Fc Epsilon RI–Neuroimmune Interplay in Pruritus Triggered by Particulate Matter in Atopic Dermatitis Patients
Abstract
1. Introduction
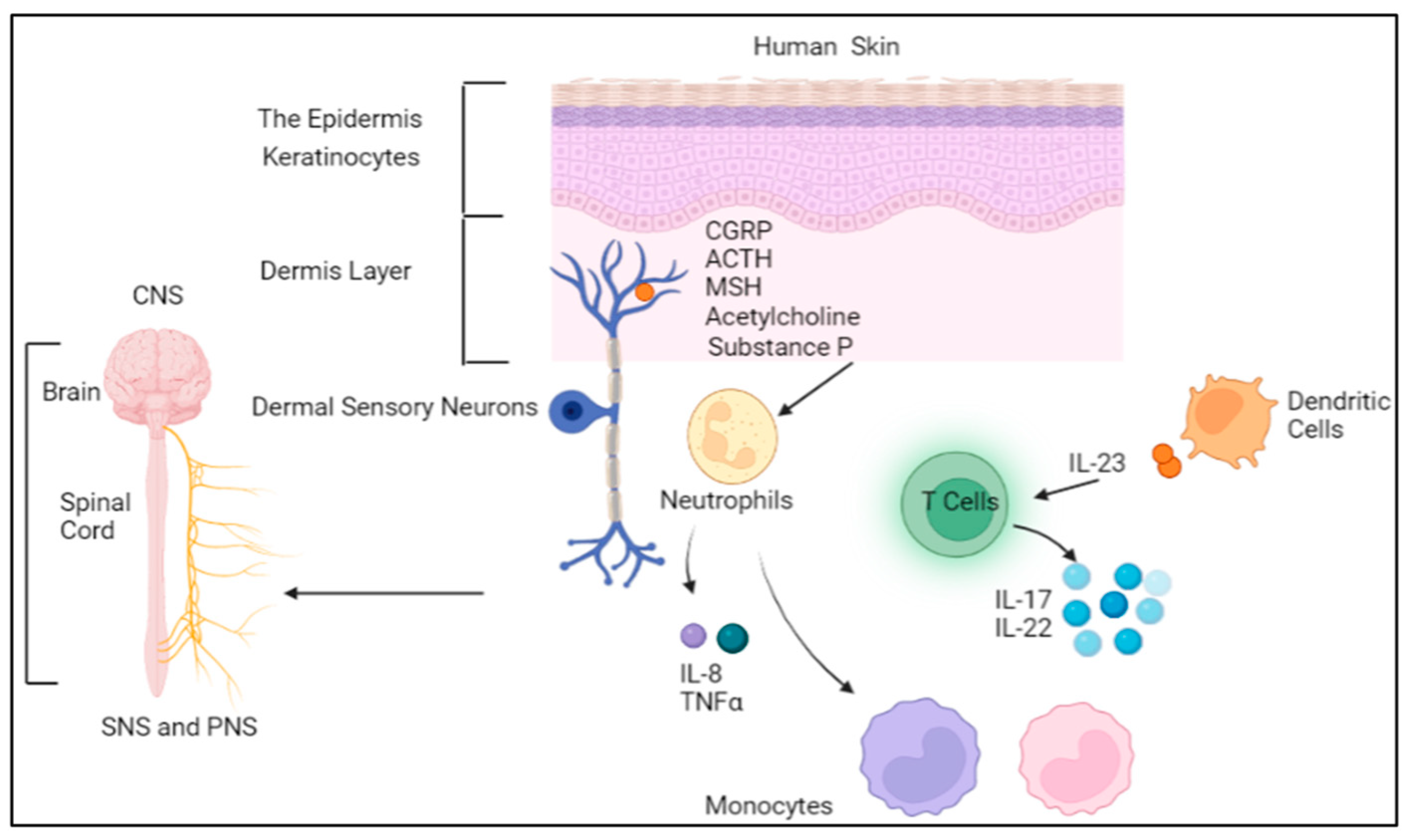
2. The Neuroimmune System and Skin
3. Neuroimmune Crosstalk in Skin Allergy
4. Association of the Neuroimmune System with Atopic Dermatitis
5. Neuronal Signaling Associated with Pruritus
5.1. The Role of Immune Cells
5.2. The Contribution of Keratinocytes
5.3. The Neuronal Contribution
6. Particulate Matter and AD Pruritus: Potential Neuroimmune Mechanisms
6.1. The Role of PM in AD Pathogenesis
6.2. Particulate Matter: Definition and Sources
6.3. Particulate Matter Triggers AD Pruritus
6.4. Particulate Matter—FcεRI–Neuroimmune Interplay in AD Pruritus
7. Potential Treatment Lines That Target the Neuroimmune-Mediated FcεRI Neuronal Signaling Pathway in AD Pruritus
8. Conclusions and Future Outlook
9. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipman, Z.M.; Labib, A.; Yosipovitch, G. Current clinical options for the management of itch in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2021, 14, 959–969. [Google Scholar] [CrossRef]
- Dawn, A.; Papoiu, A.D.P.; Chan, Y.H.; Rapp, S.R.; Rassette, N.; Yosipovitch, G. Itch characteristics in atopic dermatitis: Results of a web-based questionnaire. Br. J. Dermatol. 2009, 160, 642–644. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef]
- Ghosh, D.; Bernstein, J.A.; Hershey, G.K.K.; Rothenberg, M.E.; Mersha, T.B. Leveraging multilayered “omics” data for atopic dermatitis: A road map to precision medicine. Front. Immunol. 2018, 9, 2727. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Johansson, E.; Bernstein, J.; Chakraborty, R.; Hershey, K.; Rothernberg, M.; Mersha, T. Resolving the Etiology of Atopic Disorders by Genetic Analysis of Racial Ancestry Jayanta. J. Clin. Immunol. 2016, 138, 676–699. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef]
- Kantor, R.; Silverberg, J. Environmental risk factors and role in management of atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Rinnov, M.R.; Vestergaard, C. Disease mechanisms in atopic dermatitis: A review of aetiological factors. Acta Derm. Venereol. 2020, 100, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Trier, A.M.; Mack, M.R.; Kim, B.S. The Neuroimmune Axis in Skin Sensation, Inflammation, and Immunity. J. Immunol. 2019, 202, 2829–2835. [Google Scholar] [CrossRef]
- Kabata, H.; Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in skincare-the fascinating world of skin-brain connection: A review to explore ingredients, commercial products for skin aging, and cosmetic regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Zhu, Y.; Duan, S.; Wang, M.; Deng, Z.; Li, J. Neuroimmune Interaction: A Widespread Mutual Regulation and the Weapons for Barrier Organs. Front. Cell Dev. Biol. 2022, 10, 906755. [Google Scholar] [CrossRef] [PubMed]
- Berta, T.; Qadri, Y.; Tan, P.H.; Ji, R.R. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets 2017, 21, 695–703. [Google Scholar] [CrossRef]
- Konstantinou, G.N.; Konstantinou, G.N.; Koulias, C.; Petalas, K.; Makris, M. Further Understanding of Neuro-Immune Interactions in Allergy: Implications in Pathophysiology and Role in Disease Progression. J. Asthma Allergy 2022, 15, 1273–1291. [Google Scholar] [CrossRef]
- Sun, Y.G.; Chen, Z.F. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007, 448, 700–703. [Google Scholar] [CrossRef]
- Blome, C.; Radtke, M.A.; Eissing, L.; Augustin, M. Quality of Life in Patients with Atopic Dermatitis: Disease Burden, Measurement, and Treatment Benefit. Am. J. Clin. Dermatol. 2016, 17, 163–169. [Google Scholar] [CrossRef]
- Yarbrough, K.B.; Neuhaus, K.J.; Simpson, E.L. The effects of treatment on itch in atopic dermatitis. Dermatol. Ther. 2013, 26, 110–119. [Google Scholar] [CrossRef]
- Wang, F.; Kim, B.S. Itch: A Paradigm of Neuroimmune Crosstalk. Immunity 2020, 52, 753–766. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Cevikbas, F.; Braz, J.M.; Wang, X.; Solorzano, C.; Sulk, M.; Buhl, T.; Steinhoff, M.; Basbaum, A.I. Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 454–464.e2. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Y.; Wang, F. Sensitization Mechanisms of Chronic Itch. Int. J. Dermatol. Venereol. 2019, 2, 211–215. [Google Scholar] [CrossRef]
- Fania, L.; Moretta, G.; Antonelli, F.; Scala, E.; Abeni, D.; Albanesi, C.; Madonna, S. Multiple Roles for Cytokines in Atopic Dermatitis: From Pathogenic Mediators to Endotype-Specific Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Granstein, R.D. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav. Immun. Health 2021, 18, 100361. [Google Scholar] [CrossRef]
- Wang, Q.; Kwan, M.P.; Zhou, K.; Fan, J.; Wang, Y.; Zhan, D. The impacts of urbanization on fine particulate matter (PM2.5) concentrations: Empirical evidence from 135 countries worldwide. Environ. Pollut. 2019, 247, 989–998. [Google Scholar] [CrossRef]
- Kathuria, P.; Silverberg, J.I. Association of pollution and climate with atopic eczema in US children. Pediatr. Allergy Immunol. 2016, 27, 478–485. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Carrera, Y.I.L.; Al Hammadi, A.; Huang, Y.-H.; Llamado, L.J.; Mahgoub, E.; Tallman, A.M. Epidemiology, Diagnosis, and Treatment of Atopic Dermatitis in the Developing Countries of Asia, Africa, Latin America, and the Middle East: A Review. Dermatol. Ther. 2019, 9, 685–705. [Google Scholar] [CrossRef]
- Kaneko, Y.; Szallasi, A. Transient receptor potential [TRP] channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human dorsal root ganglia. Front. Cell. Neurosci. 2019, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Kagitani, F.; Uchida, S.; Hotta, H. Afferent nerve fibers and acupuncture. Auton. Neurosci. Basic Clin. 2010, 157, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, M.; Guo, Y.; Foreman, D.; Feng, F.; Duan, G.; Wu, W.; Zhang, W. Fine particulate matter [PM 2.5] enhances FcεRI-mediated signaling and mast cell function. Cell. Signal. 2019, 57, 102–109. [Google Scholar] [CrossRef]
- Zhu, M.; Rhee, I.; Liu, Y.; Zhang, W. Negative regulation of FcεRI-mediated signaling and mast cell function by the adaptor protein LAX. J. Biol. Chem. 2006, 281, 18408–18413. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Nishida, K.; Hirano, T.; Zhang, W. An essential role for RasGRP1 in mast cell function and IgE-mediated allergic response. J. Exp. Med. 2007, 204, 93–103. [Google Scholar] [CrossRef]
- Wu, L.C. Immunoglobulin E receptor signaling and asthma. J. Biol. Chem. 2011, 286, 32891–32897. [Google Scholar] [CrossRef]
- Cui, H.; Liu, F.; Fang, Y.; Wang, T.; Yuan, B.; Ma, C. Neuronal FcεRIα directly mediates ocular itch via IgE-immune complex in a mouse model of allergic conjunctivitis. J. Neuroinflamm. 2022, 19, 55. [Google Scholar] [CrossRef]
- Liu, F.; Shen, X.; Su, S.; Cui, H.; Fang, Y.; Wang, T.; Zhang, L.; Huang, Y.; Ma, C. Fcγ Receptor I–Coupled Signaling in Peripheral Nociceptors Mediates Joint Pain in a Rat Model of Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 1668–1678. [Google Scholar] [CrossRef]
- Yashiro, T.; Ogata, H.; Zaidi, S.F.; Lee, J.; Hayashi, S.; Yamamoto, T.; Kadowaki, M. Pathophysiological roles of neuro-immune interactions between enteric neurons and mucosal mast cells in the gut of food allergy mice. Cells 2021, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, M.; Liu, Y.; Wang, X.; Zhang, S.; Guan, L.; Hong, J.; Zhou, W.; Wu, G.; Diao, W.; et al. Signals from the TAFA4-PTEN-PU.1 axis alleviate nasal allergy by modulating the expression of FcεRI in mast cells. Clin. Exp. Immunol. 2022, 211, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Magadmi, R.; Meszaros, J.; Damanhouri, Z.A.; Seward, E.P. Secretion of mast cell inflammatory mediators is enhanced by CADM1-dependent adhesion to sensory neurons. Front. Cell. Neurosci. 2019, 13, 262. [Google Scholar] [CrossRef]
- Kaplan, G.; Avdan, Z.Y.; Avdan, U. Spaceborne Nitrogen Dioxide Observations from the Sentinel-5P TROPOMI over Turkey. Proceedings 2019, 18, 4. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Cortes, J.E.; Jiang, Q.; Wang, J.; Weng, J.; Zhu, H.; Liu, X.; Hochhaus, A.; Kim, D.W.; Radich, J.; Savona, M.; et al. Dasatinib vs. imatinib in patients with chronic myeloid leukemia in chronic phase [CML-CP] who have not achieved an optimal response to 3 months of imatinib therapy: The DASCERN randomized study. Leukemia 2020, 34, 2064–2073. [Google Scholar] [CrossRef]
- Liu, D.; Mamorska-Dyga, A. Syk inhibitors in clinical development for hematological malignancies. J. Hematol. Oncol. 2017, 10, 145. [Google Scholar] [CrossRef]
- McKeage, K.; Lyseng-Williamson, K.A. Fostamatinib in chronic immune thrombocytopenia: A profile of its use in the USA. Drugs Ther. Perspect. 2018, 34, 451–456. [Google Scholar] [CrossRef]
- Häfliger, P.; Charles, R.P. The l-type amino acid transporter LAT1—An emerging target in cancer. Int. J. Mol. Sci. 2019, 20, 2428. [Google Scholar] [CrossRef]
- Yothaisong, S.; Dokduang, H.; Anzai, N.; Hayashi, K.; Namwat, N.; Yongvanit, P.; Sangkhamanon, S.; Jutabha, P.; Endou, H.; Loilome, W. Inhibition of l-type amino acid transporter 1 activity as a new therapeutic target for cholangiocarcinoma treatment. Tumor Biol. 2017, 39, 1–14. [Google Scholar] [CrossRef]
- St-Pierre, F.; Ma, S. Use of BTK Inhibitors in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma [CLL/SLL]: A Practical Guidance. Blood Lymphat. Cancer Targets Ther. 2022, 12, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.J.; Kim, H.I.; Lee, S.T. The associated pyrazolopyrimidines PP1 and PP2 inhibit protein tyrosine kinase 6 activity and suppress breast cancer cell proliferation. Oncol. Lett. 2017, 13, 1463–1469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, K.; Zhang, Y.; Qian, L.; Wang, P. Emerging strategies to target RAS signaling in human cancer therapy. J. Hematol. Oncol. 2021, 14, 116. [Google Scholar] [CrossRef]
- Zhang, S.S.; Nagasaka, M. Spotlight on sotorasib [Amg 510] for krasg12c positive non-small cell lung cancer. Lung Cancer Targets Ther. 2021, 12, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, X.; Liu, H.; Xiao, F.; Wei, J.; You, L.; Qian, W. PDK1 inhibitor GSK2334470 exerts antitumor activity in multiple myeloma and forms a novel multitargeted combination with dual mTORC1/C2 inhibitor PP242. Oncotarget 2017, 8, 39185–39197. [Google Scholar] [CrossRef] [PubMed]
- Arnst, J.L.; Hein, A.L.; Taylor, M.A.; Palermo, N.Y.; Contreras, J.I.; Sonawane, Y.A.; Wahl, A.O.; Ouellette, M.M.; Natarajan, A.; Yan, Y. Discovery and characterization of small molecule Rac1 inhibitors. Oncotarget 2017, 8, 34586–34600. [Google Scholar] [CrossRef] [PubMed]
- Shutes, A.; Onesto, C.; Picard, V.; Leblond, B.; Schweighoffer, F.; Der, C.J. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 2007, 282, 35666–35678. [Google Scholar] [CrossRef]
- Bowyer, S.; Lee, R.; Fusi, A.; Lorigan, P. Dabrafenib and its use in the treatment of metastatic melanoma. Melanoma Manag. 2015, 2, 199–208. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Yang, S.; Wu, X.; Li, H.; Wang, Q. MEK inhibitors for the treatment of non-small cell lung cancer. J. Hematol. Oncol. 2021, 14, 147. [Google Scholar] [CrossRef]
- Khunger, A.; Khunger, M.; Velcheti, V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: Clinical evidence and experience. Ther. Adv. Respir. Dis. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- De Weerd, A.; Kho, M.; Kraaijeveld, R.; Zuiderwijk, J.; Weimar, W.; Baan, C. The protein kinase C inhibitor sotrastaurin allows regulatory T cell function. Clin. Exp. Immunol. 2014, 175, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, W.; Jacevic, V.; Franca, T.C.C.; Wang, X.; Kuca, K. Selective inhibitors for JNK signaling: A potential targeted therapy in cancer. J. Enzym. Inhib. Med. Chem. 2020, 35, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Berglöf, A.; Hamasy, A.; Meinke, S.; Palma, M.; Krstic, A.; Månsson, R.; Kimby, E.; Österborg, A.; Smith, C.I.E. Targets for Ibrutinib Beyond B Cell Malignancies. Scand. J. Immunol. 2015, 82, 208–217. [Google Scholar] [CrossRef]
- Mei, B.; Zhu, L.; Guo, Y.; Wu, T.; Ren, P.; Deng, X. Solid Form Selection and Process Development of KO-947 Drug Substances. Org. Process Res. Dev. 2021, 25, 1637–1647. [Google Scholar] [CrossRef]
- Du, G.; Wang, J.; Zhang, T.; Ding, Q.; Jia, X.; Zhao, X.; Dong, J.; Yang, X.; Lu, S.; Zhang, C.; et al. Targeting Src family kinase member Fyn by Saracatinib attenuated liver fibrosis in vitro and in vivo. Cell Death Dis. 2020, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.H.; Nygaard, H.B.; Chen, K.; Donohue, M.C.; Raman, R.; Rissman, R.A.; Brewer, J.B.; Koeppe, R.A.; Chow, T.W.; Rafii, M.S.; et al. Effect of AZD0530 on Cerebral Metabolic Decline in Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef]
- Wilhoit, T.; Patrick, J.M.; May, M.B. Alpelisib: A Novel Therapy for. J. Adv. Pract. Oncol. 2020, 11, 768–775. [Google Scholar]
- Khan, M.; Shunmugavel, A.; Dhammu, T.S.; Matsuda, F.; Singh, A.K.; Singh, I. Oral administration of cytosolic PLA2 inhibitor arachidonyl trifluoromethyl ketone ameliorates cauda equina compression injury in rats. J. Neuroinflamm. 2015, 12, 94. [Google Scholar] [CrossRef]
- Martorana, F.; Motta, G.; Pavone, G.; Motta, L.; Stella, S.; Vitale, S.R.; Manzella, L.; Vigneri, P. AKT Inhibitors: New Weapons in the Fight Against Breast Cancer? Front. Pharmacol. 2021, 12, 662232. [Google Scholar] [CrossRef]
- Lazaro, G.; Kostaras, E.; Vivanco, I. Inhibitors in AKTion: ATP-competitive vs allosteric. Biochem. Soc. Trans. 2020, 48, 933–943. [Google Scholar] [CrossRef] [PubMed]

| Allergic Condition | Study Model | Target Neurons | Target Mediator(s) | Results | Reference |
|---|---|---|---|---|---|
| Allergic Conjunctivitis | mice | Trigeminal neurons | IgE–immune complex, FcεRIα | FcεRIα was expressed in conjunctival sensory neurons; The IgE immune-activated trigeminal neurons. Both actions were recorded in the absence of any inflammation | [39] |
| PM2.5 sensitization | Bone marrow-derived mast cells (BMMC) | IL-6, IL-13, MCP1, TNFα, Syk, LAT, SLP76, PLCγ1, FcεRIα | BMMC exposed to low levels of PM2.5 showed mast cell degranulation and FcεRI-modulated cytokine production | [35] | |
| Rheumatoid Arthritis (RA) | Sprague–Dawley rats | FcεRIα | FcεRIα expression was increased in dorsal root ganglion neurons (IB4 and TRPV1) in RA rats and was reduced in knockout rats | [40] | |
| Food Allergy (FA) | BALB/c mice | Enteric neurons | FcεRIα | FcεRIα was highly expressed in enteric neurons where mast cells were allocated in FA mice | [41] |
| Allergic Rhinitis (AR) | AR patients | GINIP neurons | TAFA4, IgE, FCER1G | The transcription of Fcer1g was restricted by TAFA4 in mast cells via the TAFA4–PTEN-PU.1 axis in airway tissues, and suppressed AR symptoms | [42] |
| IgE sensitization | Bone marrow-derived mast cells (BMMC) | Dorsal root ganglion | IgE, TNFα. IL-6, CADM1 | The sensory neuron–BMMC interaction induced mast cell degranulation and enhanced the responses to antigen stimulation and the activation of FcεRI receptors | [43] |
| Target Mediator(s) | Inhibitor(s) | The Inhibitor’s Mechanism of Action | Diseases Treated by the Inhibitor | Selected References |
|---|---|---|---|---|
| IgE–FcεRI | Omalizumab | Omalizumab suppress IgE–FcεRI attachment resulting in less free FcεRI available to receptors on antigen presenting cells, mast cells, and basophils | Allergic asthma, chronic spontaneous urticaria (CSU), and nasal polyps | [44] |
| Lyn | Dasatinib | Fostamatinib inhibits the binding of CD19 with Lyn and PI3K p85 | Myeloid leukemia | [45,46] |
| Syk | Fostamatinib | Selectively targets the Syk signaling pathway by inhibiting the signal transduction of Fc-activating receptors and B-cell receptors. | Rheumatoid arthritis and immune thrombocytopenia, B-cell lymphomas | [47,48] |
| LAT1 | BCH | BCH Inhibits neutral amino acid transfer (leucine) by LAT1 | cholangiocarcinoma | [49,50] |
| Btk | Acalabrutinib | Acalabrutinib binds to cysteine 481 in the ATP-binding site of BTK, located in the kinase domain | chronic lymphocytic leukemia | [51] |
| SLP-76 | PP1, PP2 | PP1 and PP2 bind to an area of SLP-76 that does not overlap with the ATP-binding domain | PTK6-positive malignant diseases | [52] |
| Ras | Sotorasib | Sotorasib attaches to inactive KRAS covalently between cysteine 12 and acrylamide, and non-covalently between the isopropylpyridine substituent and Histidine H95, Tyrosine Y96 and glutamine Q99 | NSCLC with K-RAS (G12C) mutations | [53,54] |
| PDK1 | GSK-470 | GSK-470 suppresses the Akt1 activation by PDK1 in the presence of fat vesicles that contain PtdIns [3,4,5] P3 or an Akt 1 mutant | Multiple myeloma | [55] |
| RAC | MLS000532223 EHT1864 | Stop the activated Rac 1 and Rac-independent Tiam 1-cell transformation as well as Ras transforming proteins | Cancers | [56,57] |
| Raf-1 | Vemurafenib and Dabrafenib | Vemurafenib and Dabrafenib work on the ATP competitive binding of the active conformation of BRAF kinase | metastatic melanoma | [58] |
| MEK | Trametinib | Trametinib blocks MEK1/2 kinase activity and prevents RAF-dependent MEK phosphorylation | non-small cell lung cancer, metastatic melanoma | [59,60] |
| PKC | Sotrastaurin | Sotrastaurin inhibits the PKC α, β and the θ isoforms resulting in selective NF-κB inactivation | autoimmune disease and transplant organ rejection | [61] |
| BTK | Ibrutinib | Ibrutinib blocks and inactivates BTK though a covalent bond that connects ibrutinib with cysteine 481 in the ATP-binding site irreversibly | chronic lymphocyte Leukemia | [62,63] |
| ERK1/2 | KO-947 | KO-947 selectively inhibits ERK1/2 in the RAS/MAPK signaling pathway | Solid tumors | [64] |
| Fyn Kinase | Saracatinib | Saracatinib blocks the ATP-binding active site of the kinase domain bond | Alzheimer’s disease, Cancers | [65,66] |
| PI3K | Alpelisib | Alpelisib inhibits PIK3 selectively in the PI3K/AKT kinase signaling pathway | Breast cancer, PIK3CA-related overgrowth spectrum | [67,68] |
| cPLA2 | Arachidonyl trifluoromethyl ketone (ATK) | AKT is an arachidonic acid analog where the carboxyl group is replaced by a trifluoromethyl ketone (TFMK) group; therefore, suppressing cPLA2 | Cancers | [69] |
| AKT | Capivasertib | Capivasertib is a selective adenosine triphosphate (ATP)-competitive inhibitor of AKT | Breast cancer | [70,71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaifan, D.; Crovella, S.; Soubra, L.; Al-Nesf, M.; Steinhoff, M. Fc Epsilon RI–Neuroimmune Interplay in Pruritus Triggered by Particulate Matter in Atopic Dermatitis Patients. Int. J. Mol. Sci. 2023, 24, 11851. https://doi.org/10.3390/ijms241411851
Isaifan D, Crovella S, Soubra L, Al-Nesf M, Steinhoff M. Fc Epsilon RI–Neuroimmune Interplay in Pruritus Triggered by Particulate Matter in Atopic Dermatitis Patients. International Journal of Molecular Sciences. 2023; 24(14):11851. https://doi.org/10.3390/ijms241411851
Chicago/Turabian StyleIsaifan, Dina, Sergio Crovella, Lama Soubra, Maryam Al-Nesf, and Martin Steinhoff. 2023. "Fc Epsilon RI–Neuroimmune Interplay in Pruritus Triggered by Particulate Matter in Atopic Dermatitis Patients" International Journal of Molecular Sciences 24, no. 14: 11851. https://doi.org/10.3390/ijms241411851
APA StyleIsaifan, D., Crovella, S., Soubra, L., Al-Nesf, M., & Steinhoff, M. (2023). Fc Epsilon RI–Neuroimmune Interplay in Pruritus Triggered by Particulate Matter in Atopic Dermatitis Patients. International Journal of Molecular Sciences, 24(14), 11851. https://doi.org/10.3390/ijms241411851







