Therapeutic Effect of Enzymatically Hydrolyzed Cervi Cornu Collagen NP-2007 and Potential for Application in Osteoarthritis Treatment
Abstract
:1. Introduction
2. Results
2.1. NP-2007 Mitigated Articular Cartilage in MIA-induced OA Rats
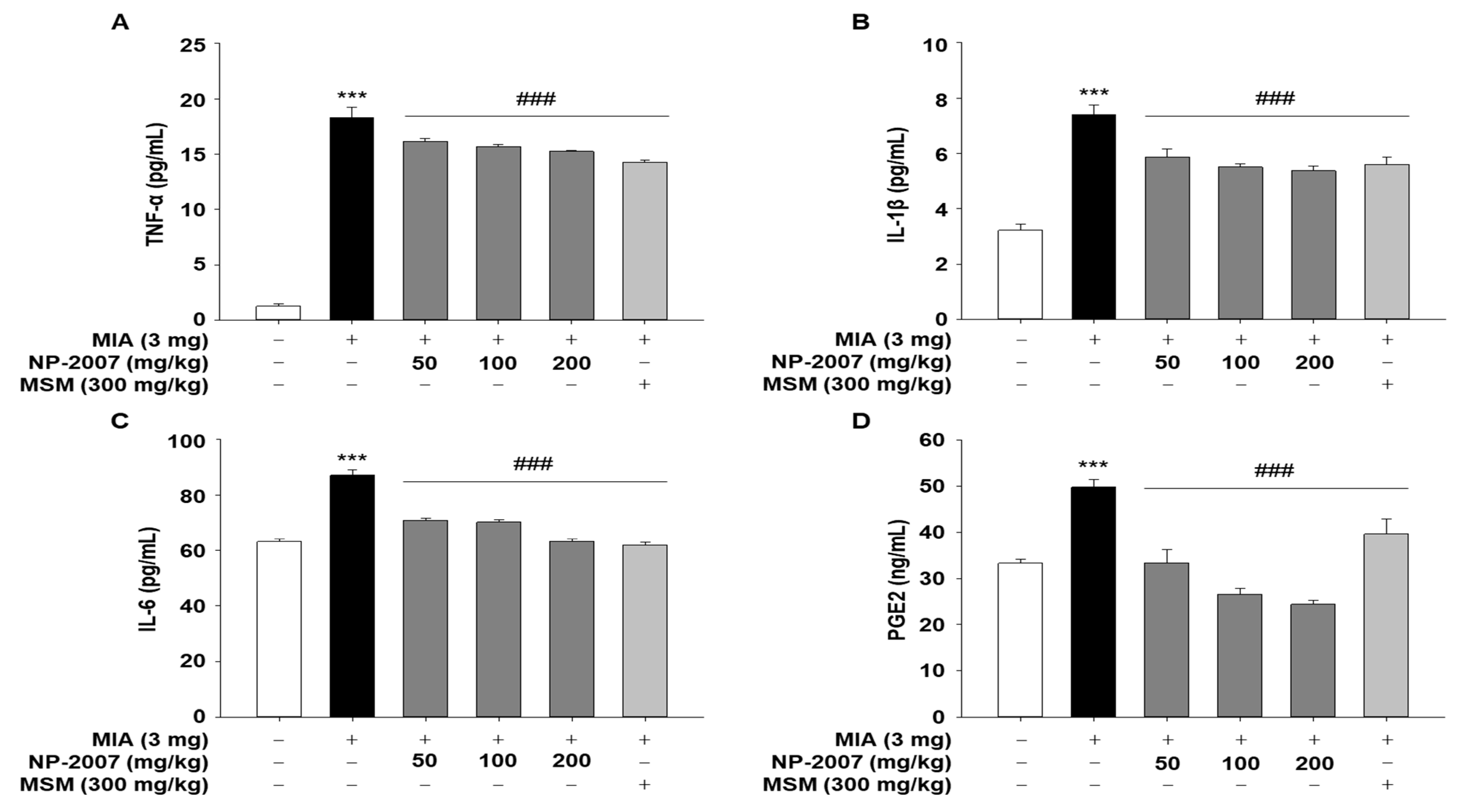
2.2. NP-2007 Alleviated Inflammation in MIA-Induced OA Rats
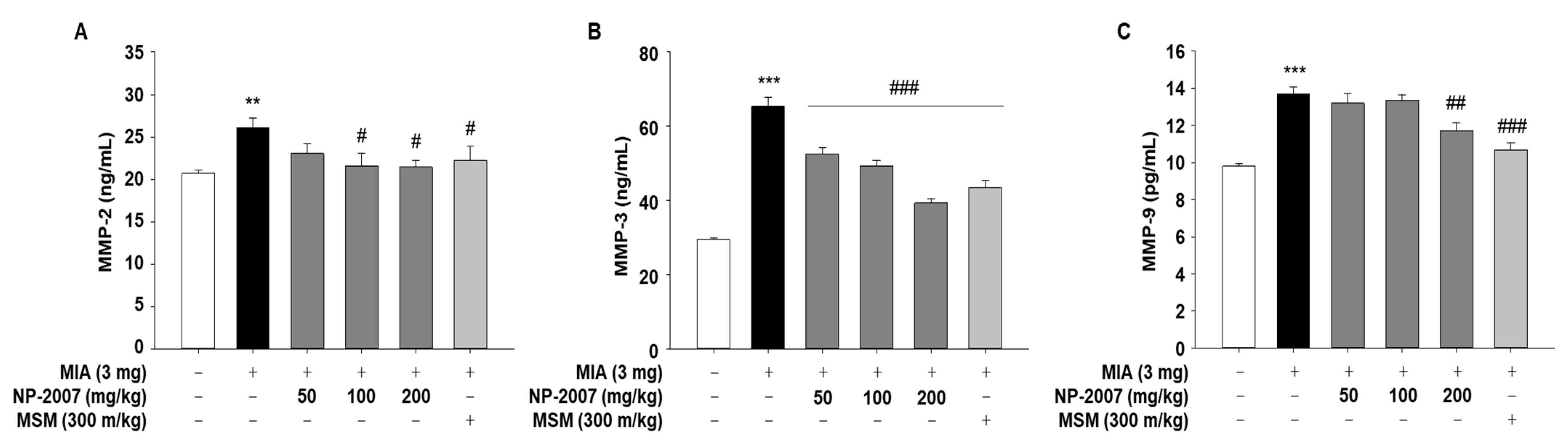
2.3. NP-2007 Modulated the Matrix Metalloprotease Levels in MIA-Induced OA Rats
2.4. NP-2007 Suppresses NF-κB Signaling in LPS-induced RAW 264.7 Macrophages
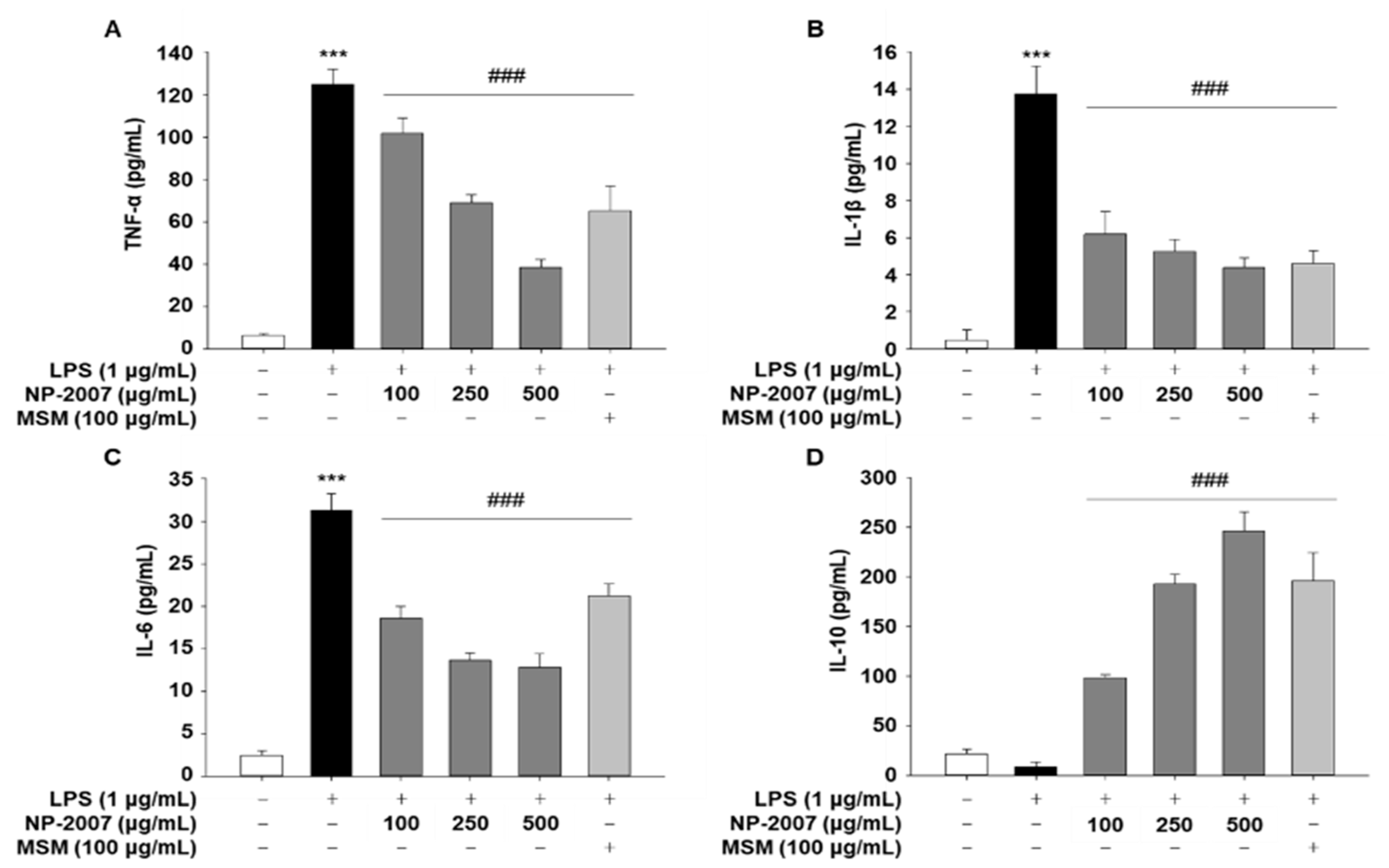
2.5. NP-2007 Suppresses Pro-Inflammatory Cytokine Production and MAPK Signaling in LPS-Induced RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Low-Molecular-Weight Cervi Cornu Collagen (NP-2007) Preparation
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Nitric Oxide (NO) Assay
4.5. Animals
4.6. Induction of Monosodium Iodoacetate (MIA)-Induced Osteoarthritis (OA) in Rats
4.7. Cytokine Assay
4.8. Immunoblotting
4.9. Histological Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steels, E.; Venkatesh, R.; Steels, E.; Vitetta, G.; Vitetta, L. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology 2019, 27, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Man, G.S.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, D.J. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat. Rev. Rheumatol. 2011, 7, 13–22. [Google Scholar] [CrossRef]
- Ragni, E.; De Luca, P.; Valli, F.; Zagra, L.; de Girolamo, L. Inflammatory Treatment Used to Mimic Osteoarthritis and Patients’ Synovial Fluid Have Divergent Molecular Impact on Chondrocytes In Vitro. Int. J. Mol. Sci. 2023, 24, 2625. [Google Scholar] [CrossRef]
- Lallana, M.J.; Feja, C.; Aguilar-Palacio, I.; Malo, S.; Rabanaque, M.J. Use of Non-Steroidal Anti-Inflammatory Drugs and Associated Gastroprotection in a Cohort of Workers. Int. J. Environ. Res. Public. Health 2018, 15, 1836. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Son, E.; Kim, S.H.; Kim, D.S. Effect of Alpinia oxyphylla extract in vitro and in a monosodium iodoacetate-induced osteoarthritis rat model. Phytomedicine 2019, 65, 153095. [Google Scholar] [CrossRef]
- Zhang, W.; Ke, C.H.; Guo, H.H.; Xiao, L. Antler stem cells and their potential in wound healing and bone regeneration. World J. Stem Cells 2021, 13, 1049–1057. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, Y.; Yang, M.; Wang, X.; Peng, Y. Velvet Antler Methanol Extracts Ameliorate Parkinson’s Disease by Inhibiting Oxidative Stress and Neuroinflammation: From C. elegans to Mice. Oxid. Med. Cell. Longev. 2021, 2021, 8864395. [Google Scholar] [CrossRef]
- Chonco, L.; Landete-Castillejos, T.; Serrano-Heras, G.; Serrano, M.P.; Perez-Barberia, F.J.; Gonzalez-Armesto, C.; Garcia, A.; de Cabo, C.; Lorenzo, J.M.; Li, C.; et al. Anti-tumour activity of deer growing antlers and its potential applications in the treatment of malignant gliomas. Sci. Rep. 2021, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Qi, L.; Tian, Y.H.; Hu, R.; Wu, L.; Meng, X.Y. Studies on the purification of polypeptide from sika antler plate and activities of antitumor. BMC Complement. Altern. Med. 2015, 15, 328. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Chen, Y.; Ni, Y.; Jiang, J.; Zhang, W.; Zhou, Q.; Zheng, X.; Wang, Q.; Fu, Z.; et al. Pilose antler polypeptides ameliorates hypoxic-ischemic encephalopathy by activated neurotrophic factors and SDF1/CXCR4 axis in rats. Acta Biochim. Biophys. Sin. 2018, 50, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.S.; Lin, H.J.; Deng, J.S.; Wu, W.T.; Huang, S.S.; Huang, G.J. Preventive Effects of Velvet Antler (Cervus elaphus) against Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting MAPK/NF-kappaB Activation and Inducing AMPK/Nrf2 Pathways. Evid. Based Complement. Altern. Med. 2018, 2018, 2870503. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.J.; Yang, H.T.; Chiang, C.C.; Lai, K.H.; Chen, Y.L.; Shih, H.L.; Kuo, J.J.; Hwang, T.L.; Lin, C.C. Deer Velvet Antler Extracts Exert Anti-Inflammatory and Anti-Arthritic Effects on Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes and Distinct Mouse Arthritis. Am. J. Chin. Med. 2022, 50, 1617–1643. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fan, M.; Choi, Y.J.; Yu, Y.; Yao, G.; Deng, Y.; Moon, S.H.; Kim, E.K. Sika deer (Cervus nippon) velvet antler extract attenuates prostate cancer in xenograft model. Biosci. Biotechnol. Biochem. 2019, 83, 348–356. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Zhang, X.L.; Xie, Q.F. Purification and identification of anti-inflammatory peptides derived from simulated gastrointestinal digests of velvet antler protein (Cervus elaphus Linnaeus). J. Food Drug Anal. 2016, 24, 376–384. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local delivery of insulin/IGF-1 for bone regeneration: Carriers, strategies, and effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef]
- Skov, K.; Oxfeldt, M.; Thogersen, R.; Hansen, M.; Bertram, H.C. Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate-A Randomized Controlled Trial. Nutrients 2019, 11, 1064. [Google Scholar] [CrossRef] [Green Version]
- Chadjichristos, C.; Ghayor, C.; Kypriotou, M.; Martin, G.; Renard, E.; Ala-Kokko, L.; Suske, G.; de Crombrugghe, B.; Pujol, J.P.; Galera, P. Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes. J. Biol. Chem. 2003, 278, 39762–39772. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Duan, G.; Fan, G.; Peng, N. Effect of Lycium barbarum polysaccharides on cell signal transduction pathways. Biomed. Pharmacother. 2022, 147, 112620. [Google Scholar] [CrossRef]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar]
- D’Arcy, Y.; Mantyh, P.; Yaksh, T.; Donevan, S.; Hall, J.; Sadrarhami, M.; Viktrup, L. Treating osteoarthritis pain: Mechanisms of action of acetaminophen, nonsteroidal anti-inflammatory drugs, opioids, and nerve growth factor antibodies. Postgrad. Med. 2021, 133, 879–894. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzi, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [PubMed] [Green Version]
- Martinez-Puig, D.; Costa-Larrion, E.; Rubio-Rodriguez, N.; Galvez-Martin, P. Collagen Supplementation for Joint Health: The Link between Composition and Scientific Knowledge. Nutrients 2023, 15, 1332. [Google Scholar] [CrossRef]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef]
- van der Kraan, P.M.; Vitters, E.L.; van de Putte, L.B.; van den Berg, W.B. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am. J. Pathol. 1989, 135, 1001–1014. [Google Scholar]
- D’Souza, W.N.; Ng, G.Y.; Youngblood, B.D.; Tsuji, W.; Lehto, S.G. A review of current animal models of osteoarthritis pain. Curr. Pharm. Biotechnol. 2011, 12, 1596–1612. [Google Scholar]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Schwager, J.; Richard, N.; Fowler, A.; Seifert, N.; Raederstorff, D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules 2016, 21, 465. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.L.; Lee, H.J.; Lee, D.-R.; Choi, B.-K.; Yang, S.H. Anti-osteoarthritic effects of Terminalia chebula fruit extract (AyuFlex®) in interleukin-1β-induced human chondrocytes and in rat models of monosodium iodoacetate (MIA)-induced osteoarthritis. Appl. Sci. 2020, 10, 8698. [Google Scholar] [CrossRef]
- Lee, D.; Baek, C.Y.; Hwang, J.H.; Kim, M.-Y. Andrographis paniculata extract relieves pain and inflammation in monosodium iodoacetate-induced osteoarthritis and acetic acid-induced writhing in animal models. Processes 2020, 8, 873. [Google Scholar] [CrossRef]
- Molnar, V.; Matisic, V.; Kodvanj, I.; Bjelica, R.; Jelec, Z.; Hudetz, D.; Rod, E.; Cukelj, F.; Vrdoljak, T.; Vidovic, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Park, M.S.; Park, S.Y.; Choi, Y.D.; Chung, J.O.; Kim, D.H.; Jung, Y.D.; Kim, H.S. Primary bile acid activates Egr-1 expression through the MAPK signaling pathway in gastric cancer. Mol. Med. Rep. 2022, 25, 129. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, V.; Stellavato, A.; Russo, R.; Cimini, D.; Valletta, M.; Alfano, A.; Pedone, P.V.; Chambery, A.; Schiraldi, C. Molecular Fingerprint of Human Pathological Synoviocytes in Response to Extractive Sulfated and Biofermentative Unsulfated Chondroitins. Int. J. Mol. Sci. 2022, 23, 15865. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Kim, S.; Kim, S.Y. Effects of Roasted Schisandra chinensis (Turcz.) Baill and Lycium chinense Mill. and Their Combinational Extracts on Antioxidant and Anti-Inflammatory Activities in RAW 264.7 Cells and in Alcohol-Induced Liver Damage Mice Model. Evid.-Based Complement. Altern. Med. 2021, 2021, 6633886. [Google Scholar] [CrossRef]
- Sim, B.Y.; Choi, H.J.; Kim, M.G.; Jeong, D.G.; Lee, D.G.; Yoon, J.M.; Kang, D.J.; Park, S.; Ji, J.G.; Joo, I.H.; et al. Effects of ID-CBT5101 in Preventing and Alleviating Osteoarthritis Symptoms in a Monosodium Iodoacetate-Induced Rat Model. J. Microbiol. Biotechnol. 2018, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-R.; Lee, S.-H.; Noh, E.-M.; Choi, B.; Seo, H.-Y.; Jang, H.; Kim, S.-Y.; Park, M.H. Therapeutic Effect of Enzymatically Hydrolyzed Cervi Cornu Collagen NP-2007 and Potential for Application in Osteoarthritis Treatment. Int. J. Mol. Sci. 2023, 24, 11667. https://doi.org/10.3390/ijms241411667
Kim H-R, Lee S-H, Noh E-M, Choi B, Seo H-Y, Jang H, Kim S-Y, Park MH. Therapeutic Effect of Enzymatically Hydrolyzed Cervi Cornu Collagen NP-2007 and Potential for Application in Osteoarthritis Treatment. International Journal of Molecular Sciences. 2023; 24(14):11667. https://doi.org/10.3390/ijms241411667
Chicago/Turabian StyleKim, Ha-Rim, Seung-Hyeon Lee, Eun-Mi Noh, Bongsuk Choi, Hyang-Yim Seo, Hansu Jang, Seon-Young Kim, and Mi Hee Park. 2023. "Therapeutic Effect of Enzymatically Hydrolyzed Cervi Cornu Collagen NP-2007 and Potential for Application in Osteoarthritis Treatment" International Journal of Molecular Sciences 24, no. 14: 11667. https://doi.org/10.3390/ijms241411667




