Stress-Induced Changes in the Endogenous Opioid System Cause Dysfunction of Pain and Emotion Regulation
Abstract
:1. Introduction
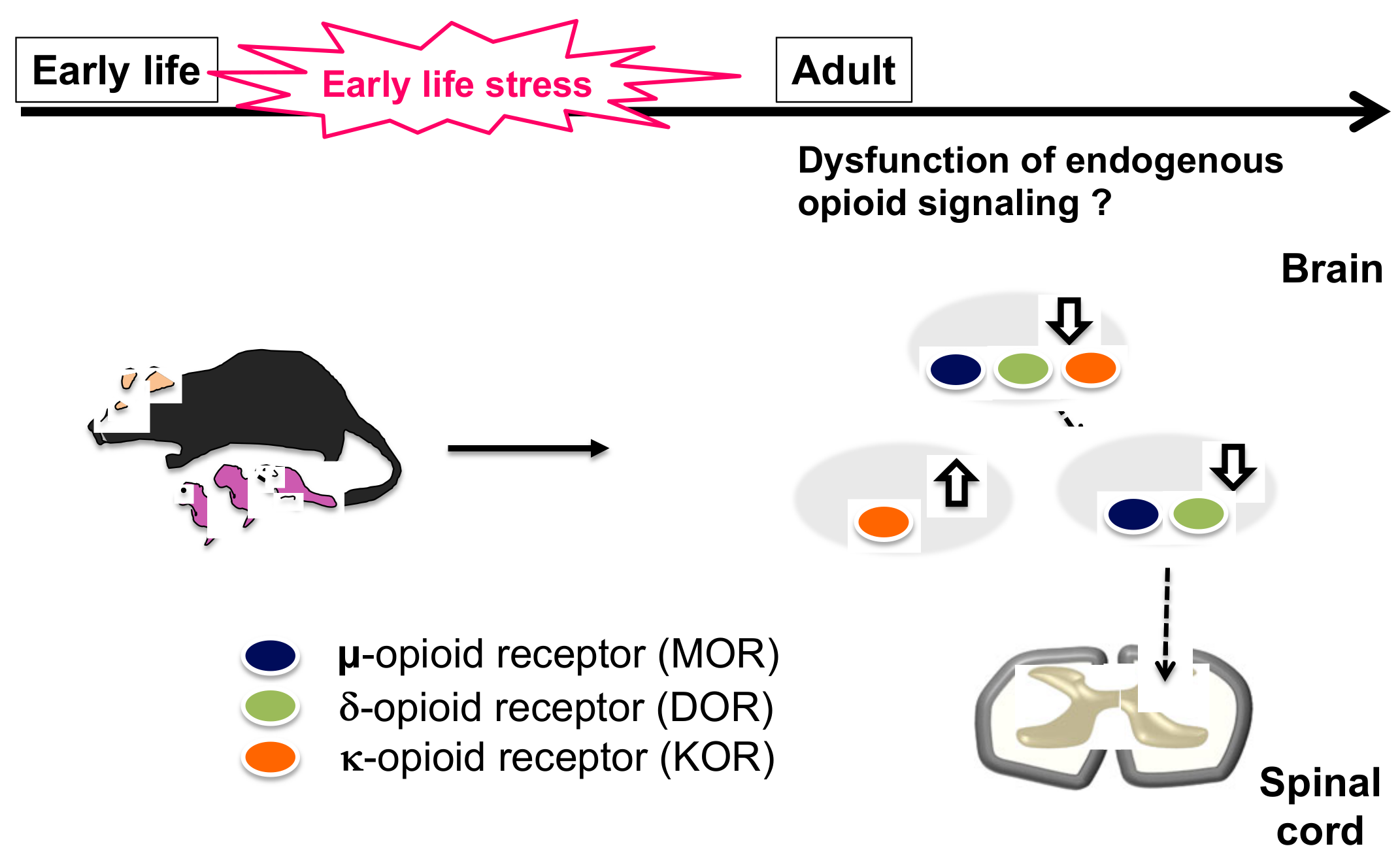
2. Early Life Stress
2.1. Influence of the Opioid Signal on ELS
2.2. Influence of ELS on Alteration of Pain Sensitivity and Chronic Pain
3. Psychosocial Stress and Psychiatric Disorders
3.1. Influence of Social Defeat Stress on Opioid Signaling
3.1.1. Social Defeat Stress and MOR
3.1.2. Social Defeat Stress and DOR
3.1.3. Social Defeat Stress and KOR
3.1.4. Social Defeat Stress and NOR
3.2. Influence of Psychosocial Stress on Alteration of Pain Sensitivity and Chronic Pain
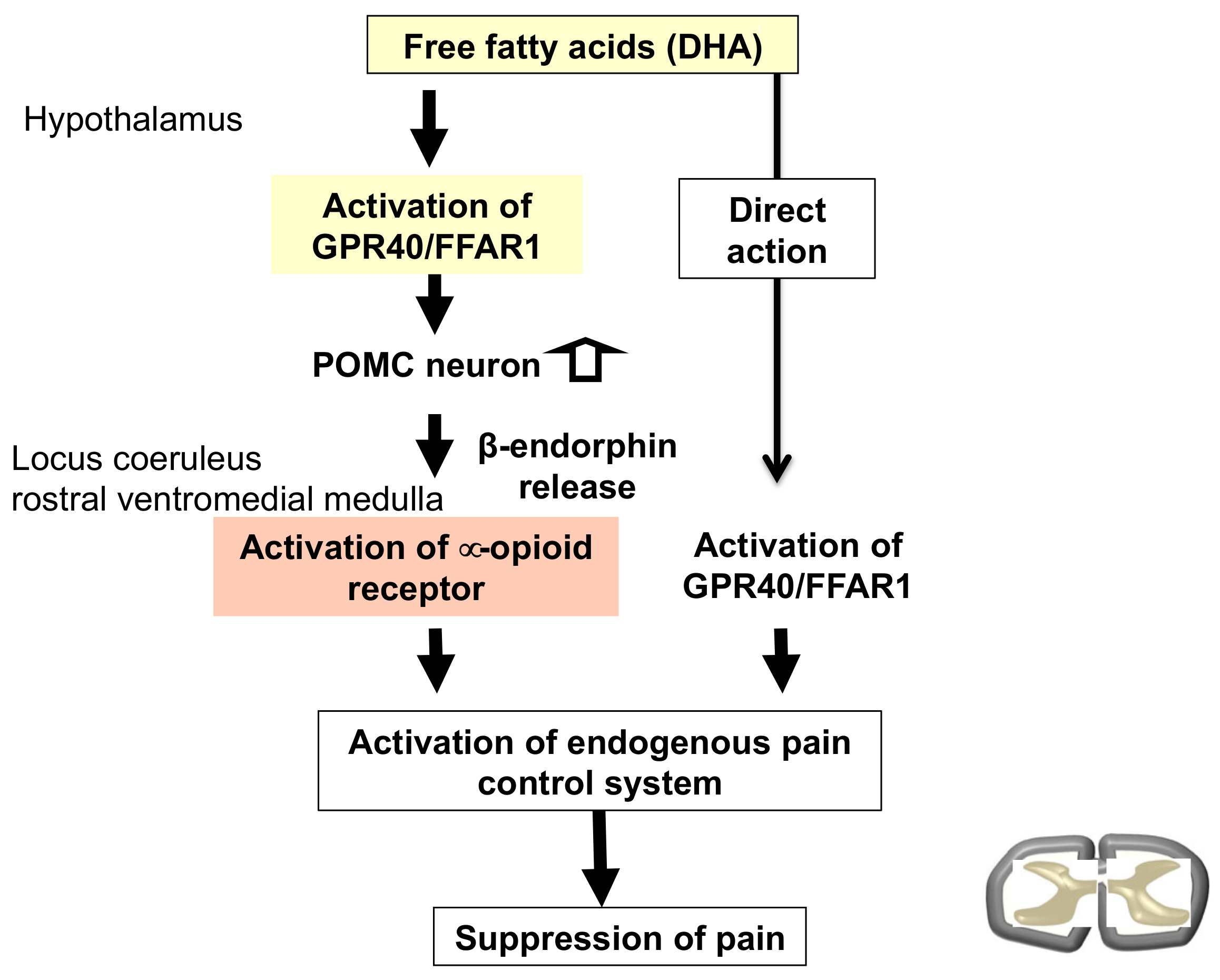
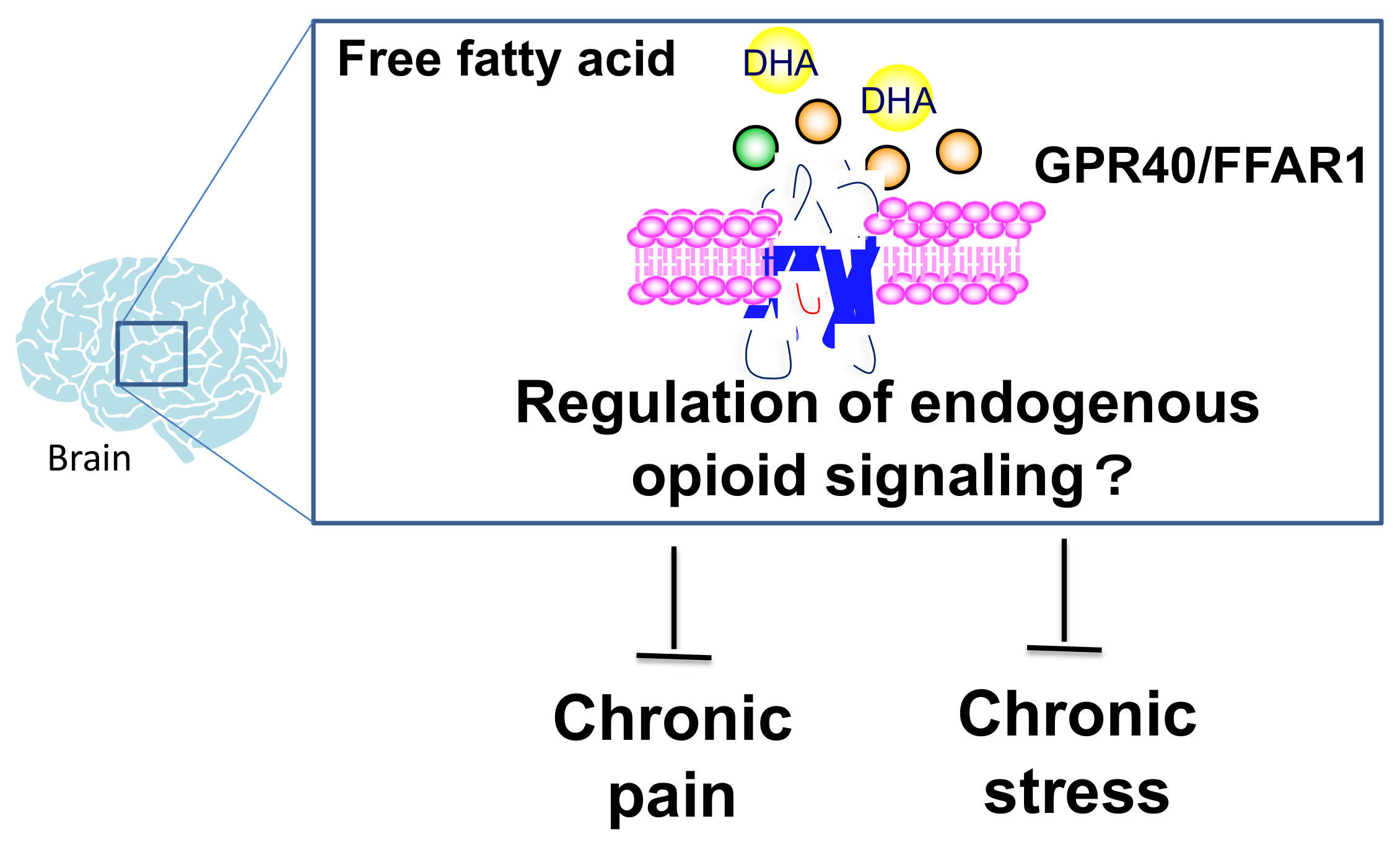
4. Involvement of Fatty Acid Receptor Signaling as a Modulator of the Opioid System
5. The Coronavirus Disease Increases Mental Illness and Chronic Pain
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hillis, S.; Mercy, J.; Amobi, A.; Kress, H. Global Prevalence of Past-year Violence against Children: A Systematic Review and Minimum Estimates. Pediatrics 2016, 137, e20154079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenewald, C.B.; Murray, C.B.; Palermo, T.M. Adverse childhood experiences and chronic pain among children and adolescents in the United States. Pain Rep. 2020, 5, e839. [Google Scholar] [CrossRef]
- Maercker, A.; Cloitre, M.; Bachem, R.; Schlumpf, Y.R.; Khoury, B.; Hitchcock, C.; Bohus, M. Complex post-traumatic stress disorder. Lancet 2022, 400, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Imbierowicz, K.; Egle, U.T. Childhood adversities in patients with fibromyalgia and somatoform pain disorder. Eur. J. Pain 2003, 7, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Power, C.; Macfarlane, G.J. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain 2009, 143, 92–96. [Google Scholar] [CrossRef]
- Anno, K.; Shibata, M.; Ninomiya, T.; Iwaki, R.; Kawata, H.; Sawamoto, R.; Kubo, C.; Kiyohara, Y.; Sudo, N.; Hosoi, M. Paternal and maternal bonding styles in childhood are associated with the prevalence of chronic pain in a general adult population: The Hisayama Study. BMC Psychiatry 2015, 15, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craner, J.R.; Lake, E.S.; Barr, A.C.; Kirby, K.E.; O’Neill, M. Childhood Adversity among Adults with Chronic Pain: Prevalence and Association with Pain-related Outcomes. Clin. J. Pain 2022, 38, 551–561. [Google Scholar] [CrossRef]
- Ciechanowski, P.; Sullivan, M.; Jensen, M.; Romano, J.; Summers, H. The relationship of attachment style to depression, catastrophizing and health care utilization in patients with chronic pain. Pain 2003, 104, 627–637. [Google Scholar] [CrossRef]
- Darcq, E.; Kieffer, B.L. Opioid receptors: Drivers to addiction? Nat. Rev. Neurosci. 2018, 19, 499–514. [Google Scholar] [CrossRef]
- Kieffer, B.L.; Evans, C.J. Opioid receptors: From binding sites to visible molecules in vivo. Neuropharmacology 2009, 56 (Suppl. 1), 205–212. [Google Scholar] [CrossRef] [Green Version]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granier, S.; Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Weis, W.I.; Kobilka, B.K. Structure of the delta-opioid receptor bound to naltrindole. Nature 2012, 485, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Wacker, D.; Mileni, M.; Katritch, V.; Han, G.W.; Vardy, E.; Liu, W.; Thompson, A.A.; Huang, X.P.; Carroll, F.I.; et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 2012, 485, 327–332. [Google Scholar] [CrossRef]
- Thompson, A.A.; Liu, W.; Chun, E.; Katritch, V.; Wu, H.; Vardy, E.; Huang, X.P.; Trapella, C.; Guerrini, R.; Calo, G.; et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 2012, 485, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Bloom, F.E.; Rossier, J.; Battenberg, E.L.; Bayon, A.; French, E.; Henriksen, S.J.; Siggins, G.R.; Segal, D.; Browne, R.; Ling, N.; et al. Beta-endorphin: Cellular localization, electrophysiological and behavioral effects. Adv. Biochem. Psychopharmacol. 1978, 18, 89–109. [Google Scholar]
- Bower, J.D.; Guest, K.P.; Morgan, B.A. Enkephalin. Synthesis of two pentapeptides isolated from porcine brain with receptor-mediated opiate agonist activity. J. Chem. Soc. Perkin Trans. 1 1976, 23, 2488–2492. [Google Scholar] [CrossRef]
- Lutz, P.E.; Gross, J.A.; Dhir, S.K.; Maussion, G.; Yang, J.; Bramoulle, A.; Meaney, M.J.; Turecki, G. Epigenetic Regulation of the Kappa Opioid Receptor by Child Abuse. Biol. Psychiatry 2018, 84, 751–761. [Google Scholar] [CrossRef]
- Nakamoto, K.; Taniguchi, A.; Tokuyama, S. Changes in opioid receptors, opioid peptides and morphine antinociception in mice subjected to early life stress. Eur. J. Pharmacol. 2020, 881, 173173. [Google Scholar] [CrossRef]
- Berube, P.; Laforest, S.; Bhatnagar, S.; Drolet, G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiol. Behav. 2013, 122, 237–245. [Google Scholar] [CrossRef]
- Gershenson, C.P. Child maltreatment, family stress, and ecological insult. Am. J. Public Health 1977, 67, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Tomoda, A.; Andersen, S.L. Neurobiological consequences of early stress and childhood maltreatment: Are results from human and animal studies comparable? Ann. N. Y. Acad. Sci. 2006, 1071, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.S.; Kennedy, M.; Kumsta, R.; Knights, N.; Golm, D.; Rutter, M.; Maughan, B.; Schlotz, W.; Kreppner, J. Child-to-adult neurodevelopmental and mental health trajectories after early life deprivation: The young adult follow-up of the longitudinal English and Romanian Adoptees study. Lancet 2017, 389, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrick, M.T.; Ford, D.C.; Ports, K.A.; Guinn, A.S. Prevalence of Adverse Childhood Experiences From the 2011-2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA Pediatr. 2018, 172, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Finkelhor, D. Screening for adverse childhood experiences (ACEs): Cautions and suggestions. Child Abus. Negl. 2018, 85, 174–179. [Google Scholar] [CrossRef]
- Oswald, L.M.; Dunn, K.E.; Seminowicz, D.A.; Storr, C.L. Early Life Stress and Risks for Opioid Misuse: Review of Data Supporting Neurobiological Underpinnings. J. Pers. Med. 2021, 11, 315. [Google Scholar] [CrossRef]
- Panksepp, J.; Herman, B.H.; Vilberg, T.; Bishop, P.; DeEskinazi, F.G. Endogenous opioids and social behavior. Neurosci. Biobehav. Rev. 1980, 4, 473–487. [Google Scholar] [CrossRef]
- Siegel, M.A.; Jensen, R.A.; Panksepp, J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behav. Neural. Biol. 1985, 44, 509–514. [Google Scholar] [CrossRef]
- Moles, A.; Kieffer, B.L.; D’Amato, F.R. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science 2004, 304, 1983–1986. [Google Scholar] [CrossRef]
- Becker, J.A.; Clesse, D.; Spiegelhalter, C.; Schwab, Y.; Le Merrer, J.; Kieffer, B.L. Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology 2014, 39, 2049–2060. [Google Scholar] [CrossRef] [Green Version]
- Pellissier, L.P.; Gandia, J.; Laboute, T.; Becker, J.A.J.; Le Merrer, J. Mu opioid receptor, social behaviour and autism spectrum disorder: Reward matters. Br. J. Pharmacol. 2018, 175, 2750–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, W.E.; Sun, H.; Costello, E.J.; Angold, A.; Heilig, M.A.; Barr, C.S. Child mu-opioid receptor gene variant influences parent-child relations. Neuropsychopharmacology 2011, 36, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karkhanis, A.N.; Rose, J.H.; Weiner, J.L.; Jones, S.R. Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology 2016, 41, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Michaels, C.C.; Holtzman, S.G. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J. Pharmacol. Exp. Ther. 2008, 325, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Kigar, S.L.; Ho, J.H.; Cuarenta, A.; Gunderson, H.C.; Baldo, B.A.; Bakshi, V.P.; Auger, A.P. Early life stress alters opioid receptor mRNA levels within the nucleus accumbens in a sex-dependent manner. Brain Res. 2019, 1710, 102–108. [Google Scholar] [CrossRef]
- Vazquez, V.; Penit-Soria, J.; Durand, C.; Besson, M.J.; Giros, B.; Dauge, V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J. Neurosci. 2005, 25, 4453–4462. [Google Scholar] [CrossRef] [Green Version]
- Andero, R. Nociceptin and the nociceptin receptor in learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 62, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, M.; Inoue, K. Effects of nocistatin on nociceptin-induced impairment of learning and memory in mice. Eur. J. Pharmacol. 1999, 367, 151–155. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Miwa, M.; Hashimoto, K.; Kawai, S.; Nomura, N. Nociceptin/orphanin FQ reverses mecamylamine-induced learning and memory impairment as well as decrease in hippocampal acetylcholine release in the rat. Brain Res. 2008, 1195, 96–103. [Google Scholar] [CrossRef]
- Ke, X.; Huang, Y.; Fu, Q.; Majnik, A.; Sampath, V.; Lane, R.H. Adverse maternal environment alters Oprl1 variant expression in mouse hippocampus. Anat. Rec. 2023, 306, 162–175. [Google Scholar] [CrossRef]
- Nishinaka, T.; Nakamoto, K.; Tokuyama, S. Enhancement of nerve-injury-induced thermal and mechanical hypersensitivity in adult male and female mice following early life stress. Life Sci. 2015, 121, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Niwa, M.; Matsumoto, Y.; Mouri, A.; Ozaki, N.; Nabeshima, T. Vulnerability in early life to changes in the rearing environment plays a crucial role in the aetiopathology of psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 459–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kubota, A.; Iwama, T.; Wada, T.; Yasui, M.; Fujibayashi, K.; Takagi, H. Comparison of analgesic potencies of mu, delta and kappa agonists locally applied to various CNS regions relevant to analgesia in rats. Life Sci. 1983, 33 (Suppl. 1), 689–692. [Google Scholar] [CrossRef] [PubMed]
- Shaham, Y. Effect of stress on opioid-seeking behavior: Evidence from studies with rats. Ann. Behav. Med. 1996, 18, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Shaham, Y. Immobilization stress-induced oral opioid self-administration and withdrawal in rats: Role of conditioning factors and the effect of stress on “relapse” to opioid drugs. Psychopharmacology 1993, 111, 477–485. [Google Scholar] [CrossRef]
- Shaham, Y.; Alvares, K.; Nespor, S.M.; Grunberg, N.E. Effect of stress on oral morphine and fentanyl self-administration in rats. Pharmacol. Biochem. Behav. 1992, 41, 615–619. [Google Scholar] [CrossRef]
- Ide, S.; Satoyoshi, H.; Minami, M.; Satoh, M. Amelioration of the reduced antinociceptive effect of morphine in the unpredictable chronic mild stress model mice by noradrenalin but not serotonin reuptake inhibitors. Mol. Pain 2015, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Tidmarsh, L.V.; Harrison, R.; Ravindran, D.; Matthews, S.L.; Finlay, K.A. The Influence of Adverse Childhood Experiences in Pain Management: Mechanisms, Processes, and Trauma-Informed Care. Front. Pain Res. 2022, 3, 923866. [Google Scholar] [CrossRef]
- Nicolson, N.A.; Davis, M.C.; Kruszewski, D.; Zautra, A.J. Childhood maltreatment and diurnal cortisol patterns in women with chronic pain. Psychosom. Med. 2010, 72, 471–480. [Google Scholar] [CrossRef]
- Gardoki-Souto, I.; Redolar-Ripoll, D.; Fontana, M.; Hogg, B.; Castro, M.J.; Blanch, J.M.; Ojeda, F.; Solanes, A.; Radua, J.; Valiente-Gomez, A.; et al. Prevalence and Characterization of Psychological Trauma in Patients with Fibromyalgia: A Cross-Sectional Study. Pain Res. Manag. 2022, 2022, 2114451. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.H.; Clohessy, D.S.; Stone, A.L.; Darnall, B.D.; Wilson, A.C. Adverse Childhood Experiences in Mothers with Chronic Pain and Intergenerational Impact on Children. J. Pain 2019, 20, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Luecken, L.J.; Zautra, A.J. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin. J. Pain 2005, 21, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Burke, N.N.; Llorente, R.; Marco, E.M.; Tong, K.; Finn, D.P.; Viveros, M.P.; Roche, M. Maternal deprivation is associated with sex-dependent alterations in nociceptive behavior and neuroinflammatory mediators in the rat following peripheral nerve injury. J. Pain 2013, 14, 1173–1184. [Google Scholar] [CrossRef]
- Low, L.A.; Schweinhardt, P. Early life adversity as a risk factor for fibromyalgia in later life. Pain Res. Treat. 2012, 2012, 140832. [Google Scholar] [CrossRef] [Green Version]
- Louwies, T.; Mohammadi, E.; Greenwood-Van Meerveld, B. Epigenetic mechanisms underlying stress-induced visceral pain: Resilience versus vulnerability in a two-hit model of early life stress and chronic adult stress. Neurogastroenterol. Motil. 2023, 35, e14558. [Google Scholar] [CrossRef]
- Louwies, T.; Greenwood-Van Meerveld, B. Sex differences in the epigenetic regulation of chronic visceral pain following unpredictable early life stress. Neurogastroenterol. Motil. 2020, 32, e13751. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E., III; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, H.; Ohara, A.; Sasaki, K.; Abe, H.; Hattori, H.; Hall, F.S.; Uhl, G.R.; Sora, I. Decreased response to social defeat stress in mu-opioid-receptor knockout mice. Pharmacol. Biochem. Behav. 2011, 99, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Briand, L.A.; Hilario, M.; Dow, H.C.; Brodkin, E.S.; Blendy, J.A.; Berton, O. Mouse model of OPRM1 (A118G) polymorphism increases sociability and dominance and confers resilience to social defeat. J. Neurosci. 2015, 35, 3582–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikulina, E.M.; Hammer, R.P., Jr.; Miczek, K.A.; Kream, R.M. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. Neuroreport 1999, 10, 3015–3019. [Google Scholar] [CrossRef] [PubMed]
- Nikulina, E.M.; Arrillaga-Romany, I.; Miczek, K.A.; Hammer, R.P., Jr. Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: Time course of mu-opioid receptor mRNA and FosB/DeltaFosB immunoreactivity. Eur. J. Neurosci. 2008, 27, 2272–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, C.E.; Herschel, D.J.; Lasek, A.W.; Hammer, R.P., Jr.; Nikulina, E.M. Knockdown of ventral tegmental area mu-opioid receptors in rats prevents effects of social defeat stress: Implications for amphetamine cross-sensitization, social avoidance, weight regulation and expression of brain-derived neurotrophic factor. Neuropharmacology 2015, 89, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, T.; Konno, K.; Uchigashima, M.; Yanagawa, Y.; Sora, I.; Minami, M.; Watanabe, M. GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis. Eur. J. Neurosci. 2014, 39, 1796–1809. [Google Scholar] [CrossRef]
- Garzon, M.; Pickel, V.M. Ultrastructural localization of enkephalin and mu-opioid receptors in the rat ventral tegmental area. Neuroscience 2002, 114, 461–474. [Google Scholar] [CrossRef]
- Sesack, S.R.; Pickel, V.M. Ultrastructural relationships between terminals immunoreactive for enkephalin, GABA, or both transmitters in the rat ventral tegmental area. Brain Res. 1995, 672, 261–275. [Google Scholar] [CrossRef]
- Dacher, M.; Nugent, F.S. Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area. Neuropharmacology 2011, 61, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Pascoe, J.E.; Williams, K.L.; Mukhopadhyay, P.; Rice, K.C.; Woods, J.H.; Ko, M.C. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology 2008, 33, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Hoehe, M.; Duka, T.; Doenicke, A. Human studies on the mu opiate receptor agonist fentanyl: Neuroendocrine and behavioral responses. Psychoneuroendocrinology 1988, 13, 397–408. [Google Scholar] [CrossRef]
- Iyengar, S.; Kim, H.S.; Wood, P.L. Mu-, delta-, kappa- and epsilon-opioid receptor modulation of the hypothalamic-pituitary-adrenocortical (HPA) axis: Subchronic tolerance studies of endogenous opioid peptides. Brain Res. 1987, 435, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Charboneau, R.; Barke, R.A.; Loh, H.H.; Roy, S. Mu-opioid receptor mediates chronic restraint stress-induced lymphocyte apoptosis. J. Immunol. 2002, 169, 3630–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filliol, D.; Ghozland, S.; Chluba, J.; Martin, M.; Matthes, H.W.; Simonin, F.; Befort, K.; Gaveriaux-Ruff, C.; Dierich, A.; LeMeur, M.; et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 2000, 25, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.A.; Hoshaw, B.A.; Unterwald, E.M. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br. J. Pharmacol. 2006, 147, 864–872. [Google Scholar] [CrossRef]
- Broom, D.C.; Jutkiewicz, E.M.; Folk, J.E.; Traynor, J.R.; Rice, K.C.; Woods, J.H. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology 2002, 26, 744–755. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, A.; Sugiyama, A.; Nemoto, T.; Fujii, H.; Wada, K.; Oka, J.; Nagase, H.; Yamada, M. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav. Brain Res. 2011, 223, 271–279. [Google Scholar] [CrossRef]
- Henry, M.S.; Gendron, L.; Tremblay, M.E.; Drolet, G. Enkephalins: Endogenous Analgesics with an Emerging Role in Stress Resilience. Neural Plast. 2017, 2017, 1546125. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.S.; Bisht, K.; Vernoux, N.; Gendron, L.; Torres-Berrio, A.; Drolet, G.; Tremblay, M.E. Delta Opioid Receptor Signaling Promotes Resilience to Stress Under the Repeated Social Defeat Paradigm in Mice. Front. Mol. Neurosci. 2018, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.; Chandra, R.; Francis, T.C.; Dias, C.; Cheer, J.F.; Lobo, M.K. Reduced nucleus accumbens enkephalins underlie vulnerability to social defeat stress. Neuropsychopharmacology 2019, 44, 1876–1885. [Google Scholar] [CrossRef]
- Yoshioka, T.; Yamada, D.; Segi-Nishida, E.; Nagase, H.; Saitoh, A. KNT-127, a selective delta opioid receptor agonist, shows beneficial effects in the hippocampal dentate gyrus of a chronic vicarious social defeat stress mouse model. Neuropharmacology 2023, 232, 109511. [Google Scholar] [CrossRef]
- Chavkin, C.; James, I.F.; Goldstein, A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 1982, 215, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Land, B.B.; Bruchas, M.R.; Lemos, J.C.; Xu, M.; Melief, E.J.; Chavkin, C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008, 28, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, J.P.; Li, S.; Valdez, J.; Chavkin, T.A.; Chavkin, C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 2006, 31, 1241–1248. [Google Scholar] [CrossRef]
- Wells, A.M.; Ridener, E.; Bourbonais, C.A.; Kim, W.; Pantazopoulos, H.; Carroll, F.I.; Kim, K.S.; Cohen, B.M.; Carlezon, W.A., Jr. Effects of Chronic Social Defeat Stress on Sleep and Circadian Rhythms Are Mitigated by Kappa-Opioid Receptor Antagonism. J. Neurosci. 2017, 37, 7656–7668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zan, G.Y.; Sun, X.; Wang, Y.J.; Liu, R.; Wang, C.Y.; Du, W.J.; Guo, L.B.; Chai, J.R.; Li, Q.L.; Liu, Z.Q.; et al. Amygdala dynorphin/kappa opioid receptor system modulates depressive-like behavior in mice following chronic social defeat stress. Acta Pharmacol. Sin. 2022, 43, 577–587. [Google Scholar] [CrossRef]
- Fontaine, H.M.; Silva, P.R.; Neiswanger, C.; Tran, R.; Abraham, A.D.; Land, B.B.; Neumaier, J.F.; Chavkin, C. Stress decreases serotonin tone in the nucleus accumbens in male mice to promote aversion and potentiate cocaine preference via decreased stimulation of 5-HT(1B) receptors. Neuropsychopharmacology 2022, 47, 891–901. [Google Scholar] [CrossRef]
- Le, A.D.; Funk, D.; Coen, K.; Tamadon, S.; Shaham, Y. Role of kappa-Opioid Receptors in the Bed Nucleus of Stria Terminalis in Reinstatement of Alcohol Seeking. Neuropsychopharmacology 2018, 43, 838–850. [Google Scholar] [CrossRef] [Green Version]
- Ehrich, J.M.; Messinger, D.I.; Knakal, C.R.; Kuhar, J.R.; Schattauer, S.S.; Bruchas, M.R.; Zweifel, L.S.; Kieffer, B.L.; Phillips, P.E.; Chavkin, C. Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons. J. Neurosci. 2015, 35, 12917–12931. [Google Scholar] [CrossRef]
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef]
- Bruchas, M.R.; Land, B.B.; Chavkin, C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010, 1314, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Haun, H.L.; Lebonville, C.L.; Solomon, M.G.; Griffin, W.C.; Lopez, M.F.; Becker, H.C. Dynorphin/Kappa Opioid Receptor Activity within the Extended Amygdala Contributes to Stress-Enhanced Alcohol Drinking in Mice. Biol. Psychiatry 2022, 91, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Krystal, A.D.; Pizzagalli, D.A.; Smoski, M.; Mathew, S.J.; Nurnberger, J., Jr.; Lisanby, S.H.; Iosifescu, D.; Murrough, J.W.; Yang, H.; Weiner, R.D.; et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating kappa-opioid antagonism as a treatment for anhedonia. Nat. Med. 2020, 26, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Rorick-Kehn, L.M.; Witkin, J.M.; Statnick, M.A.; Eberle, E.L.; McKinzie, J.H.; Kahl, S.D.; Forster, B.M.; Wong, C.J.; Li, X.; Crile, R.S.; et al. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 2014, 77, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.; Montkowski, A.; Schulz, S.; Stube, E.M.; Knaudt, K.; Jenck, F.; Moreau, J.L.; Nothacker, H.P.; Civelli, O.; Reinscheid, R.K. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 10444–10449. [Google Scholar] [CrossRef]
- Rizzi, A.; Molinari, S.; Marti, M.; Marzola, G.; Calo, G. Nociceptin/orphanin FQ receptor knockout rats: In vitro and in vivo studies. Neuropharmacology 2011, 60, 572–579. [Google Scholar] [CrossRef]
- Gavioli, E.C.; Calo, G. Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol. Ther. 2013, 140, 10–25. [Google Scholar] [CrossRef]
- Gavioli, E.C.; Holanda, V.A.D.; Ruzza, C. NOP Ligands for the Treatment of Anxiety and Mood Disorders; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 254, pp. 233–257. [Google Scholar] [CrossRef]
- Ozawa, A.; Brunori, G.; Mercatelli, D.; Wu, J.; Cippitelli, A.; Zou, B.; Xie, X.S.; Williams, M.; Zaveri, N.T.; Low, S.; et al. Knock-In Mice with NOP-eGFP Receptors Identify Receptor Cellular and Regional Localization. J. Neurosci. 2015, 35, 11682–11693. [Google Scholar] [CrossRef] [Green Version]
- Goeldner, C.; Reiss, D.; Kieffer, B.L.; Ouagazzal, A.M. Endogenous nociceptin/orphanin-FQ in the dorsal hippocampus facilitates despair-related behavior. Hippocampus 2010, 20, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Ubaldi, M.; Cannella, N.; Borruto, A.M.; Petrella, M.; Micioni Di Bonaventura, M.V.; Soverchia, L.; Stopponi, S.; Weiss, F.; Cifani, C.; Ciccocioppo, R. Role of Nociceptin/Orphanin FQ-NOP Receptor System in the Regulation of Stress-Related Disorders. Int. J. Mol. Sci. 2021, 22, 12956. [Google Scholar] [CrossRef]
- Camara, A.B.; Brandao, I.A. Behavioral and neurochemical effects of nociceptin/orphanin FQ receptor activation in the social defeat protocol. Behav. Neurosci. 2023, 137, 52–66. [Google Scholar] [CrossRef]
- Der-Avakian, A.; D’Souza, M.S.; Potter, D.N.; Chartoff, E.H.; Carlezon, W.A., Jr.; Pizzagalli, D.A.; Markou, A. Social defeat disrupts reward learning and potentiates striatal nociceptin/orphanin FQ mRNA in rats. Psychopharmacology 2017, 234, 1603–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.K.; Devine, D.P. Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides 2009, 43, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccocioppo, R.; de Guglielmo, G.; Hansson, A.C.; Ubaldi, M.; Kallupi, M.; Cruz, M.T.; Oleata, C.S.; Heilig, M.; Roberto, M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: Significance for anxiety-like behaviors. J. Neurosci. 2014, 34, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nativio, P.; Pascale, E.; Maffei, A.; Scaccianoce, S.; Passarelli, F. Effect of stress on hippocampal nociceptin expression in the rat. Stress 2012, 15, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Statnick, M.A.; Rorick-Kehn, L.M.; Pintar, J.E.; Ansonoff, M.; Chen, Y.; Tucker, R.C.; Ciccocioppo, R. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol. Ther. 2014, 141, 283–299. [Google Scholar] [CrossRef] [Green Version]
- Witkin, J.M.; Wallace, T.L.; Martin, W.J. Therapeutic Approaches for NOP Receptor Antagonists in Neurobehavioral Disorders: Clinical Studies in Major Depressive Disorder and Alcohol Use Disorder with BTRX-246040 (LY2940094); Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 254, pp. 399–415. [Google Scholar] [CrossRef]
- Rivat, C.; Becker, C.; Blugeot, A.; Zeau, B.; Mauborgne, A.; Pohl, M.; Benoliel, J.J. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain 2010, 150, 358–368. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Liu, S.; Fang, H.; Zhang, Y.; Furmanski, O.; Skinner, J.; Xing, Y.; Johns, R.A.; Huganir, R.L.; et al. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J. Neurosci. 2014, 34, 13737–13746. [Google Scholar] [CrossRef]
- Arora, V.; Martin, T.J.; Aschenbrenner, C.A.; Hayashida, K.; Kim, S.A.; Parker, R.A.; Eisenach, J.C.; Peters, C.M. Psychosocial Stress Delays Recovery of Postoperative Pain Following Incisional Surgery in the Rat. Neuroscience 2018, 382, 35–47. [Google Scholar] [CrossRef]
- Aizawa, F.; Nakamoto, K.; Tokuyama, S. The involvement of free fatty acid-GPR40/FFAR1 signaling in chronic social defeat stress-induced pain prolongation in C57BL/6J male mice. Psychopharmacology 2018, 235, 2335–2347. [Google Scholar] [CrossRef]
- Suarez-Roca, H.; Leal, L.; Silva, J.A.; Pinerua-Shuhaibar, L.; Quintero, L. Reduced GABA neurotransmission underlies hyperalgesia induced by repeated forced swimming stress. Behav. Brain Res. 2008, 189, 159–169. [Google Scholar] [CrossRef]
- Miczek, K.A.; Thompson, M.L.; Shuster, L. Opioid-like analgesia in defeated mice. Science 1982, 215, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Coderre, T.J.; Rollman, G.B. Stress analgesia: Effects of PCPA, yohimbine, and naloxone. Pharmacol. Biochem. Behav. 1984, 21, 681–686. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Suplita, R.L.; Bolton, N.M.; Neely, M.H.; Fegley, D.; Mangieri, R.; Krey, J.F.; Walker, J.M.; Holmes, P.V.; Crystal, J.D.; et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 2005, 435, 1108–1112. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K.; Nishinaka, T.; Mankura, M.; Fujita-Hamabe, W.; Tokuyama, S. Antinociceptive effects of docosahexaenoic acid against various pain stimuli in mice. Biol. Pharm. Bull. 2010, 33, 1070–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, K.; Nishinaka, T.; Ambo, A.; Mankura, M.; Kasuya, F.; Tokuyama, S. Possible involvement of beta-endorphin in docosahexaenoic acid-induced antinociception. Eur. J. Pharmacol. 2011, 666, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [Green Version]
- Nishizaki, H.; Matsuoka, O.; Kagawa, T.; Kobayashi, A.; Watanabe, M.; Moritoh, Y. Erratum. SCO-267, a GPR40 Full Agonist, Stimulates Islet and Gut Hormone Secretion and Improves Glycemic Control in Humans. Diabetes 2021, 70, 2364–2376. [Google Scholar] [CrossRef]
- Ho, J.D.; Chau, B.; Rodgers, L.; Lu, F.; Wilbur, K.L.; Otto, K.A.; Chen, Y.; Song, M.; Riley, J.P.; Yang, H.C.; et al. Structural basis for GPR40 allosteric agonism and incretin stimulation. Nat. Commun. 2018, 9, 1645. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K.; Nishinaka, T.; Matsumoto, K.; Kasuya, F.; Mankura, M.; Koyama, Y.; Tokuyama, S. Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res. 2012, 1432, 74–83. [Google Scholar] [CrossRef]
- Nakamoto, K.; Aizawa, F.; Miyagi, K.; Yamashita, T.; Mankura, M.; Koyama, Y.; Kasuya, F.; Hirasawa, A.; Kurihara, T.; Miyata, A.; et al. Dysfunctional GPR40/FFAR1 signaling exacerbates pain behavior in mice. PLoS ONE 2017, 12, e0180610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, K.; Nishinaka, T.; Sato, N.; Aizawa, F.; Yamashita, T.; Mankura, M.; Koyama, Y.; Kasuya, F.; Tokuyama, S. The activation of supraspinal GPR40/FFA1 receptor signalling regulates the descending pain control system. Br. J. Pharmacol. 2015, 172, 1250–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, K.; Aizawa, F.; Nishinaka, T.; Tokuyama, S. Regulation of prohormone convertase 2 protein expression via GPR40/FFA1 in the hypothalamus. Eur. J. Pharmacol. 2015, 762, 459–463. [Google Scholar] [CrossRef]
- Aizawa, F.; Sato, S.; Yamazaki, F.; Yao, I.; Yamashita, T.; Nakamoto, K.; Kasuya, F.; Setou, M.; Tokuyama, S. N-3 fatty acids modulate repeated stress-evoked pain chronicity. Brain Res. 2019, 1714, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munster, V.J.; Koopmans, M.; van Doremalen, N.; van Riel, D.; de Wit, E. A Novel Coronavirus Emerging in China—Key Questions for Impact Assessment. N. Engl. J. Med. 2020, 382, 692–694. [Google Scholar] [CrossRef]
- Pfefferbaum, B.; North, C.S. Mental Health and the COVID-19 Pandemic. N. Engl. J. Med. 2020, 383, 510–512. [Google Scholar] [CrossRef]
- Jernslett, M.; Anastassiou-Hadjicharalambous, X.; Lioupi, C.; Syros, I.; Kapatais, A.; Karamanoli, V.; Evgeniou, E.; Messas, K.; Palaiokosta, T.; Papathanasiou, E.; et al. Disentangling the associations between past childhood adversity and psychopathology during the COVID-19 pandemic: The mediating roles of specific pandemic stressors and coping strategies. Child Abus. Negl. 2022, 129, 105673. [Google Scholar] [CrossRef]
- Cuartas, J. Heightened risk of child maltreatment amid the COVID-19 pandemic can exacerbate mental health problems for the next generation. Psychol. Trauma 2020, 12, S195–S196. [Google Scholar] [CrossRef]
- Letourneau, N.; Luis, M.A.; Kurbatfinski, S.; Ferrara, H.J.; Pohl, C.; Marabotti, F.; Hayden, K.A. COVID-19 and family violence: A rapid review of literature published up to 1 year after the pandemic declaration. EClinicalMedicine 2022, 53, 101634. [Google Scholar] [CrossRef]
- Kim, A.W.; Nyengerai, T.; Mendenhall, E. Evaluating the mental health impacts of the COVID-19 pandemic: Perceived risk of COVID-19 infection and childhood trauma predict adult depressive symptoms in urban South Africa. Psychol. Med. 2022, 52, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Calvano, C.; Engelke, L.; Di Bella, J.; Kindermann, J.; Renneberg, B.; Winter, S.M. Families in the COVID-19 pandemic: Parental stress, parent mental health and the occurrence of adverse childhood experiences-results of a representative survey in Germany. Eur. Child Adolesc. Psychiatry 2022, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meulders, A.; Vlaeyen, J.W.S.; Evers, A.W.M.; Koke, A.J.A.; Smeets, R.; Van Zundert, J.H.M.; Verbunt, J.; Van Ryckeghem, D.M.L. Chronic primary pain in the COVID-19 pandemic: How uncertainty and stress impact on functioning and suffering. Pain 2022, 163, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Hauser, W.; Cohen, S.P.; Fitzcharles, M.A. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain 2020, 161, 1694–1697. [Google Scholar] [CrossRef]
- Karos, K.; McParland, J.L.; Bunzli, S.; Devan, H.; Hirsh, A.; Kapos, F.P.; Keogh, E.; Moore, D.; Tracy, L.M.; Ashton-James, C.E. The social threats of COVID-19 for people with chronic pain. Pain 2020, 161, 2229–2235. [Google Scholar] [CrossRef]
| Behavioral and Functional Changes via Opioid Receptor after Exposure of EARLY life Stress | |||
|---|---|---|---|
| Pre-Clinical/Clinical | Model | Changes of Behabioral Phenotype and Each Opioid Receptor Expression in Stressed Mice | References No. |
| Pre-clinical | ELS model mice induced by maternal separation and social isolation (MSSI) | MSSI mice decreased MOR, DOR, and KOR mRNA expression in the periaqueductal gray matter. In contrast, the expression of KOR mRNA was significantly increased in the amygdala of MSSI mice | [19] |
| Pre-clinical | Morphine or Naloxone treated mice | Morphine, a MOR agonist, decreased proximity maintenance time in socially housed animals, increases play, whereas naloxonean, an opioid antagonist, reduces play and disrupts pup-retrieval | [27] |
| Pre-clinical | Naloxone treated mice | Naloxone, an opioid antagonist, decreased play, social behavior and decreased food consumption | [28] |
| Pre-clinical | MOR knockout mice | MOR knockout mice reduced maternal attachment in mouse pups. Deficits in social behavior | [29] |
| Pre-clinical | MOR knockout mice | MOR knockout mice decreased social interaction, and exacerbation of anxiety, which are the core and secondary symptoms of autism spectrum disorder | [30,31] |
| Pre-clinical | Rats with social isolation stress during adolescence | Stressed rats exhibited greater ethanol intake and preference, and induced increases in KOR function contribute to the dysregulation of NAc dopamine | [33] |
| Pre-clinical | Rats with maternal separation stress | Early postnatal stress can significantly alter the rewarding or aversive value of MOR agonist (morphine) and KOR agonists (spiradoline) when measured using place conditioning | [34] |
| Pre-clinical | Male and female rats subjected to a neonatal predator odor exposure (POE) paradigm | POE down-regulated neonatal MOR and KOR mRNA levels in neonatal females, but upregulated MOR and DOR mRNA levels in juvenile females, but not male mice | [35] |
| Pre-clinical | Rats with maternal separation stress | A significant decrease in preproenkephalin mRNA expression was observed in the nucleus accumbens and the caudate-putamen nucleus of deprived rats | [36] |
| Pre-clinical | The western diet and the environmental stress exposure to the mother during gestation | Mice showed the increased hippocampal Oprl1 mRNA and Oprl1 variants | [40] |
| Clinical | The downregulation of KOR in the anterior insula of Postmortem samples | A history of child abuse was explicitly associated with the downregulation of KOR and decreased DNA methylation in the second intron of Kappa gene in the anterior insula | [18] |
| Clinical | The child MOR variant OPRM1 A118G | The child MOR variant OPRM1 A118G improved parent-child relationships | [32] |
| Early Life Stress and Pain | |||
| Pre-Clinical/Clinical | Model | Changes of Behabioral Phenotype and Each Opioid Receptor Expression in Stressed Mice | References No. |
| Pre-clinical | Mice with maternal separation and social isolation with partial sciatic nerve ligation | MSSI mice with partial sciatic nerve ligation experienced an exacerbation of neuropathic pain compared to non-stressed mice | [19] |
| Pre-clinical | Mice with maternal separation and social isolation with L5–L6 spinal nerve ligation | Maternal deprivated female, but not male, rats exhibit enhanced nociceptive responding following peripheral nerve injury | [54] |
| Pre-clinical | Mice with maternal separation and social isolation with partial sciatic nerve ligation | Early Life Adversity as a Risk Factor for Fibromyalgia in Later Life | [55] |
| Pre-clinical | Mice with maternal separation and social isolation with partial sciatic nerve ligation | Women with a history of ELS have a higher risk of developing irritable bowel syndrome | [56,57] |
| Clinical | Cross-sectional analysis of the 2016–2017 National Survey of Children’s Health | Children with exposure to 1 or more ACEs had higher rates of chronic pain as compared to those with no reported ACEs. Children and adolescents with ACEs had increased risk for chronic pain | [2] |
| Clinical | Data from the 1958 British Birth Cohort Study | Children who had resided in institutional care experienced an increase in the risk of chronic widespread pain at adulthood as did those who experienced maternal death and familial financial hardship | [5] |
| Clinical | Self-report (Childhood Trauma Questionnaire-short form | In women with chronic pain, self-reported childhood maltreatment was associated with higher diurnal cortisol levels | [49] |
| Clinical | Eighty-eight females in A Cross-Sectional Study | Fibromyalgia patients having suffered traumatic events throughout their lifespan, especially in childhood and early adolescence. All patients showed clinically relevant levels of anxiety, depression, insomnia, suicidal thoughts, and pain, as well as somatic comorbidities and poor quality of life | [50] |
| Clinical | A sample of mothers with chronic pain and their 8- to 12-year-old children in A Cross-Sectional Study | Higher maternal adverse childhood experiences (ACEs) scores corresponded with depressive symptoms and greater anxiety, greater sleep disturbances and fatigue, and greater pain intensity in mothers | [51] |
| Clinical | Meta analysis review | Children who experienced childhood abuse and neglect had worse pain symptoms than those who did not experience early life stress (ELS) | [52] |
| Social Defeat Stress and Opioid | |||
| Pre-Clinical/Clinical | Model | Changes of Behabioral Phenotype and Each Opioid Receptor Expression in Stressed Mice | References No. |
| Pre-clinical | Sprague-Dawley rats | Enkephaline mRNA expression was lower in the basolateral nucleus of the amygdala in vulnerable. Dynorphin mRNA is increased only within the dorsal and medial shell of the NAc of vulnerable rats | [20] |
| Pre-clinical | Rats exposed to restraint stress | N/OFQ and NOR mRNA expression is increased in the hippocampus | [37] |
| Pre-clinical | Mouse model of Oprm1 (A118G) polymorphism | Mouse model of Oprm1 (A118G) polymorphism increased sociability and dominance and confers resilience to social defeat | [61] |
| Pre-clinical | Lentivirus-mediated MOR knockdown in the VTA | Social defeat stress increased MOR mRNA expression and leads to an increased capacity for MOR activation in the ventral tegmental area (VTA). Social stress exposure induced social avoidance were prevented by VTA MOR knockdown | [62,63,64] |
| Pre-clinical | MOR deficient mice | MOR deficient mice did not exhibit socially aversive behavior and reduced activity of the hypothalamic-pituitary-adrenocortical axis associated with the stress response and improvement in symptoms such as stress-induced depression-like behavior | [72] |
| Pre-clinical | Mice with DOR agonist or antagonist | DOR agonists showed anxiolytic and antidepressant effects, whereas DOR antagonists or DOR deficient mice exhibited enhanced anxiety-like behaviors | [73,74,75,76] |
| Pre-clinical | Adult C57BL/6J mice | SDS-induced susceptible mice showed reduced enkephalin levels in the NAc. DOR agonist, SNC80, improved sds-induced reduction of social behavior | [79] |
| Pre-clinical | Adult C57BL/6J mice | Repeated administrations to cVSDS mice with a selective DOP agonist, KNT-127 improved their decreased social interaction behaviors and increased serum corticosterone levels. | [80] |
| Pre-clinical | Adult C57BL/6J mice | KOR antagonist nor-binaltorphimine blocked the stress-induced analgesia, and significantly reduced the stress-induced immobility on the second and third days of SDS exposure. In contrast, prodynorphin gene-disrupted mice improved these effects | [83] |
| Pre-clinical | Adult C57BL/6J mice | KOR antagonist JDTic reduced stress effects on both sleep and circadian rhythms | [84] |
| Pre-clinical | Adult C57BL/6J mice | KOR antagonist norBNI or KOR knockout mice could prevent the development of social avoidance induced by chronic social defeat stress | [85] |
| Pre-clinical | N/OFQ knockout (−/−) and NOR−/− mice | N/OFQ knockout (−/−) and NOR−/− mice showed higher anxiety behavior | [94,95] |
| Pre-clinical | Adult C57BL/6J mice | NOR inhibitors are known to exhibit antidepressant-like effects | [96,97,98,99] |
| Pre-clinical | Acute exposure to social defeat stress in rats | Stressed mice enhanced NOR receptor expression in the amygdala | [103] |
| Clinical | Patients diagnosed with postpartum depression, major depressive disorder and bipolar disorder | Patientss showed increased plasma levels of N/OFQ | [106,107] |
| Social Defeat Stress and Pain | |||
| Pre-Clinical/Clinical | Model | Changes of Behabioral Phenotype and Each Opioid Receptor Expression in Stressed Mice | References No. |
| Pre-clinical | Social defeat stress model mice with incision pain | Social defeat stress decreases the mechanical pain threshold. Increasing inflammatory factors, such as COX-2 and iNOS, in the spinal cord. | [108] |
| Pre-clinical | Social defeat stress model mice | Social defeat stress enhanced plantar incision-induced AMPA receptor GluA1 phosphorylation at the Ser831 site in the spinal cord. | [109] |
| Pre-clinical | Social defeat stress model mice | Social defeat stress displayed delayed resolution of mechanical hypersensitivity and increased micro-glial activation and neuronal sensitization within the ipsilateral spinal cord. | [110] |
| Pre-clinical | Social defeat stress model mice | Mice subjected to repeated social defeat stress showed a dramatically longer prolongation of postsurgical pain through decreasing brain fatty acid signaling. | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamoto, K.; Tokuyama, S. Stress-Induced Changes in the Endogenous Opioid System Cause Dysfunction of Pain and Emotion Regulation. Int. J. Mol. Sci. 2023, 24, 11713. https://doi.org/10.3390/ijms241411713
Nakamoto K, Tokuyama S. Stress-Induced Changes in the Endogenous Opioid System Cause Dysfunction of Pain and Emotion Regulation. International Journal of Molecular Sciences. 2023; 24(14):11713. https://doi.org/10.3390/ijms241411713
Chicago/Turabian StyleNakamoto, Kazuo, and Shogo Tokuyama. 2023. "Stress-Induced Changes in the Endogenous Opioid System Cause Dysfunction of Pain and Emotion Regulation" International Journal of Molecular Sciences 24, no. 14: 11713. https://doi.org/10.3390/ijms241411713
APA StyleNakamoto, K., & Tokuyama, S. (2023). Stress-Induced Changes in the Endogenous Opioid System Cause Dysfunction of Pain and Emotion Regulation. International Journal of Molecular Sciences, 24(14), 11713. https://doi.org/10.3390/ijms241411713




