. J. Ethnopharmacol. 2011, 137, 893–901. [Google Scholar] [CrossRef]
- Zhao, D.-D.; Jiang, L.-L.; Li, H.-Y.; Yan, P.-F.; Zhang, Y.-L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as therapeutic drugs and pharmaceutical agents. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2005; pp. 197–227. [Google Scholar] [CrossRef]
- Phulara, S.; Chaturvedi, P.; Gupta, P. Isoprenoid-Based Biofuels: Homologous Expression and Heterologous Expression in Prokaryotes. Appl. Environ. Microbiol. 2016, 82, 5730–5740. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, R.; Yang, Q.; Zhang, J.; Zhao, Y.; Zheng, Y.; Yang, J. Recent advances in the biosynthesis of isoprenoids in engineered Saccharomyces cerevisiae. Adv. Appl. Microbiol. 2020, 114, 1–35. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biot. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.; Smita, S.S.; Singh, A.K.; Shanker, K.; Nagegowda, D.A. Heteromeric and Homomeric Geranyl Diphosphate Synthases from Catharanthus roseus and Their Role in Monoterpene Indole Alkaloid Biosynthesis. Mol. Plant 2013, 6, 1531–1549. [Google Scholar] [CrossRef] [Green Version]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Boutanaev, A.M.; Moses, T.; Zi, J.; Nelson, D.R.; Mugford, S.T.; Peters, R.J.; Osbourn, A. Investigation of terpene diversification across multiple sequenced plant genomes. Proc. Natl. Acad. Sci. USA 2015, 122, E81–E88. [Google Scholar] [CrossRef] [PubMed]
- Demissie, Z.; Woolfson, K.; Fang, Y.; Yang, Q.; Vincenzo, D. The ATP binding cassette transporter, VmTPT2/VmABCG1, is involved in export of the monoterpenoid indole alkaloid, vincamine in Vinca minor leaves. Phytochemistry 2019, 140, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shi, P.; He, Q.; Shen, Q.; Tang, Y.; Pan, Q.; Ma, Y.; Yan, T.; Chen, M.; Hao, X.; et al. AaPDR3, a PDR Transporter 3, Is Involved in Sesquiterpene beta-Caryophyllene Transport in Artemisia annua. Front. Plant Sci. 2017, 8, 723. [Google Scholar] [CrossRef]
- Shibata, Y.; Ojika, M.; Sugiyama, A.; Yazaki, K.; Jones, D.A.; Kawakita, K.; Takemoto, D. The Full-Size ABCG Transporters Nb-ABCG1 and Nb-ABCG2 Function in Pre- and Postinvasion Defense against Phytophthora infestans in Nicotiana benthamiana. Plant Cell 2016, 28, 1163–1181. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, J.; Meyer-Gauen, G.; Croteau, R. Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. USA 1998, 95, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wu, P.; Jia, Y.; Wei, X.; Xu, L.; Yang, Y.; Qiu, D.; Chen, Y.; Li, M.; Jiang, H.; et al. Genome-wide analysis of the terpene synthase gene family in physic nut (Jatropha curcas L.) and functional identification of six terpene synthases. Tree Genet. Genomes 2016, 12, 97. [Google Scholar] [CrossRef]
- Alicandri, E.; Paolacci, A.R.; Osadolor, S.; Sorgonà, A.; Badiani, M.; Ciaffi, M. On the Evolution and Functional Diversity of Terpene Synthases in the Pinus Species: A Review. J. Mol. Evol. 2020, 88, 253–283. [Google Scholar] [CrossRef]
- Christianson, D. Structural biology and chemistry of the terpenoid cyclases. Chem. Rev. 2006, 106, 3412–3442. [Google Scholar] [CrossRef]
- Prosser, I.; Altug, I.G.; Phillips, A.L.; König, W.A.; Bouwmeester, H.J.; Beale, M.H. Enantiospecific (+)- and (−)-germacrene D synthases, cloned from goldenrod, reveal a functionally active variant of the universal isoprenoid-biosynthesis aspartate-rich motif. Arch. Biochem. Biophys. 2004, 432, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Köllner, T.G.; Wiggins, G.; Grant, J.; Degenhardt, J.; Chen, F. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J. 2008, 55, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, W.; Lin, Y.-J.; Pickett, J.A.; Birkett, M.A.; Wu, K.; Wang, G.; Zhou, J.-J. Expression of lima bean terpene synthases in rice enhances recruitment of a beneficial enemy of a major rice pest. Plant Cell Environ. 2018, 41, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.-F.; Liu, J.-J.; He, Z.-R.; Wang, F.-M.; Yang, H.; Yan, Y.-F.; Gao, M.-J.; Gruber, M.Y.; Wan, X.-C.; Wei, S. Implementation of CsLIS/NES in linalool biosynthesis involves transcript splicing regulation in Camellia sinensis. Plant Cell Environ. 2018, 41, 176–186. [Google Scholar] [CrossRef]
- Richter, A.; Schaff, C.; Zhang, Z.; Lipka, A.E.; Tian, F.; Köllner, T.G.; Schnee, C.; Preiß, S.; Irmisch, S.; Jander, G.; et al. Characterization of Biosynthetic Pathways for the Production of the Volatile Homoterpenes DMNT and TMTT in Zea mays. Plant Cell 2016, 28, 2651–2665. [Google Scholar] [CrossRef] [Green Version]
- Brillada, C.; Nishihara, M.; Shimoda, T.; Garms, S.; Boland, W.; Maffei, M.E.; Arimura, G. Metabolic engineering of the C16 homoterpene TMTT in Lotus japonicus through overexpression of (E,E)-geranyllinalool synthase attracts generalist and specialist predators in different manners. New Phytol. 2013, 200, 1200–1211. [Google Scholar] [CrossRef]
- Guitton, Y.; Nicolè, F.; Moja, S.; Valot, N.; Legrand, S.; Jullien, F.; Legendre, L. Differential accumulation of volatile terpene and terpene synthase mRNAs during lavender (Lavandula angustifolia and L. x intermedia) inflorescence development. Physiol. Plant. 2010, 138, 150–163. [Google Scholar] [CrossRef]
- Irmisch, S.; Jiang, Y.; Chen, F.; Gershenzon, J.; Köllner, T.G. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa). BMC Plant Biol. 2014, 14, 270. [Google Scholar] [CrossRef] [Green Version]
- Macías, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.C.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Wray, G.A.; Hahn, M.W.; Abouheif, E.; Balhoff, J.P.; Pizer, M.; Rockman, M.V.; Romano, L.A. The evolution of transcriptional regulation in eukaryotes. Mol. Biol. Evol. 2003, 20, 1377–1419. [Google Scholar] [CrossRef] [Green Version]
- Rushton, P.J.; Bokowiec, M.T.; Han, S.; Zhang, H.; Brannock, J.F.; Chen, X.; Laudeman, T.W.; Timko, M.P. Tobacco Transcription Factors: Novel Insights into Transcriptional Regulation in the Solanaceae. Plant Physiol. 2008, 147, 280–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuizen, N.J.; Chen, X.; Wang, M.Y.; Matich, A.J.; Perez, R.L.; Allan, A.C.; Green, S.A.; Atkinson, R.G. Natural variation in monoterpene synthesis in kiwifruit: Transcriptional regulation of terpene synthases by NAC and ETHYLENE-INSENSITIVE3-like transcription factors. Plant Physiol. 2015, 167, 1243–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, G.-J.; Xue, X.-Y.; Mao, Y.-B.; Wang, L.-J.; Chen, X.-Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yan, T.; Shen, Q.; Lu, X.; Pan, Q.; Huang, Y.; Tang, Y.; Fu, X.; Liu, M.; Jiang, W.; et al. Glandular trichome-specific WRKY 1 promotes artemisinin biosynthesis in Artemisia annua. New Phytol. 2017, 214, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-X.; Li, J.-X.; Yang, C.-Q.; Hu, W.-L.; Wang, L.-J.; Chen, X.-Y. The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol. Plant 2012, 5, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Fu, X.; Lv, Z.; Lu, X.; Shen, Q.; Zhang, L.; Zhu, M.; Wang, G.; Sun, X.; Liao, Z.; et al. A basic leucine zipper transcription factor, AabZIP1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Mol. Plant 2015, 8, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Wang, Y.; Liu, Y.; Peng, B.; Zhang, L.; Tang, K.; Chen, W. The SPB-Box Transcription Factor AaSPL2 Positively Regulates Artemisinin Biosynthesis in Artemisia annua L. Front. Plant Sci. 2019, 10, 409. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, Y.; Shen, S.; Yin, X.; Klee, H.; Zhang, B.; Chen, K. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit. J. Exp. Bot. 2017, 68, 4929–4938. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-H.; Wang, J.-W.; Wang, S.; Wang, J.-Y.; Chen, X.-Y. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Mertens, J.; Pollier, J.; Bossche, R.V.; Lopez-Vidriero, I.; Franco-Zorrilla, J.M.; Goossens, A. The bHLH Transcription Factors TSAR1 and TSAR2 Regulate Triterpene Saponin Biosynthesis in Medicago truncatula. Plant Physiol. 2016, 170, 194–210. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.A.; Wang, Q.; Dhar, N.; Kumar, N.; Venkatesh, P.N.; Rajan, C.; Panicker, D.; Sridhar, V.; Mao, H.-Z.; Sarojam, R. Spearmint R2R3-MYB transcription factor MsMYB negatively regulates monoterpene production and suppresses the expression of geranyl diphosphate synthase large subunit (MsGPPS.LSU). Plant Biotechnol. J. 2017, 15, 1105–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Reddy, V.A.; Panicker, D.; Mao, H.-Z.; Kumar, N.; Rajan, C.; Venkatesh, P.N.; Chua, N.-H.; Sarojam, R. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor MsYABBY5 from spearmint (Mentha spicata). Plant Biotechnol. J. 2016, 14, 1619–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akagi, A.; Fukushima, S.; Okada, K.; Jiang, C.-J.; Yoshida, R.; Nakayama, A.; Shimono, M.; Sugano, S.; Yamane, H.; Takatsuji, H. WRKY45-dependent priming of diterpenoid phytoalexin biosynthesis in rice and the role of cytokinin in triggering the reaction. Plant Mol. Biol. 2014, 86, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.; Matsumoto, T.; Okada, A.; Komiyama, K.; Chujo, T.; Yoshikawa, H.; Nojiri, H.; Yamane, H.; Okada, K. Identification of target genes of the bZIP transcription factor OsTGAP1, whose overexpression causes elicitor-induced hyperaccumulation of diterpenoid phytoalexins in rice cells. PLoS ONE 2014, 9, e105823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.; Nishizawa, Y.; Minami, E.; Nojiri, H.; Yamane, H.; Okada, K. Overexpression of the bZIP transcription factor OsbZIP79 suppresses the production of diterpenoid phytoalexin in rice cells. J. Plant Physiol. 2015, 173, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, Y.; Xu, J.; Li, Y.; Luo, H.; Zhu, Y.; Liu, M.; Wu, Q.; Song, J.; Sun, C.; et al. Discovery of WRKY transcription factors through transcriptome analysis and characterization of a novel methyl jasmonate-inducible PqWRKY1 gene from Panax quinquefolius. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 114, 269–277. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Hung, Y.-C.; Tsai, W.-C.; Chen, W.-H.; Chen, H.-H. PbbHLH4 regulates floral monoterpene biosynthesis in Phalaenopsis orchids. J. Exp. Bot. 2018, 69, 4363–4377. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Wang, Y.; Shi, M.; Hao, X.; Zhao, W.; Wang, Y.; Ren, J.; Kai, G. Transcription Factor SmWRKY1 Positively Promotes the Biosynthesis of Tanshinones in Salvia miltiorrhiza. Front. Plant Sci. 2018, 9, 554. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Hao, X.; Shi, M.; Fu, R.; Wang, Y.; Zhang, Y.; Zhou, W.; Feng, Y.; Makunga, N.P.; Kai, G. Tanshinone production could be increased by the expression of SmWRKY2 in Salvia miltiorrhiza hairy roots. Plant Sci. 2019, 284, 1–8. [Google Scholar] [CrossRef]
- Ding, K.; Pei, T.; Bai, Z.; Jia, Y.; Ma, P.; Liang, Z. SmMYB36, a Novel R2R3-MYB Transcription Factor, Enhances Tanshinone Accumulation and Decreases Phenolic Acid Content in Salvia miltiorrhiza Hairy Roots. Sci. Rep. 2017, 7, 5104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ji, A.; Xu, Z.; Luo, H.; Song, J. The AP2/ERF transcription factor SmERF128 positively regulates diterpenoid biosynthesis in Salvia miltiorrhiza. Plant Mol. Biol. 2019, 100, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol. 2014, 55, 119–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, P.; Zhang, M.; Fu, C.; Yu, L. Functional analysis of a WRKY transcription factor involved in transcriptional activation of the DBAT gene in Taxus chinensis. Plant Biol. 2013, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Li, F.; Chen, Z.; Li, X.; Zhu, L.; Wang, G.; Yu, J.; Huang, D.; Lang, Z. The maize transcription factor EREB58 mediates the jasmonate-induced production of sesquiterpene volatiles. Plant J. 2015, 84, 296–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotewold, E. Transcription factors for predictive plant metabolic engineering: Are we there yet? Curr. Opin. Biotechnol. 2008, 19, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Hauser, A.; Froidevaux, J.-C. (E)-4,8-dimethyl-1,3,7-nonatriene and (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, two unusual hydrocarbons from cardamom oil. Tetrahedron Lett. 1986, 27, 2111–2112. [Google Scholar] [CrossRef]
- Azuma, H.; Toyota, M.; Asakawa, Y. Intraspecific Variation of Floral Scent Chemistry in Magnolia kobus DC (Magnoliaceae). J. Plant Res. 2001, 114, 411–422. [Google Scholar] [CrossRef]
- Kaiser, R. Trapping, Investigation and Reconstitution of Flower Scents. In Perfumes; Springer: Dordrecht, The Netherlands, 1994; pp. 213–250. [Google Scholar] [CrossRef]
- Svensson, G.P.; Hickman, M.O., Jr.; Bartram, S.; Boland, W.; Pellmyr, O.; Raguso, R.A. Chemistry and geographic variation of floral scent in Yucca filamentosa (Agavaceae). Am. J. Bot. 2005, 92, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- Svensson, G.P.; Pellmyr, O.; Raguso, R.A. Strong Conservation of Floral Scent Composition in Two Allopatric Yuccas. J. Chem. Ecol. 2006, 32, 2657–2665. [Google Scholar] [CrossRef]
- Liu, D.; Huang, X.; Jing, W.; An, X.; Zhang, Q.; Zhang, H.; Zhou, J.; Zhang, Y.; Guo, Y. Identification and functional analysis of two P450 enzymes of Gossypium hirsutum involved in DMNT and TMTT biosynthesis. Plant Biotechnol. J. 2017, 16, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.A.; Matthes, M.C.; Chamberlain, K.; Woodcock, C.M.; Mohib, A.; Webster, B.; Smart, L.E.; Birkett, M.A.; Pickett, J.A.; Napier, J.A. cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc. Natl. Acad. Sci. USA 2008, 105, 4553–4558. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.-I.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.-I.; Ozawa, R.; Horiuchi, J.-I.; Nishioka, T.; Takabayashi, J. Plant–plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem. Syst. Ecol. 2001, 29, 1049–1061. [Google Scholar] [CrossRef]
- Jing, T.; Du, W.; Gao, T.; Wu, Y.; Zhang, N.; Zhao, M.; Jin, J.; Wang, J.; Schwab, W.; Wan, X.; et al. Herbivore-induced DMNT catalyzed by CYP82D47 plays an important role in the induction of JA-dependent herbivore resistance of neighboring tea plants. Plant Cell Environ. 2020, 44, 1178–1191. [Google Scholar] [CrossRef]
- Adams, S.; Che, D.; Qin, G.; Farouk, M.H.; Hailong, J.; Rui, H. Novel Biosynthesis, Metabolism and Physiological Functions of L-Homoarginine. Curr. Protein Pept. Sci. 2019, 20, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Shuo, X.; Lu, W. Progress of Heterologous Biosynthesis of Terpenoids in Engineered Corynebacterium glutamicum. China Biotechnol. 2019, 39, 91–96. [Google Scholar] [CrossRef]
- Lee, S.; Badieyan, S.; Bevan, D.R.; Herde, M.; Gatz, C.; Tholl, D. Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 21205–21210. [Google Scholar] [CrossRef]
- Kfir, R.; Overholt, W.A.; Khan, Z.R.; Polaszek, A. Biology and management of economically important lepidopteran cereal stem borers in Africa. Annu. Rev. Entomol. 2022, 47, 701–731. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.R.; Midega, C.A.O.; Pittchar, J.O.; Murage, A.W.; Birkett, M.; Bruce, T.; Pickett, J.A. Achieving food security for one million sub-Saharan African poor through push-pull innovation by 2020. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20120284. [Google Scholar] [CrossRef] [Green Version]
- Touchet, S.; Chamberlain, K.; Woodcock, C.M.; Miller, D.J.; Birkett, M.A.; Pickett, J.A.; Allemann, R.K. Novel olfactory ligands via terpene synthases. Chem. Commun. 2015, 51, 7550–7553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickett, J.A.; Khan, Z.R. Plant volatile-mediated signalling and its application in agriculture: Successes and challenges. New Phytol. 2016, 212, 856–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappers, I.F.; Hoogerbrugge, H.; Bouwmeester, H.J.; Dicke, M. Variation in Herbivory-induced Volatiles Among Cucumber (Cucumis sativus L.) Varieties has Consequences for the Attraction of Carnivorous Natural Enemies. J. Chem. Ecol. 2011, 37, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wang, L.; Zhou, F.; Li, C.; Ma, W.; Chen, H.; Wang, G.; Pickett, J.A.; Zhou, J.; Lin, Y. Overexpression of the homoterpene synthase gene, oscyp92c21, increases emissions of volatiles mediating tritrophic interactions in rice. Plant Cell Environ. 2020, 44, 948–963. [Google Scholar] [CrossRef] [PubMed]
| Species | Terpene | Genes | TF | Reference |
|---|---|---|---|---|
| Actinidia arguta | Terpinolene | AaTPS1 | AaNAC | [73] |
| Arabidopsis thaliana | (E)-β-caryophyllene | AtTPS11; AtTPS21 | AtMYC2 | [74] |
| Artemisia annua | Artemisinin | AaGSW1; | AaWRKY1; AaERF1; AaERF2; AabZIP1; AaSPL2 | [75,76,77,78] |
| Citrus sinensis | (E)-geraniol | CitTPS16 | CitERF71 | [79] |
| Gossypium arboreum | Gossypol | CAD1-A | GaWRKY 1 | [80] |
| Medicago truncatula | Saponin | TSAR1; TSAR2 | TSAR1; TSAR2 | [81] |
| Mentha spicata | α-Pinene; β-Pinene; eucalyptol; linalyl acetate; α-bergamotene; germacrene D; γ-muurolene; β-copaene; Limonene | MsGPPS; MsNTT | MsMYB; MsYABBY5 | [82,83] |
| Oryza sativa | Phytoalexins | OsDXS3 | OsWRKY45; OsTGAP1; OsbZIP79 | [84,85,86] |
| Panax quinquefolius (Arabidopsis thaliana) | Ginsenoside | AtHMGR; AtFPS2; AtSQS1; AtSQE2 | PqWRKY1 | [87] |
| Phalaenopsis bellina | Geraniol; linanol | PbGDPS; PbGDPS2; PbTPS5&7&9&10 | PbbHLH4; PbbHLH6 | [88] |
| Salvia miltiorrhiza | Tanshinones | SmCPS1; SmKSL1; SmCYP76AH1 | SmWRKY1; SmWRKY2; SmMYB36; SmERF128 | [89,90,91,92] |
| Solanum lycopersicum | Carotenoid | SlACS2; SIACS4 | SINAC4 | [93] |
| Taxus chinensis | Taxol | TcDBAT | TcWRKY1 | [94] |
| Zea mays | (E)-β-farnesene | ZmTPS10 | ZmEREB58 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. Int. J. Mol. Sci. 2023, 24, 11561. https://doi.org/10.3390/ijms241411561
Li C, Zha W, Li W, Wang J, You A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. International Journal of Molecular Sciences. 2023; 24(14):11561. https://doi.org/10.3390/ijms241411561
Chicago/Turabian StyleLi, Changyan, Wenjun Zha, Wei Li, Jianyu Wang, and Aiqing You. 2023. "Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance" International Journal of Molecular Sciences 24, no. 14: 11561. https://doi.org/10.3390/ijms241411561
APA StyleLi, C., Zha, W., Li, W., Wang, J., & You, A. (2023). Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. International Journal of Molecular Sciences, 24(14), 11561. https://doi.org/10.3390/ijms241411561






