Neoadjuvant Immunotherapy: A Promising New Standard of Care
Abstract
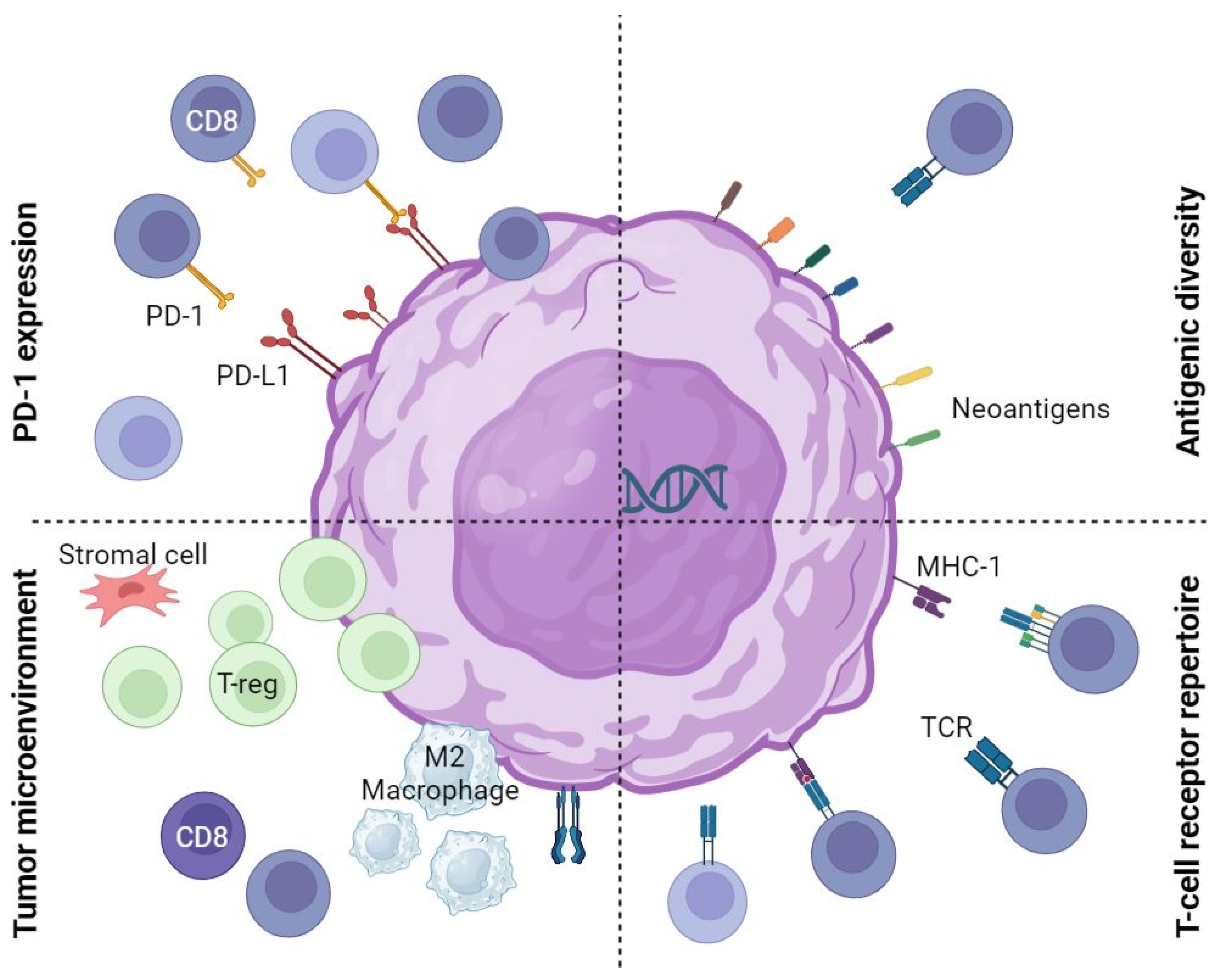
:1. Introduction
2. Non-Small Cell Lung Cancer
3. Melanoma
4. Bladder Cancer
5. Triple-Negative Breast Cancer
6. Gastric Cancer
7. Colorectal Cancer
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: A Comprehensive Review of Registration Trials and Future Considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant Ipilimumab versus Placebo after Complete Resection of High-Risk Stage III Melanoma (EORTC 18071): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R. Harnessing the Immune System for the Treatment of Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Chocry, M.; Gibbons, D.L.; Sepesi, B.; Cascone, T. Emerging Biomarkers for Neoadjuvant Immune Checkpoint Inhibitors in Operable Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 590–606. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Furness, A.J.S.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal Neoantigens Elicit T Cell Immunoreactivity and Sensitivity to Immune Checkpoint Blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; O’Donnell, J.S.; Yan, J.; Madore, J.; Allen, S.; Smyth, M.J.; Teng, M.W.L. Timing of Neoadjuvant Immunotherapy in Relation to Surgery Is Crucial for Outcome. Oncoimmunology 2019, 8, e1581530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banna, G.L.; Addeo, A.; Zygoura, P.; Tsourti, Z.; Popat, S.; Curioni-Fontecedro, A.; Nadal, E.; Shah, R.; Pope, A.; Fisher, P.; et al. A Prognostic Score for Patients with Malignant Pleural Mesothelioma (MPM) Receiving Second-Line Immunotherapy or Chemotherapy in the ETOP 9–15 PROMISE-Meso Phase III Trial. Lung Cancer Amst. Neth. 2022, 169, 77–83. [Google Scholar] [CrossRef]
- Banna, G.L.; Friedlaender, A.; Tagliamento, M.; Mollica, V.; Cortellini, A.; Rebuzzi, S.E.; Prelaj, A.; Naqash, A.R.; Auclin, E.; Garetto, L.; et al. Biological Rationale for Peripheral Blood Cell-Derived Inflammatory Indices and Related Prognostic Scores in Patients with Advanced Non-Small-Cell Lung Cancer. Curr. Oncol. Rep. 2022, 24, 1851–1862. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Prelaj, A.; Friedlaender, A.; Cortellini, A.; Addeo, A.; Genova, C.; Naqash, A.R.; Auclin, E.; Mezquita, L.; Banna, G.L. Prognostic Scores Including Peripheral Blood-Derived Inflammatory Indices in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Crit. Rev. Oncol. Hematol. 2022, 179, 103806. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Cortellini, A.; Cortinovis, D.L.; Tiseo, M.; Aerts, J.G.J.V.; Barbieri, F.; Giusti, R.; Bria, E.; Grossi, F.; Pizzutilo, P.; et al. The Lung Immuno-Oncology Prognostic Score (LIPS-3): A Prognostic Classification of Patients Receiving First-Line Pembrolizumab for PD-L1 ≥ 50% Advanced Non-Small-Cell Lung Cancer. ESMO Open 2021, 6, 100078. [Google Scholar] [CrossRef]
- Ballman, K.V. Biomarker: Predictive or Prognostic? J. Clin. Oncol. 2015, 33, 3968–3971. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 38, 1–10. [Google Scholar] [CrossRef]
- Addeo, A.; Banna, G.L.; Weiss, G.J. Tumor Mutation Burden—From Hopes to Doubts. JAMA Oncol. 2019, 5, 934–935. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after Surgery in Patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Crinò, L.; Dooms, C.; Douillard, J.Y.; Faivre-Finn, C.; Lim, E.; Rocco, G.; Senan, S.; Schil, P.V.; Veronesi, G.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Early-Stage Non-Small-Cell Lung Cancer Consensus on Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2014, 25, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Rosell, R.; Lena, M.D.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Groumellec, A.L.; Lorusso, V.; et al. Adjuvant Vinorelbine plus Cisplatin versus Observation in Patients with Completely Resected Stage IB–IIIA Non-Small-Cell Lung Cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A Randomised Controlled Trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-analysis Collaborative Group Preoperative Chemotherapy for Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet Lond. Engl. 2014, 383, 1561–1571. [CrossRef] [Green Version]
- Westeel, V.; Quoix, E.; Puyraveau, M.; Lavolé, A.; Braun, D.; Laporte, S.; Bigay-Game, L.; Pujol, J.-L.; Ozenne, G.; Rivière, A.; et al. A Randomised Trial Comparing Preoperative to Perioperative Chemotherapy in Early-Stage Non-Small-Cell Lung Cancer (IFCT 0002 Trial). Eur. J. Cancer 2013, 49, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, N.; Gao, S.; Xue, Q.; Ying, J.; Wang, S.; Tao, X.; Zhao, J.; Mao, Y.; Wang, B.; et al. Neoadjuvant PD-1 Inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 816–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, D.; Lee, J.; Kris, M.; Wistuba, I.; Kwiatkowski, D.; Owen, D.; Bunn, P.; Johnson, B.; Oezkan, F.; Tang, Y.; et al. OA06.06 Clinical/Biomarker Data for Neoadjuvant Atezolizumab in Resectable Stage IB-IIIB NSCLC: Primary Analysis in the LCMC3 Study. J. Thorac. Oncol. 2021, 16, S115–S116. [Google Scholar] [CrossRef]
- Besse, B.; Adam, J.; Cozic, N.; Chaput-Gras, N.; Planchard, D.; Mezquita, L.; Masip, J.R.; Lavaud, P.; Naltet, C.; Gazzah, A.; et al. 1215O—SC Neoadjuvant Atezolizumab (A) for Resectable Non-Small Cell Lung Cancer (NSCLC): Results from the Phase II PRINCEPS Trial. Ann. Oncol. 2020, 31, S794–S795. [Google Scholar] [CrossRef]
- Wislez, M.; Mazieres, J.; Lavole, A.; Zalcman, G.; Carre, O.; Egenod, T.; Caliandro, R.; Dubos-Arvis, C.; Jeannin, G.; Molinier, O.; et al. Neoadjuvant Durvalumab for Resectable Non-Small-Cell Lung Cancer (NSCLC): Results from a Multicenter Study (IFCT-1601 IONESCO). J. Immunother. Cancer 2022, 10, e005636. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant Nivolumab or Nivolumab plus Ipilimumab in Operable Non-Small Cell Lung Cancer: The Phase 2 Randomized NEOSTAR Trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant Chemotherapy and Nivolumab in Resectable Non-Small-Cell Lung Cancer (NADIM): An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Rothschild, S.I.; Zippelius, A.; Eboulet, E.I.; Savic Prince, S.; Betticher, D.; Bettini, A.; Früh, M.; Joerger, M.; Lardinois, D.; Gelpke, H.; et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non–Small-Cell Lung Cancer—A Multicenter Single-Arm Phase II Trial. J. Clin. Oncol. 2021, 39, 2872–2880. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Chaft, J.E.; Oezkan, F.; Kris, M.G.; Bunn, P.A.; Wistuba, I.I.; Kwiatkowski, D.J.; Owen, D.H.; Tang, Y.; Johnson, B.E.; Lee, J.M.; et al. Neoadjuvant Atezolizumab for Resectable Non-Small Cell Lung Cancer: An Open-Label, Single-Arm Phase II Trial. Nat. Med. 2022, 28, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Provencio-Pulla, M.; Nadal, E.; Larriba, J.L.G.; Martinez-Marti, A.; Bernabé, R.; Bosch-Barrera, J.; Casal, J.; Calvo, V.; Insa, A.; Aix, S.P.; et al. Nivolumab + Chemotherapy versus Chemotherapy as Neoadjuvant Treatment for Resectable Stage IIIA NSCLC: Primary Endpoint Results of Pathological Complete Response (pCR) from Phase II NADIM II Trial. J. Clin. Oncol. 2022, 40, 8501. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant Atezolizumab and Chemotherapy in Patients with Resectable Non-Small-Cell Lung Cancer: An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020, 11, 167. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Abstract CT005: AEGEAN: A Phase 3 Trial of Neoadjuvant Durvalumab + Chemotherapy Followed by Adjuvant Durvalumab in Patients with Resectable NSCLC. Cancer Res. 2023, 83, CT005. [Google Scholar] [CrossRef]
- Lu, S.; Wu, L.; Zhang, W.; Zhang, P.; Wang, W.; Fang, W.; Xing, W.; Chen, Q.; Mei, J.; Yang, L.; et al. Perioperative Toripalimab + Platinum-Doublet Chemotherapy vs Chemotherapy in Resectable Stage II/III Non-Small Cell Lung Cancer (NSCLC): Interim Event-Free Survival (EFS) Analysis of the Phase III Neotorch Study. J. Clin. Oncol. 2023, 41, 425126. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [Green Version]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef]
- Tarhini, A.; Lin, Y.; Lin, H.; Rahman, Z.; Vallabhaneni, P.; Mendiratta, P.; Pingpank, J.F.; Holtzman, M.P.; Yusko, E.C.; Rytlewski, J.A.; et al. Neoadjuvant Ipilimumab (3 Mg/Kg or 10 Mg/Kg) and High Dose IFN-α2b in Locally/Regionally Advanced Melanoma: Safety, Efficacy and Impact on T-Cell Repertoire. J. Immunother. Cancer 2018, 6, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A Single Dose of Neoadjuvant PD-1 Blockade Predicts Clinical Outcomes in Resectable Melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant Immune Checkpoint Blockade in High-Risk Resectable Melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus Adjuvant Ipilimumab plus Nivolumab in Macroscopic Stage III Melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the Optimal Combination Dosing Schedule of Neoadjuvant Ipilimumab plus Nivolumab in Macroscopic Stage III Melanoma (OpACIN-Neo): A Multicentre, Phase 2, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef]
- Reijers, I.L.M.; Dimitriadis, P.; Rozeman, E.A.; Krijgsman, O.; Cornelissen, S.; Bosch, L.J.W.; Broeks, A.; Menzies, A.M.; van de Wiel, B.A.; Scolyer, R.A.; et al. The Interferon-Gamma (IFN-Y) Signature from Baseline Tumor Material Predicts Pathologic Response after Neoadjuvant Ipilimumab (IPI) + Nivolumab (NIVO) in Stage III Melanoma. J. Clin. Oncol. 2022, 40, 9539. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Hoefsmit, E.P.; Reijers, I.L.M.; Saw, R.P.M.; Versluis, J.M.; Krijgsman, O.; Dimitriadis, P.; Sikorska, K.; van de Wiel, B.A.; Eriksson, H.; et al. Survival and Biomarker Analyses from the OpACIN-Neo and OpACIN Neoadjuvant Immunotherapy Trials in Stage III Melanoma. Nat. Med. 2021, 27, 256–263. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; Li, X. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-Small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int. J. Biol. Sci. 2022, 18, 3845–3858. [Google Scholar] [CrossRef]
- Blank, C.U.; Reijers, I.L.M.; Versluis, J.M.; Menzies, A.M.; Dimitriadis, P.; Wouters, M.W.; Saw, R.P.M.; Klop, W.M.C.; Pennington, T.E.; Bosch, L.J.W.; et al. LBA39 Personalized Combination of Neoadjuvant Domatinostat, Nivolumab (NIVO) and Ipilimumab (IPI) in Stage IIIB-D Melanoma Patients (Pts) Stratified according to the Interferon-Gamma Signature (IFN-γ Sign): The DONIMI Study. Ann. Oncol. 2021, 32, S1315. [Google Scholar] [CrossRef]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tezlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant Relatlimab and Nivolumab in Resectable Melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef]
- Danielli, R.; Patuzzo, R.; Di Giacomo, A.M.; Gallino, G.; Maurichi, A.; Di Florio, A.; Cutaia, O.; Lazzeri, A.; Fazio, C.; Miracco, C.; et al. Intralesional Administration of L19-IL2/L19-TNF in Stage III or Stage IVM1a Melanoma Patients: Results of a Phase II Study. Cancer Immunol. Immunother. 2015, 64, 999–1009. [Google Scholar] [CrossRef]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; van den Heuvel, N.M.J.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized Response-Directed Surgery and Adjuvant Therapy after Neoadjuvant Ipilimumab and Nivolumab in High-Risk Stage III Melanoma: The PRADO Trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Milowsky, M.I.; Rumble, R.B.; Booth, C.M.; Gilligan, T.; Eapen, L.J.; Hauke, R.J.; Boumansour, P.; Lee, C.T. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2016, 34, 1945–1952. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; Santis, M.D.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Gravis, G.; Fléchon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulié, M.; Allory, Y.; et al. Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Nonmetastatic Muscle-Invasive Bladder Cancer: Results of the GETUG-AFU V05 VESPER Trial. J. Clin. Oncol. 2022, 40, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Tully, C.M.; Zabor, E.C.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; Donat, S.M.; Russo, P.; Ostrovnaya, I.; Regazzi, A.M.; et al. Neoadjuvant Gemcitabine-Cisplatin Plus Radical Cystectomy-Pelvic Lymph Node Dissection for Muscle-Invasive Bladder Cancer: A 12-Year Experience. Clin. Genitourin. Cancer 2020, 18, 387–394. [Google Scholar] [CrossRef]
- Rose, T.L.; Harrison, M.R.; Deal, A.M.; Ramalingam, S.; Whang, Y.E.; Brower, B.; Dunn, M.; Osterman, C.K.; Heiling, H.M.; Bjurlin, M.A.; et al. Phase II Study of Gemcitabine and Split-Dose Cisplatin Plus Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Bladder Cancer. J. Clin. Oncol. 2021, 39, 3140–3148. [Google Scholar] [CrossRef]
- Gupta, S.; Gibb, E.; Sonpavde, G.P.; Gupta, S.; Maughan, B.L.; Agarwal, N.; McGregor, B.A.; Weight, C.; Wei, X.X.; Einstein, D.J.; et al. Biomarker Analysis and Updated Clinical Follow-up from BLASST-1 (Bladder Cancer Signal Seeking Trial) of Nivolumab, Gemcitabine, and Cisplatin in Patients with Muscle-Invasive Bladder Cancer (MIBC) Undergoing Cystectomy. J. Clin. Oncol. 2022, 40, 528. [Google Scholar] [CrossRef]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical Efficacy and Biomarker Analysis of Neoadjuvant Atezolizumab in Operable Urothelial Carcinoma in the ABACUS Trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef]
- Gao, J.; Navai, N.; Alhalabi, O.; Siefker-Radtke, A.; Campbell, M.T.; Tidwell, R.S.; Guo, C.C.; Kamat, A.M.; Matin, S.F.; Araujo, J.C.; et al. Neoadjuvant PD-L1 plus CTLA-4 Blockade in Patients with Cisplatin-Ineligible Operable High-Risk Urothelial Carcinoma. Nat. Med. 2020, 26, 1845–1851. [Google Scholar] [CrossRef]
- Grande, E.; Guerrero, F.; Puente, J.; Galante, I.; Duran, I.; Dominguez, M.; Alonso Gordoa, T.; Burgos, J.; Font, A.; Pinto, A.; et al. DUTRENEO Trial: A Randomized Phase II Trial of DUrvalumab and TREmelimumab versus Chemotherapy as a NEOadjuvant Approach to Muscle-Invasive Urothelial Bladder Cancer (MIBC) Patients (Pts) Prospectively Selected by an Interferon (INF)-Gamma Immune Signature. J. Clin. Oncol. 2020, 38, 5012. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef] [Green Version]
- Padua, T.C.D.; Basile, G.; Bandini, M.; Raggi, D.; Marandino, L.; Giannatempo, P.; Colombo, R.; Colecchia, M.; Lucianò, R.; Moschini, M.; et al. 1738P Three-Year Follow-up Update and Survival Outcomes of the PURE-01 Study. Ann. Oncol. 2022, 33, S1331–S1332. [Google Scholar] [CrossRef]
- Basile, G.; Bandini, M.; Gibb, E.A.; Ross, J.S.; Raggi, D.; Marandino, L.; Costa de Padua, T.; Crupi, E.; Colombo, R.; Colecchia, M.; et al. Neoadjuvant Pembrolizumab and Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Cancer: 3-Year Median Follow-Up Update of PURE-01 Trial. Clin. Cancer Res. 2022, 28, 5107–5114. [Google Scholar] [CrossRef]
- Szabados, B.; Kockx, M.; Assaf, Z.J.; van Dam, P.-J.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Pous, A.F.; Gravis, G.; et al. Final Results of Neoadjuvant Atezolizumab in Cisplatin-Ineligible Patients with Muscle-Invasive Urothelial Cancer of the Bladder. Eur. Urol. 2022, 82, 212–222. [Google Scholar] [CrossRef]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative Ipilimumab plus Nivolumab in Locoregionally Advanced Urothelial Cancer: The NABUCCO Trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, M.; Solovyov, A.; Lihm, J.; Quinlan, C.; Whiting, K.; Li, H.; Al-Ahmadie, H.A.; Teo, M.Y.; Aggen, D.H.; Ostrovnaya, I.; et al. Biomarkers of Response to Neoadjuvant Atezolizumab with Gemcitabine and Cisplatin in Muscle-Invasive Bladder Cancer. J. Clin. Oncol. 2022, 40, 4584. [Google Scholar] [CrossRef]
- Galsky, M.D.; Daneshmand, S.; Chan, K.G.; Dorff, T.B.; Cetnar, J.P.; Neil, B.O.; D’souza, A.; Mamtani, R.; Kyriakopoulos, C.; Garcia, P.; et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Nivolumab with Selective Bladder Sparing in Patients with Muscle- Invasive Bladder Cancer (MIBC): HCRN GU 16-257. J. Clin. Oncol. 2021, 39, 4503. [Google Scholar] [CrossRef]
- Sonpavde, G.; Necchi, A.; Gupta, S.; Steinberg, G.D.; Gschwend, J.E.; Van Der Heijden, M.S.; Garzon, N.; Ibrahim, M.; Raybold, B.; Liaw, D.; et al. ENERGIZE: A Phase III Study of Neoadjuvant Chemotherapy Alone or with Nivolumab With/Without Linrodostat Mesylate for Muscle-Invasive Bladder Cancer. Future Oncol. 2019, 16, 4359–4368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez Chanza, N.; Carnot, A.; Barthélémy, P.; Casert, V.; Staudacher, L.; Van Den Brande, J.; Sautois, B.; Vanhaudenarde, V.; Seront, E.; Debien, V.; et al. Avelumab as the Basis of Neoadjuvant Regimen in Platinum-Eligible and -Ineligible Patients with Nonmetastatic Muscle-Invasive Bladder Cancer: AURA (Oncodistinct-004) Trial. J. Clin. Oncol. 2022, 40, 4517. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the Benefits of Therapy for Early-Stage Breast Cancer: The St. Gallen International Consensus Guidelines for the Primary Therapy of Early Breast Cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant Atezolizumab in Combination with Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy versus Placebo and Chemotherapy in Patients with Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Pathologic Complete Response (pCR) to Neoadjuvant Treatment with or without Atezolizumab in Triple-Negative, Early High-Risk and Locally Advanced Breast Cancer: NeoTRIP Michelangelo Randomized Study☆. Ann. Oncol. 2022, 33, 534–543. [Google Scholar] [CrossRef]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A Randomised Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer: Clinical Results and Biomarker Analysis of GeparNuevo Study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Karn, T.; Denkert, C.; Weber, K.E.; Holtrich, U.; Hanusch, C.; Sinn, B.V.; Higgs, B.W.; Jank, P.; Sinn, H.P.; Huober, J.; et al. Tumor Mutational Burden and Immune Infiltration as Independent Predictors of Response to Neoadjuvant Immune Checkpoint Inhibition in Early TNBC in GeparNuevo. Ann. Oncol. 2020, 31, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Schneeweiss, A.; Huober, J.; Braun, M.; Rey, J.; Blohmer, J.-U.; Furlanetto, J.; Zahm, D.-M.; Hanusch, C.; Thomalla, J.; et al. Neoadjuvant Durvalumab Improves Survival in Early Triple-Negative Breast Cancer Independent of Pathological Complete Response☆. Ann. Oncol. 2022, 33, 1149–1158. [Google Scholar] [CrossRef]
- Sharma, P.; Stecklein, S.R.; Yoder, R.; Staley, J.M.; Schwensen, K.; O’Dea, A.; Nye, L.E.; Elia, M.; Satelli, D.; Crane, G.; et al. Clinical and Biomarker Results of Neoadjuvant Phase II Study of Pembrolizumab and Carboplatin plus Docetaxel in Triple-Negative Breast Cancer (TNBC) (NeoPACT). J. Clin. Oncol. 2022, 40, 513. [Google Scholar] [CrossRef]
- Sinn, B.V.; Loibl, S.; Hanusch, C.A.; Zahm, D.-M.; Sinn, H.-P.; Untch, M.; Weber, K.; Karn, T.; Becker, C.; Marmé, F.; et al. Immune-Related Gene Expression Predicts Response to Neoadjuvant Chemotherapy but Not Additional Benefit from PD-L1 Inhibition in Women with Early Triple-Negative Breast Cancer. Clin. Cancer Res. 2021, 27, 2584–2591. [Google Scholar] [CrossRef]
- Foldi, J.; Silber, A.; Reisenbichler, E.; Singh, K.; Fischbach, N.; Persico, J.; Adelson, K.; Katoch, A.; Horowitz, N.; Lannin, D.; et al. Neoadjuvant Durvalumab plus Weekly Nab-Paclitaxel and Dose-Dense Doxorubicin/Cyclophosphamide in Triple-Negative Breast Cancer. NPJ Breast Cancer 2021, 7, 9. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.M.; Yau, C.; Wulfkuhle, J.; Brown-Swigart, L.; Gallagher, R.I.; Lee, P.R.E.; Zhu, Z.; Magbanua, M.J.; Sayaman, R.; O’Grady, N.; et al. Redefining Breast Cancer Subtypes to Guide Treatment Prioritization and Maximize Response: Predictive Biomarkers across 10 Cancer Therapies. Cancer Cell 2022, 40, 609–623.e6. [Google Scholar] [CrossRef]
- Gonzalez-Ericsson, P.I.; Wulfkhule, J.D.; Gallagher, R.I.; Sun, X.; Axelrod, M.L.; Sheng, Q.; Luo, N.; Gomez, H.; Sanchez, V.; Sanders, M.; et al. Tumor-Specific Major Histocompatibility-II Expression Predicts Benefit to Anti–PD-1/L1 Therapy in Patients With HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2021, 27, 5299–5306. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; Acosta, A.D.J.; Doi, T.; Longo, F.; et al. Pembrolizumab in Microsatellite Instability High or Mismatch Repair Deficient Cancers: Updated Analysis from the Phase II KEYNOTE-158 Study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Tougeron, D.; Piessen, G.; de la Fouchardière, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2022, 41, 255–265. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Liu, D.; Wang, X.; Xu, C.; Yu, Y.; Cui, Y.; Tang, C.; Li, Q.; Sun, J.; et al. The Neo-PLANET Phase II Trial of Neoadjuvant Camrelizumab plus Concurrent Chemoradiotherapy in Locally Advanced Adenocarcinoma of Stomach or Gastroesophageal Junction. Nat. Commun. 2022, 13, 6807. [Google Scholar] [CrossRef]
- Merck Provides Update on Phase 3 KEYNOTE-585 Trial in Locally Advanced Resectable Gastric and Gastroesophageal Junction (GEJ) Adenocarcinoma. Available online: https://www.merck.com/news/merck-provides-update-on-phase-3-keynote-585-trial-in-locally-advanced-resectable-gastric-and-gastroesophageal-junction-gej-adenocarcinoma/ (accessed on 3 July 2023).
- Imfinzi Plus Chemotherapy Significantly Improved Pathologic Complete Response in Gastric and Gastroesophageal Junction Cancers in MATTERHORN Phase III Trial. Available online: https://www.astrazeneca.com/media-centre/press-releases/2023/imfinzi-plus-chemotherapy-significantly-improved-pathologic-complete-response-in-gastric-and-gastroesophageal-junction-cancers-in-matterhorn-phase-iii-trial.html (accessed on 3 July 2023).
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up†. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Morton, D. FOxTROT: An International Randomised Controlled Trial in 1053 Patients Evaluating Neoadjuvant Chemotherapy (NAC) for Colon Cancer. On Behalf of the FOxTROT Collaborative Group. Ann. Oncol. 2019, 30, v198. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Verschoor, Y.L.; van den Berg, J.; Sikorska, K.; Beets, G.; Lent, A.V.; Grootscholten, M.C.; Aalbers, A.; Buller, N.; Marsman, H.; et al. LBA7 Neoadjuvant Immune Checkpoint Inhibition in Locally Advanced MMR-Deficient Colon Cancer: The NICHE-2 Study. Ann. Oncol. 2022, 33, S1389. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Tsukada, Y.; Inamori, K.; Togashi, Y.; Koyama, S.; Kotani, D.; Fukuoka, S.; Yuki, S.; Komatsu, Y.; Homma, S.; et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability–High Locally Advanced Rectal Cancer. Clin. Cancer Res. 2022, 28, 1136–1146. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Hong, S.C.; Eng, C.; Yao, X.; Cho, M.T.; You, Y.N.; Das, P.; Chakravarthy, A.B.; O’Dwyer, P.J. EA2201: An ECOG-ACRIN Phase II Study of Neoadjuvant Nivolumab plus Ipilimumab and Short Course Radiation in MSI-H/dMMR Rectal Tumors. J. Clin. Oncol. 2022, 40, TPS3644. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, M.; Zhang, P.; Li, G.; Liu, T.; Li, X.; Cai, K.; Nie, X.; Wang, J.; Liu, J.; et al. Phase II, Single-Arm Trial of Preoperative Short-Course Radiotherapy Followed by Chemotherapy and Camrelizumab in Locally Advanced Rectal Cancer. J. Immunother. Cancer 2021, 9, e003554. [Google Scholar] [CrossRef]
- Papke, D.J.; Yurgelun, M.B.; Noffsinger, A.E.; Turner, K.O.; Genta, R.M.; Redston, M. Prevalence of Mismatch-Repair Deficiency in Rectal Adenocarcinomas. N. Engl. J. Med. 2022, 387, 1714–1716. [Google Scholar] [CrossRef]
- Montemurro, F.; Cosimo, S.D. Pathological Complete Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Breast 2014, 23, 690–691. [Google Scholar] [CrossRef]
- Ren, S.; Xu, A.; Lin, Y.; Camidge, D.R.; Di Maio, M.; Califano, R.; Hida, T.; Rossi, A.; Guibert, N.; Zhu, C.; et al. A Narrative Review of Primary Research Endpoints of Neoadjuvant Therapy for Lung Cancer: Past, Present and Future. Transl. Lung Cancer Res. 2021, 10, 3264–3275. [Google Scholar] [CrossRef]
- Cottrell, T.R.; Thompson, E.D.; Forde, P.M.; Stein, J.E.; Duffield, A.S.; Anagnostou, V.; Rekhtman, N.; Anders, R.A.; Cuda, J.D.; Illei, P.B.; et al. Pathologic Features of Response to Neoadjuvant Anti-PD-1 in Resected Non-Small-Cell Lung Carcinoma: A Proposal for Quantitative Immune-Related Pathologic Response Criteria (irPRC). Ann. Oncol. 2018, 29, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; He, B.; Wang, F.; Zhong, Y.; Wang, T.; Liu, Z.; Yang, M.; Yu, B.; Deng, J.; Sun, X.; et al. Deep Learning for Predicting Major Pathological Response to Neoadjuvant Chemoimmunotherapy in Non-Small Cell Lung Cancer: A Multicentre Study. EBioMedicine 2022, 86, 104364. [Google Scholar] [CrossRef]
- Cui, Y.; Lin, Y.; Zhao, Z.; Long, H.; Zheng, L.; Lin, X. Comprehensive 18F-FDG PET-Based Radiomics in Elevating the Pathological Response to Neoadjuvant Immunochemotherapy for Resectable Stage III Non-Small-Cell Lung Cancer: A Pilot Study. Front. Immunol. 2022, 13, 994917. [Google Scholar] [CrossRef]
- Groheux, D.; Ferrer, L.; Vargas, J.; Martineau, A.; Teixeira, L.; Menu, P.; Bertheau, P.; Gallinato, O.; Colin, T.; Lehmann-Che, J. Multimodal Machine Learning Model Prediction of Complete Pathological Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. J. Clin. Oncol. 2022, 40, 601. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune Induction Strategies in Metastatic Triple-Negative Breast Cancer to Enhance the Sensitivity to PD-1 Blockade: The TONIC Trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
| Study Name or Number | Phase | Regimen | Positive Biomarker | Non-Contributing Biomarker | Negative Biomarker |
|---|---|---|---|---|---|
| Checkmate 816 | III | Chemotherapy ± nivolumab | PD-L1, non-squamous histology, ctDNA clearance | TMB | - |
| LCMC3 | II | Chemotherapy ± atezolizumab | PD-L1, CD8 | TMB, squamous histology | NKG2A expression |
| NEOSTAR | II | Nivolumab ± ipilimumab | PD-L1, ctDNA clearance | TCR repertoire | - |
| NADIM | II | Chemotherapy ± nivolumab | - | PD-L1, TMB | - |
| SAKK16/14 | II | Chemotherapy + durvalumab | - | PD-L1 | - |
| NCT02716038 | II | Chemotherapy + atezolizumab | - | PD-L1, squamous histology | STK11mut subtypes |
| Study Name or Number | Phase | Regimen | Positive Biomarker | Non-Contributing Biomarker | Negative Biomarker |
|---|---|---|---|---|---|
| OPACIN-NEO | II | Nivolumab + ipilimumab | 10 gene IFN-gamma score, TMB | PD-L1 | VEGFR-2 |
| NCT02519322 | II | Nivolumab ± ipilimumab | PD-L1, CD8 infiltration | TMB | - |
| OPACIN | Ib | Nivolumab + ipilimumab | CD8 infiltration, T cell expansion, 10 gene IFN-gamma score | - | - |
| NCT02434354 | Ib | Pembrolizumab | 18 gene IFN-gamma score | TMB | - |
| Study Name or Number | Phase | Regimen | Positive Biomarker | Non-Contributing Biomarker | Negative Biomarker |
|---|---|---|---|---|---|
| DUTRENEO | II | Chemotherapy + durvalumab + tremelimumab | PD-L1, tumour immune score (TIS), TLS | - | - |
| PURE-01 | II | Pembrolizumab | PD-L1, TMB, IFN gamma expression | - | - |
| ABACUS | II | Atezolizumab | PD-L1, CD8-granzyme B expression, tGE8 signature | TMB | Stromal TGF-beta, FAP |
| HCRN GU16-257 | II | Chemotherapy + nivolumab | TMB | - | - |
| NCT02989584 | II | Chemotherapy + atezolizumab | TMB, tGE8 signature | PD-L1 | - |
| BLASST1 | II | Chemotherapy + nivolumab | TMB | PD-L1 | - |
| NABUCCO | Ib | Ipilimumab + nivolumab | PD-L1, TMB | tGE8 signature, sTILs, TLS | Stromal TGF-beta, FAP |
| NCT02812420 | I | Durvalumab + tremelimumab | TLS | PD-L1, TMBtGE8 signature | Stromal TGF-beta, FAP |
| Study Name or Number | Phase | Regimen | Positive Biomarker | Non-Contributing Biomarker |
|---|---|---|---|---|
| KEYNOTE-522 | III | Chemotherapy ± pembrolizumab | - | PD-L1 |
| IMPASSION031 | III | Chemotherapy ± atezolizumab | - | PD-L1 |
| NeoTRIP | III | Chemotherapy ± atezolizumab | PD-L1, sTILs | - |
| GeparNUEVO | II | Chemotherapy ± durvalumab | PD-L1, sTILs | TMB |
| NeoPACT | II | Chemotherapy + pembrolizumab | sTILs, DetermaIO profile | - |
| I-SPY-2 | II | Chemotherapy + pembrolizumab | Dendritic cell signature, STAT1/chemokine signature, MHCII expression | - |
| Study Name or Number | Phase | Regimen | Positive Biomarker | Non-Contributing Biomarker |
|---|---|---|---|---|
| GERCOR NEONIPIGA | II | Ipilimumab + nivolumab | MSI | PD-L1 CPS |
| DANTE | IIb | Chemotherapy ± atezolizumab | PD-L1 CPS, MSI | - |
| NEO-PLANET | II | Chemoradiotherapy + camrelizumab | TMB | PD-L1 |
| Study Name or Number | Phase | Arms | Positive Biomarker | Non-Contributing Biomarker |
|---|---|---|---|---|
| NICHE-1 | I | Ipilimumab + nivolumab | MSI | TMB, TCR clonality, IFN-gamma score, TLS |
| NICHE-2 | II | Ipilimumab + nivolumab | MSI | - |
| NCT04165772 | II | Dostarlimab | MSI | - |
| VOLTAGE | II | Chemoradiotherapy then nivolumab | MSI, TMB, CD8 TILs, consensus molecular subtype 1 | - |
| NCT04231552 | II | Chemoradiotherapy then camrelizumab | TMB | - |
| Study Name or Number | Cancer Type | Arms | Adjuvant | Primary Endpoint(s) |
|---|---|---|---|---|
| Checkmate 77T | NSCLC | Neoadjuvant chemotherapy ± nivolumab | Nivolumab | EFS |
| IMpower030 | NSCLC | Neoadjuvant chemotherapy ± atezolizumab | Atezolizumab | MPR |
| NADINA | Melanoma | Neoadjuvant ipilimumab-nivolumab versus adjuvant nivolumab | Nivolumab (control arm) | EFS |
| NeoDREAM | Melanoma | Neoadjuvant intralesional Daromun + adjuvant therapy versus adjuvant therapy | Investigator’s choice of adjuvant therapy | RFS |
| NIAGARA | Bladder | Neoadjuvant chemotherapy ± durvalumab | Durvalumab | pCR and EFS |
| Keynote 905 | Bladder | Neoadjuvant pembrolizumab ± enfortumab vedotin | Pembrolizumab ± enfortumab vedotin | pCR and EFS |
| GeparDouze | TNBC | Neoadjuvant chemotherapy ± atezolizumab | Atezolizumab | pCR and EFS |
| MATTERHORN | Gastric-gastro-oesophageal | Perioperative chemotherapy ± durvalumab | Chemotherapy ± durvalumab | EFS |
| Keynote 585 | Gastric-gastro-oesophageal | Perioperative chemotherapy ± pembrolizumab | Chemotherapy ± pembrolizumab | pCR and EFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boydell, E.; Sandoval, J.L.; Michielin, O.; Obeid, M.; Addeo, A.; Friedlaender, A. Neoadjuvant Immunotherapy: A Promising New Standard of Care. Int. J. Mol. Sci. 2023, 24, 11849. https://doi.org/10.3390/ijms241411849
Boydell E, Sandoval JL, Michielin O, Obeid M, Addeo A, Friedlaender A. Neoadjuvant Immunotherapy: A Promising New Standard of Care. International Journal of Molecular Sciences. 2023; 24(14):11849. https://doi.org/10.3390/ijms241411849
Chicago/Turabian StyleBoydell, Emma, Jose L. Sandoval, Olivier Michielin, Michel Obeid, Alfredo Addeo, and Alex Friedlaender. 2023. "Neoadjuvant Immunotherapy: A Promising New Standard of Care" International Journal of Molecular Sciences 24, no. 14: 11849. https://doi.org/10.3390/ijms241411849






