Study of the Effect of Baicalin from Scutellaria baicalensis on the Gastrointestinal Tract Normoflora and Helicobacter pylori
Abstract
:1. Introduction
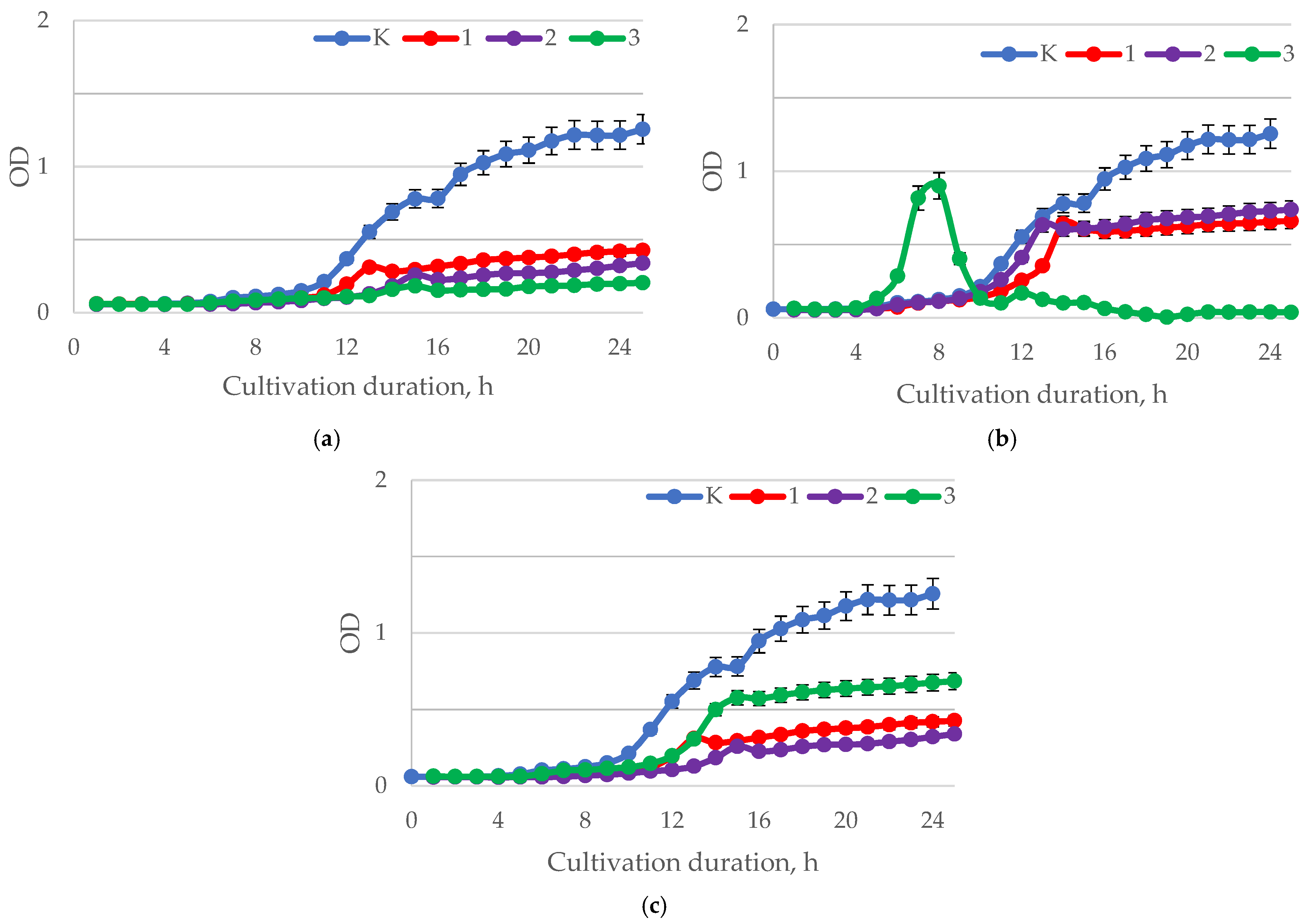
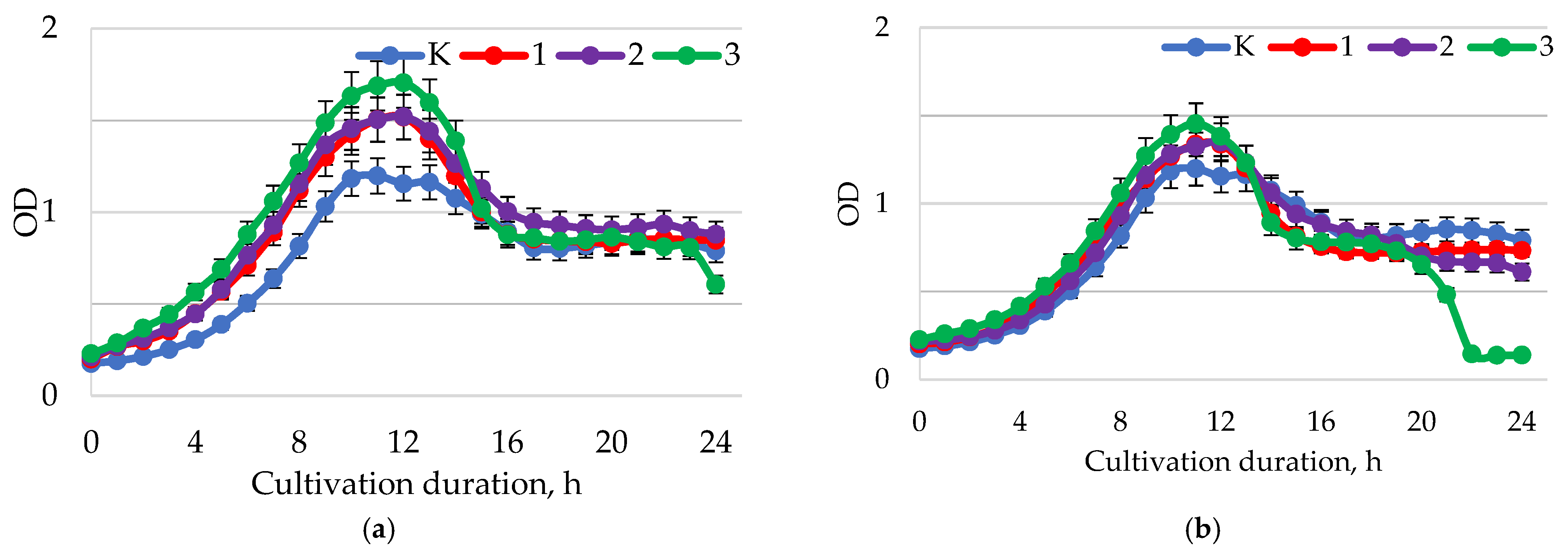
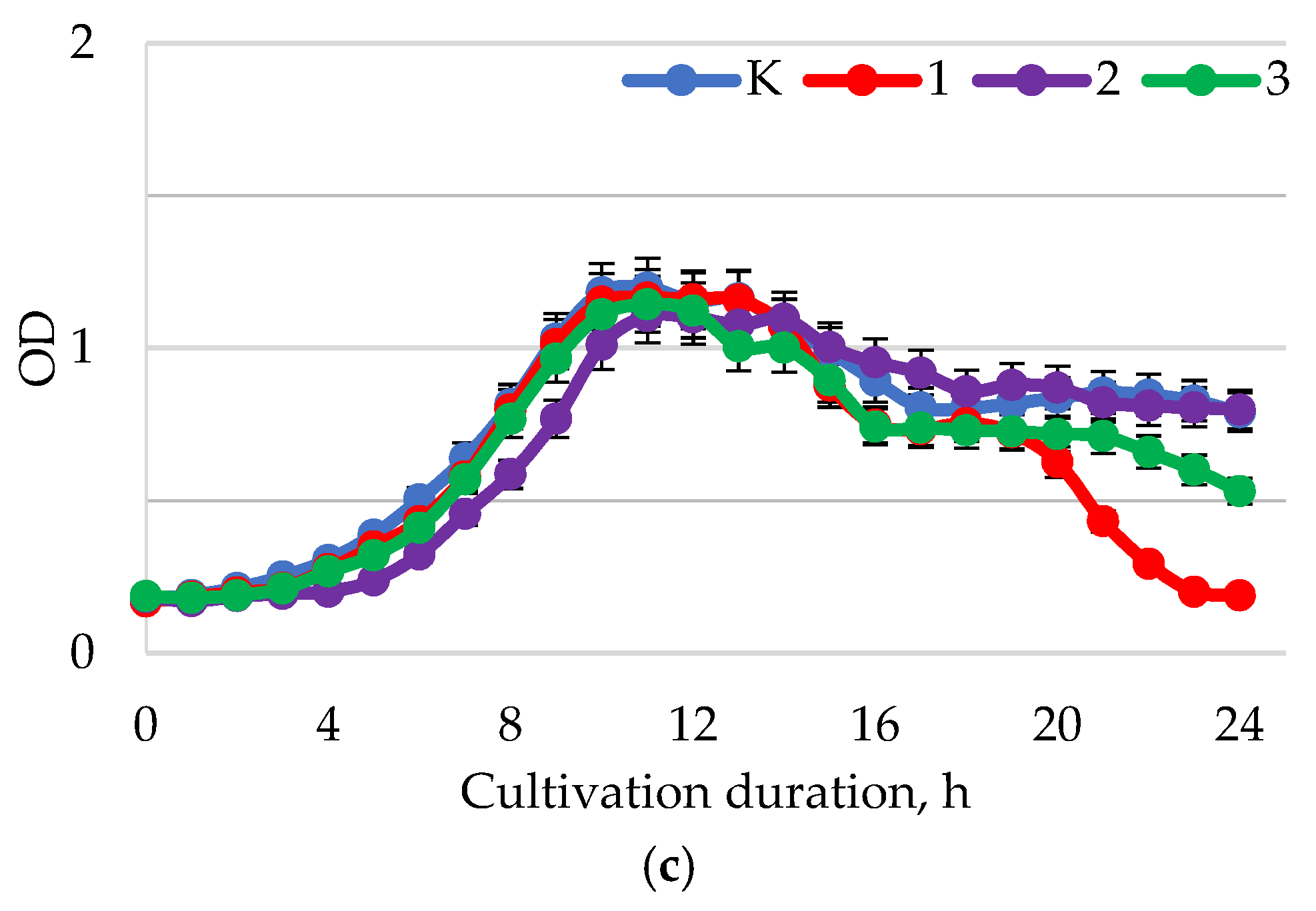
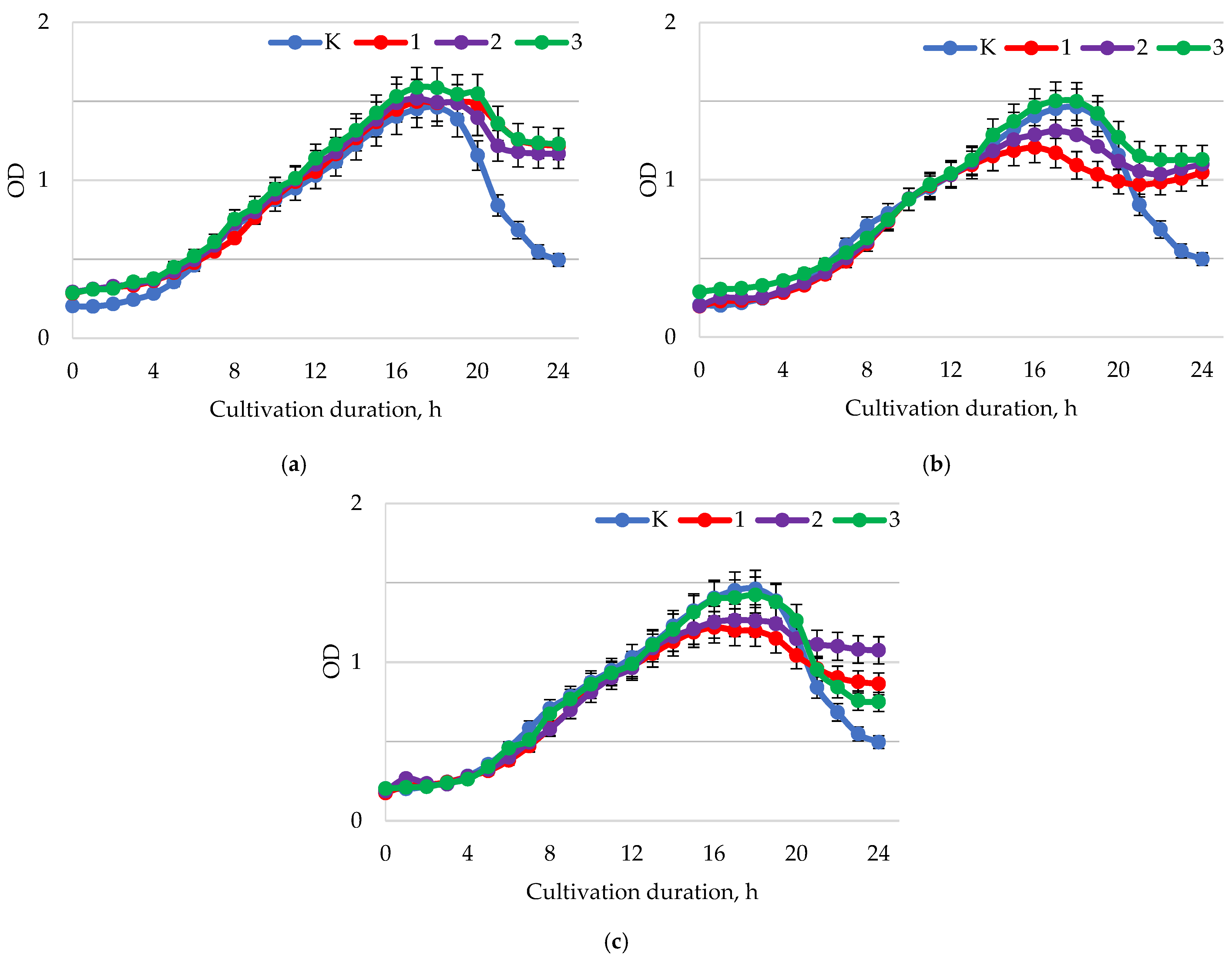
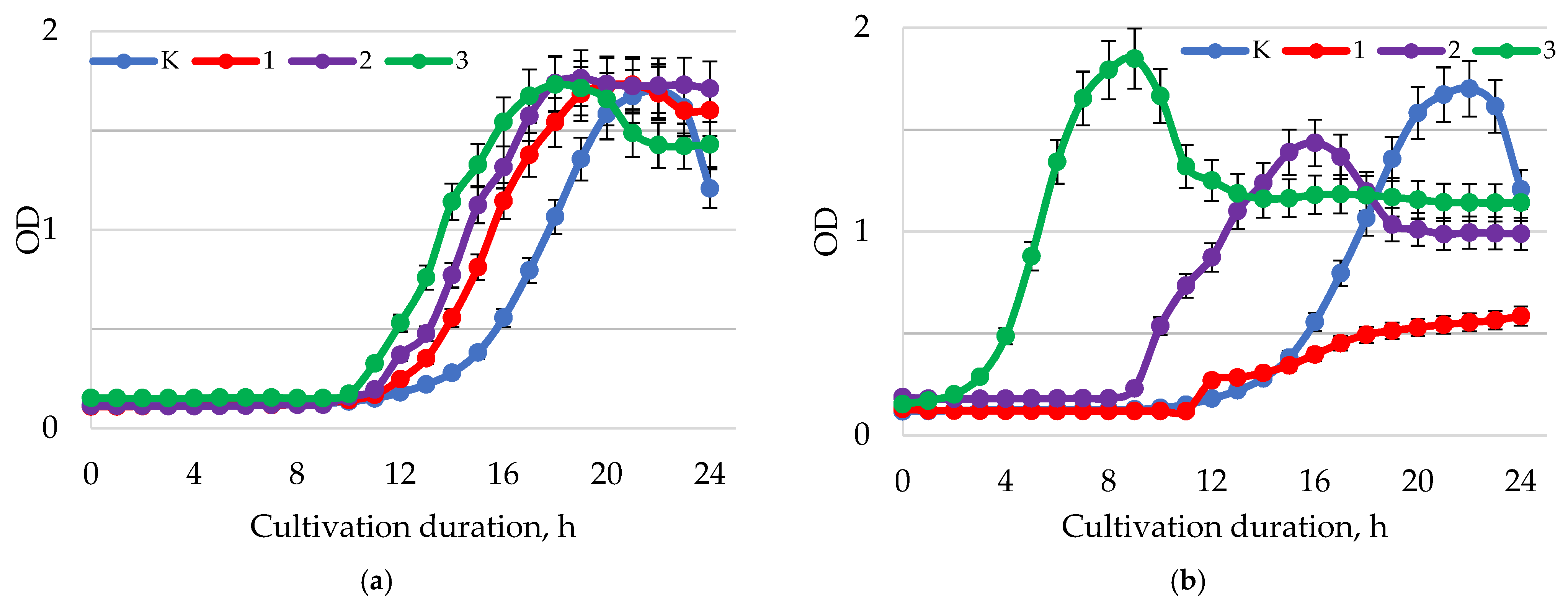
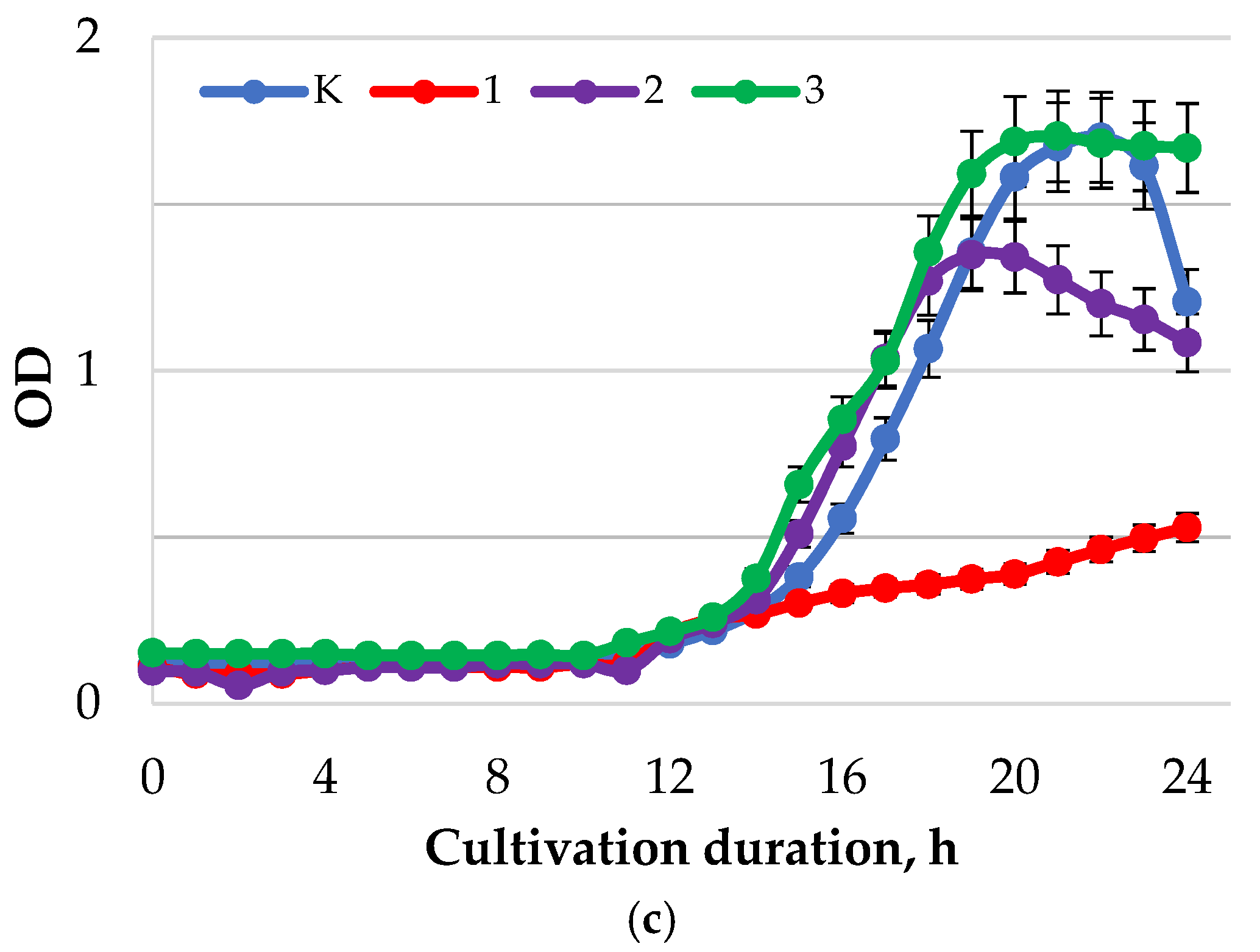
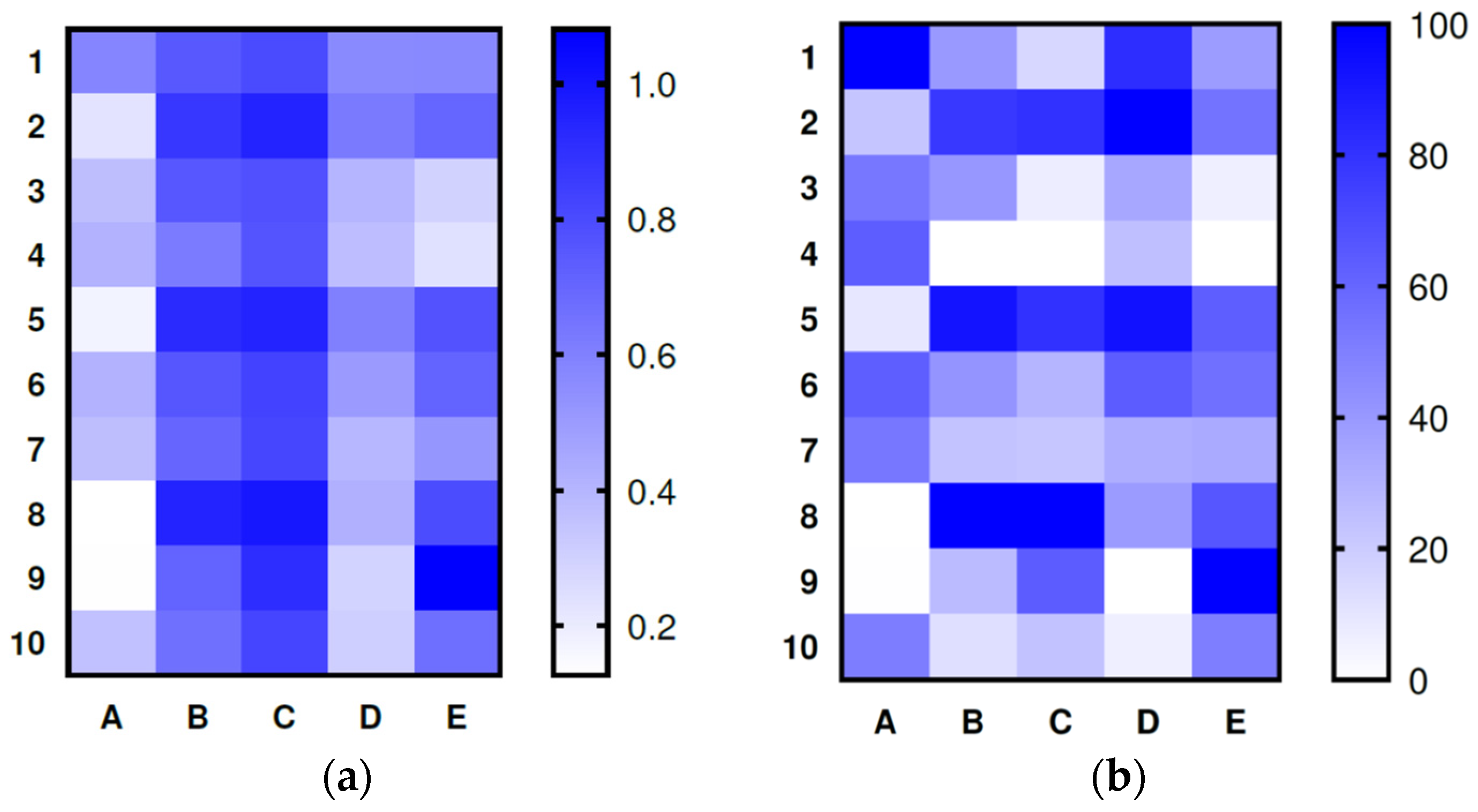
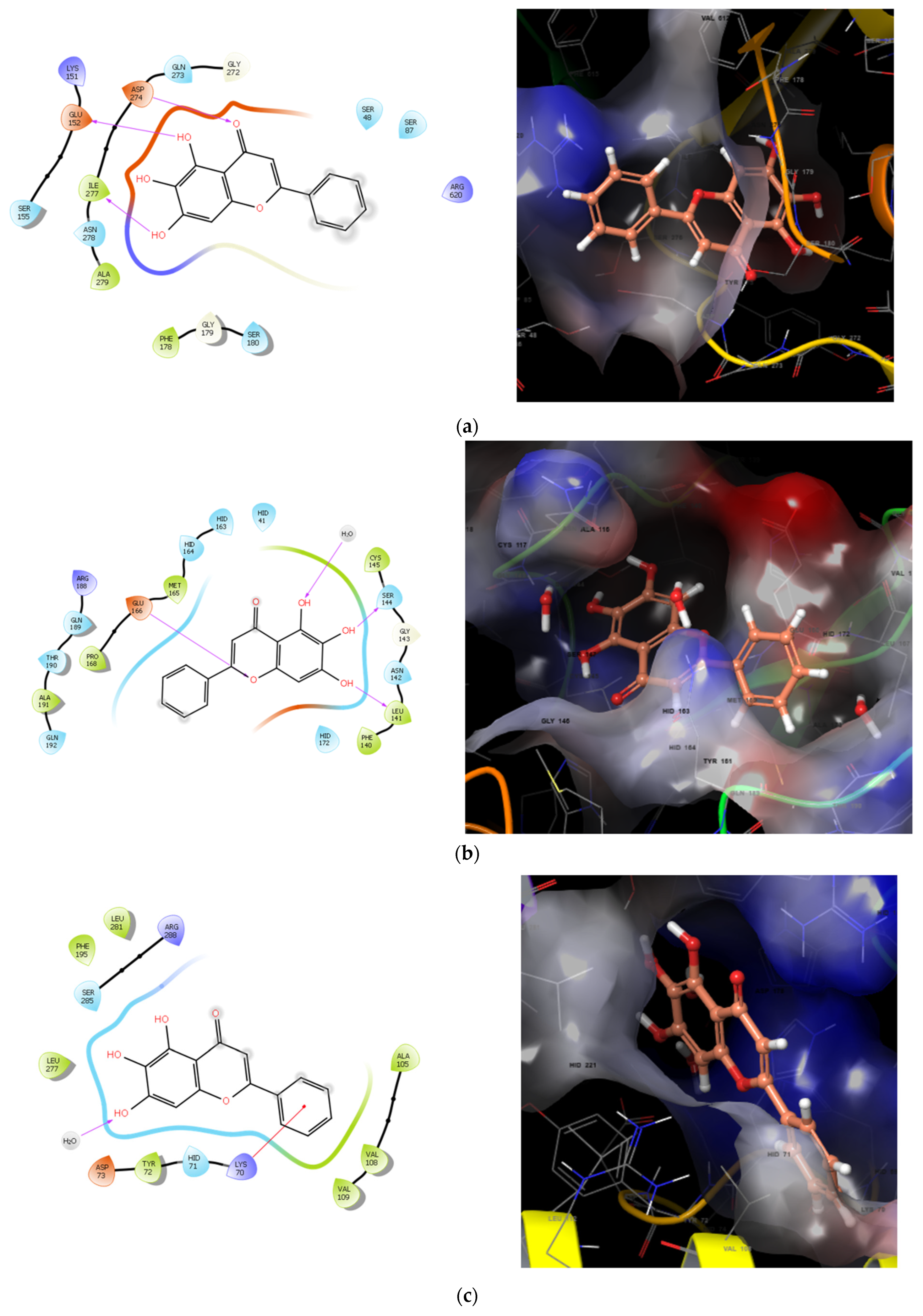
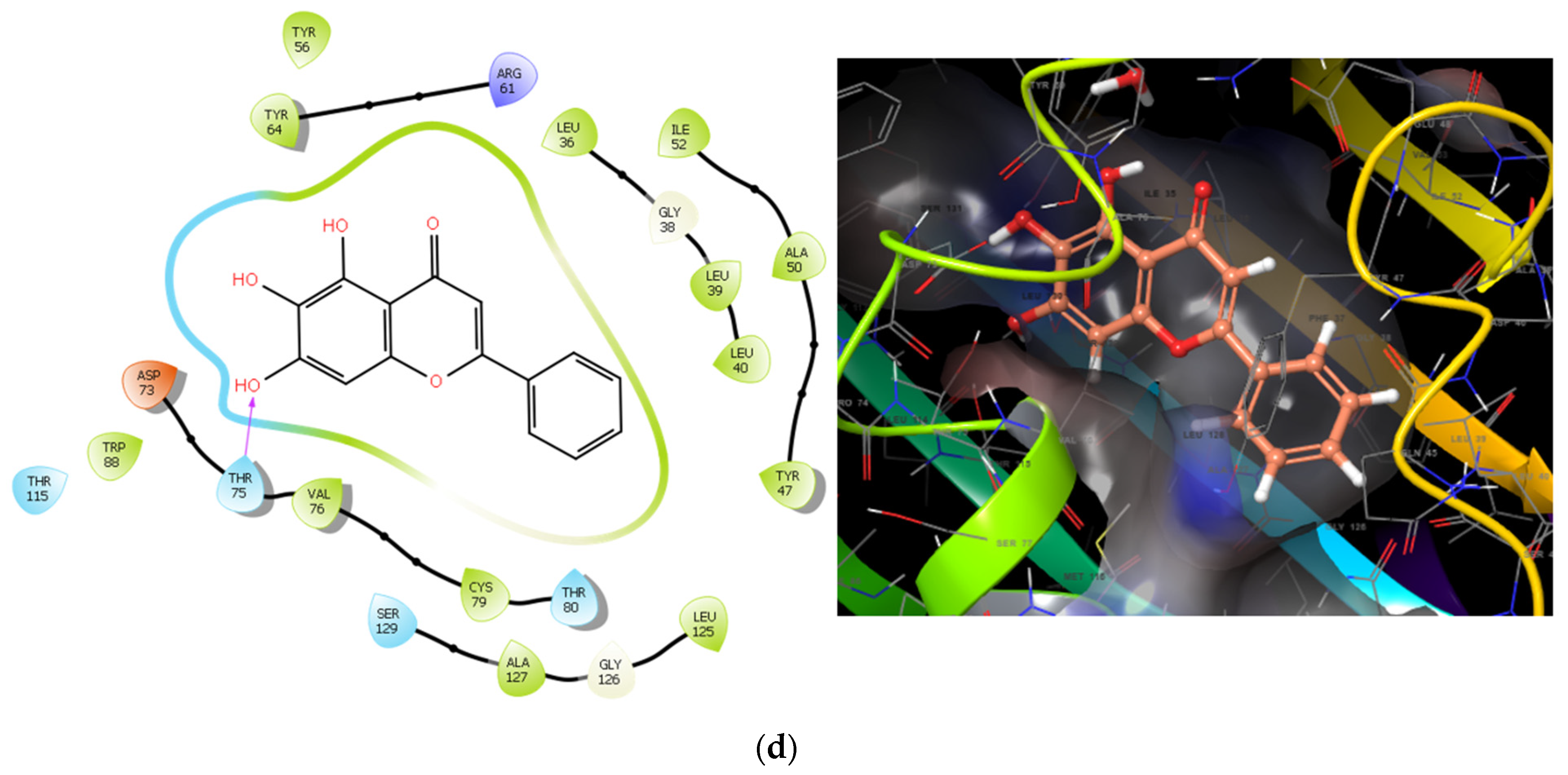
2. Results
3. Discussion
4. Materials and Methods
4.1. Scutellaria Baicalensis Extraction
4.2. Isolation of Polyphenols from the Scutellaria Baicalensis Extracts
4.3. Isolation of Baicalin from the Scutellaria Baicalensis Extracts
4.4. Determination of the Antimicrobial Properties of Baicalin
4.5. Determination of the Active Acidity of Baicalin Solutions
4.6. Docking Method
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Samples | H. pylori | L. casei | L. brevis | B. longum | B. adolescentis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | I* | I | I* | I | I* | I | I* | I | I* | |
| Y1 | 0.000003 | 0.084389 | 0.000005 | 0.000031 | 0.000090 | 0.000001 | 0.007557 | 0.000084 | 0.000000 | 0.007557 |
| Y2 | 0.000056 | 0.133983 | 0.000006 | 0.000035 | 0.000176 | 0.000006 | 0.020219 | 0.000689 | 0.000000 | 0.020219 |
| Y3 | 0.000060 | 0.128850 | 0.000002 | 0.000031 | 0.000013 | 0.000003 | 0.057831 | 1.000000 | 0.000000 | 0.057831 |
| Y4 | 0.000000 | 0.060581 | 0.000022 | 0.000099 | 0.000024 | 0.000001 | 0.013243 | 0.005520 | 0.000000 | 0.013243 |
| Y5 | 0.000074 | 0.143633 | 0.000004 | 0.000058 | 0.000073 | 0.000002 | 0.256614 | 1.000000 | 0.032460 | 0.256614 |
| Y6 | 0.000015 | 0.130982 | 0.004144 | 0.001492 | 0.000116 | 0.000001 | 0.00167 | 0.000014 | 0.000000 | 0.001670 |
| Y7 | 0.000013 | 0.180837 | 0.000000 | 0.000009 | 0.000041 | 0.000001 | 0.040699 | 0.000993 | 0.000045 | 0.040699 |
| Y8 | 0.163307 | 0.557292 | 0.000005 | 0.000045 | 0.000180 | 0.000001 | 0.829892 | 1.000000 | 0.643357 | 0.829892 |
| Y9 | 0.000006 | 0.080849 | 0.000028 | 0.000085 | 0.000000 | 0.000003 | 0.001318 | 0.039540 | 0.000000 | 0.001318 |
References
- Alarcón, T.; Llorca, L.; Perez-Perez, G. Impact of the Microbiota and Gastric Disease Development by Helicobacter pylori. Curr. Top. Microbiol. Immunol. 2017, 400, 253–275. [Google Scholar] [CrossRef]
- Diaconu, S.; Predescu, A.; Moldoveanu, A.; Pop, C.S.; Fierbințeanu-Braticevici, C. Helicobacter pylori infection: Old and new. J. Med. Life 2017, 10, 112–117. [Google Scholar] [PubMed]
- Matsumoto, H.; Shiotani, A.; Graham, D.Y. Current and Future Treatment of Helicobacter pylori Infections. Adv. Exp. Med. Biol. 2019, 1149, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- Ji, J.; Yang, H. Using Probiotics as Supplementation for Helicobacter pylori Antibiotic Therapy. Int. J. Mol. Sci. 2020, 21, 1136. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C. Medicinal plant activity on Helicobacter pylori related diseases. World J. Gastroenterol. 2014, 20, 10368–10382. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.F.; Hsu, P.I. Second-line rescue treatment of Helicobacter pylori infection: Where are we now? World J. Gastroenterol. 2018, 24, 4548–4553. [Google Scholar] [CrossRef]
- Lin, J.; Huang, W.W. A systematic review of treating Helicobacter pylori infection with Traditional Chinese Medicine. World J. Gastroenterol. 2009, 15, 4715–4719. [Google Scholar] [CrossRef]
- Sheh, A.; Fox, J.G. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes 2013, 4, 505–531. [Google Scholar] [CrossRef] [Green Version]
- Schulz, C.; Koch, N.; Schütte, K.; Pieper, D.H.; Malfertheiner, P. H. pylori and its modulation of gastrointestinal microbiota. J. Dig. Dis. 2015, 16, 109–117. [Google Scholar] [CrossRef]
- Hold, G.L.; Hansen, R. Impact of the Gastrointestinal Microbiome in Health and Disease: Co-evolution with the Host Immune System. Curr. Top. Microbiol. Immunol. 2019, 421, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Kurbanova, M.; Voroshilin, R.; Kozlova, O.; Atuchin, V. Effect of Lactobacteria on Bioactive Peptides and Their Sequence Identification in Mature Cheese. Microorganisms 2022, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Cho, I.K.; Lee, C.H.; Song, G.G.; Lim, J.H. Clinical Outcomes of Standard Triple Therapy Plus Probiotics or Concomitant Therapy for Helicobacter pylori Infection. Gut Liver 2018, 12, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Nabavi-Rad, A.; Sadeghi, A.; Asadzadeh Aghdaei, H.; Yadegar, A.; Smith, S.M.; Zali, M.R. The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut Microbes 2022, 14, 2108655. [Google Scholar] [CrossRef]
- Vesnina, A.D.; Prosekov, A.Y.; Kozlova, O.V.; Kurbanova, M.G.; Kozlenko, E.A.; Golubtsova, Y.V. Razrabotka probioticheskogo konsorciuma dlya lyudej s onkologicheskimi zabolevaniyami [Development of a probiotic consortium for people with cancer]. Vestn. VGUIT 2021, 83, 219–232. (In Russian) [Google Scholar]
- Boyanova, L.; Gergova, G.; Markovska, R.; Yordanov, D.; Mitov, I. Bacteriocin-like inhibitory activities of seven Lactobacillus delbrueckii subsp. bulgaricus strains against antibiotic susceptible and resistant Helicobacter pylori strains. Lett. Appl. Microbiol. 2017, 65, 469–474. [Google Scholar] [CrossRef]
- Asyakina, L.; Atuchin, V.; Drozdova, M.; Kozlova, O.; Prosekov, A. Ex Vivo and In Vitro Antiaging and Antioxidant Extract Activity of the Amelanchier ovalis from Siberia. Int. J. Mol. Sci. 2022, 23, 15156. [Google Scholar] [CrossRef]
- Rueda-Robles, A.; Rubio-Tomás, T.; Plaza-Diaz, J.; Álvarez-Mercado, A.I. Impact of Dietary Patterns on H. pylori Infection and the Modulation of Microbiota to Counteract Its Effect. A Narrative Review. Pathogens 2021, 10, 875. [Google Scholar] [CrossRef]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 7343–7350. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Escamilla-García, E.; de la Garza-Ramos, M.A.; Tamez-Guerra, P.; Gomez-Flores, R.; Urbina-Ríos, C.S. In Vitro Antimicrobial Activity and Downregulation of Virulence Gene Expression on Helicobacter pylori by Reuterin. Probiotics Antimicrob. Proteins 2018, 10, 168–175. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tsai, W.H.; Wu, H.Y.; Chen, C.Y.; Yeh, W.L.; Chen, Y.H.; Hsu, H.Y.; Chen, W.W.; Chen, Y.W.; Chang, W.W.; et al. Probiotic Lactobacillus spp. act Against Helicobacter pylori-induced Inflammation. J. Clin. Med. 2019, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Xie, Q.; Xu, D.; Guo, Y.; Tao, X.; Wei, H.; Wan, C. Antagonistics of Lactobacillus plantarum ZDY2013 against Helicobacter pylori SS1 and its infection in vitro in human gastric epithelial AGS cells. J. Biosci. Bioeng. 2018, 126, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Aiba, Y.; Ishikawa, H.; Tokunaga, M.; Komatsu, Y. Anti-Helicobacter pylori activity of non-living, heat-killed form of lactobacilli including Lactobacillus johnsonii No.1088. FEMS Microbiol. Lett. 2017, 364, fnx102. [Google Scholar] [CrossRef]
- Vesnina, A.; Prosekov, A.; Atuchin, V.; Minina, V.; Ponasenko, A. Tackling Atherosclerosis via Selected Nutrition. Int. J. Mol. Sci. 2022, 23, 8233. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Lu, C.W.; Lin, C.J. Treatment of Helicobacter pylori infection: Current status and future concepts. World J. Gastroenterol. 2014, 20, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A. Plants against Helicobacter pylori to combat resistance: An ethnopharmacological review. Biotechnol. Rep. 2020, 26, e00470. [Google Scholar] [CrossRef]
- Braude, M.R.; Bassily, R. Drug-induced liver injury secondary to Scutellaria baicalensis (Chinese skullcap). Intern. Med. J. 2019, 49, 544–546. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Baicalein as a potent neuroprotective agent: A review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Lin, H.; Qu, Y.; Shang, C.; Wang, Y.; Lu, Y.; Cui, X. Regulatory Mechanisms of Baicalin in Cardiovascular Diseases: A Review. Front. Pharmacol. 2020, 11, 583200. [Google Scholar] [CrossRef] [PubMed]
- Milentyeva, I.S.; Fedorova, A.M.; Larichev, T.A.; Altshuler, O.G. Biologically active compounds in Scutellaria baicalensis L. callus extract: Phytochemical analysis and isolation. Foods Raw Mater. 2023, 11, 172–186. [Google Scholar] [CrossRef]
- Yang, Y.; Asyakina, L.K.; Babich, O.O.; Dyshlyuk, L.S.; Sukhikh, S.A.; Popov, A.D.; Kostyushina, N. Physicochemical properties and biological activity of extracts of dried biomass of callus and suspension cells and in vitro root cultures. Food Process. Tech. Technol. 2020, 50, 480–492. (In Russian) [Google Scholar] [CrossRef]
- Anateva, T.N.; Bashilova, O.A.; Beberina, I.V.; Belyakova, N.P.; Bobyleva, E.V.; Borodina, V.N.; Borisova, V.S.; Borisova, I.N.; Brodovskaya-Kantakueen, I.V.; Bykova, A.N.; et al. Atlas of Habitats and Resources of Medicinal Plants; The Main Directorate of Geodesy and Cartography under the Council of Ministers of the USSR: Moscow, Russia, 1980; p. 340. Available online: https://bigenc.ru/b/atlas-arealov-i-resursov-le-6a4493 (accessed on 25 January 2023).
- Babich, O.; Sukhikh, S.; Prosekov, A.; Asyakina, L.; Ivanova, S. Medicinal Plants to Strengthen Immunity during a Pandemic. Pharmaceuticals 2020, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yu, M.; Shi, M.; Bo, P.; Gu, X.; Zhang, Z. Baicalin and its aglycone: A novel approach for treatment of metabolic disorders. Pharmacol. Rep. 2020, 72, 13–23. [Google Scholar] [CrossRef]
- Ibrahim, A.; Nasr, M.; El-Sherbiny, I.M. Baicalin as an emerging magical nutraceutical molecule: Emphasis on pharmacological properties and advances in pharmaceutical delivery. J. Drug Deliv. Sci. Technol. 2022, 70, 103269. [Google Scholar] [CrossRef]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem. Biophys Res. Commun. 2020, 531, 320–327. [Google Scholar] [CrossRef]
- Zhi, H.J.; Zhu, H.Y.; Zhang, Y.Y.; Lu, Y.; Li, H.; Chen, D.F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 2019, 57, 105–116. [Google Scholar] [CrossRef]
- Wang, H.; Ma, X.; Cheng, Q.; Wang, L.; Zhang, L. Deep Eutectic Solvent-Based Ultrahigh Pressure Extraction of Baicalin from Scutellaria baicalensis Georgi. Molecules 2018, 23, 3233. [Google Scholar] [CrossRef] [Green Version]
- Teplova, V.V.; Isakova, E.P.; Kljajn, O.I.; Dergacheva, D.I.; Gessler, N.N.; Derjabina, J.I. Prirodnye polifenoly: Biologicheskaja aktivnost’, farmakologicheskij potencial, puti metabolicheskoj inzhenerii (obzor) [Natural polyphenols: Biological activity, pharmacological potential, metabolic engineering pathways (review)]. Appl. Biochem. Microbiol. 2018, 54, 215–235. (In Russian) [Google Scholar] [CrossRef]
- Huang, Y.Q.; Huang, G.R.; Wu, M.H.; Tang, H.Y.; Huang, Z.S.; Zhou, X.H.; Yu, W.Q.; Su, J.W.; Mo, X.Q.; Chen, B.P.; et al. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: Treatment of Helicobacter pylori-induced multidrug resistance. World J. Gastroenterol. 2015, 21, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Casado, J.; Lanas, Á. Fighting the Antibiotic Crisis: Flavonoids as Promising Antibacterial Drugs Against Helicobacter pylori Infection. Front. Cell. Infect. Microbiol. 2021, 11, 709749. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-E.; Su, C.-H.; Yang, J.-S.; Lu, C.-C.; Hou, Y.-C.; Wu, J.-B.; Hsu, Y.-M. Baicalin, Baicalein, and Lactobacillus rhamnosus JB3 Alleviated Helicobacter pylori Infections in Vitro and in Vivo. J. Food Sci. 2018, 83, 3118–3125. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zheng, P.Y.; Yang, P.C. Efflux pump gene hefA of Helicobacter pylori plays an important role in multidrug resistance. World J. Gastroenterol. 2008, 14, 5217–5222. [Google Scholar] [CrossRef] [PubMed]
- Zang, T.; Yang, D.; Meng, X. Baicalin protects against gastroduodenal ulcers via the modulation of Nrf2 expression: Experimental, biochemical, and histological analyses. Pharmacol. Rep. 2017, 69, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Zheng, R.B.; Xie, J.H.; Su, J.Y.; Huang, X.Q.; Wang, Y.H.; Zheng, Y.F.; Mo, Z.Z.; Wu, X.L.; Wu, D.W.; et al. Biological evaluation and molecular docking of baicalin and scutellarin as Helicobacter pylori urease inhibitors. J. Ethnopharmacol. 2015, 162, 69–78. [Google Scholar] [CrossRef]
- Zabokritsky, N.A. Kratkij obzor sovremennogo sostoyaniya rynka farmakologicheskih preparatov (otechestvennyh i importnyh) na osnove probioticheskih bakterij [A brief review of the current state of the market for pharmacological preparations (domestic and imported) based on probiotic bacteria]. J. Sci. Artic. Health Educ. 21st Century 2015, 17, 3–15. (In Russian) [Google Scholar]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V. Calcium and strontium thiobarbiturates with discrete and polymeric structures. J. Coord. Chem. 2013, 66, 4119–4130. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V.; Sidorenko, M.Y.; Dmitrushkov, M.S. Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2 2H2O and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe). Polyhedron 2014, 70, 71–76. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Molokeev, M.S.; Oreshonkov, A.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Azarapin, N.O.; Andreev, O.V.; Razumkova, I.A.; Atuchin, V.V. Crystal structure, vibrational, spectroscopic and thermochemical properties of double sulfate crystalline hydrate [CsEu(H2O)3(SO4)2]·H2O and its thermal dehydration product CsEu(SO4)2. Crystals 2021, 11, 1027. [Google Scholar] [CrossRef]
- Wu, J.; Hu, D.; Wang, K.X. Study of Scutellaria baicalensis and Baicalin against antimicrobial susceptibility of Helicobacter pylori strains in vitro. Zhong Yao Cai 2008, 31, 707–710. [Google Scholar]
- Asyakina, L.K.; Eremeeva, N.I.; Dyshlyuk, L.S. Selection of optimal parameters for the ex-traction of a complex of biologically active compounds from suspension cultures of medicinal plants of the Siberian Federal District. Bull. KrasGAU 2021, 8, 176–187. [Google Scholar] [CrossRef]
- Wan, F.; Wang, M.; Zhong, R.; Chen, L.; Han, H.; Liu, L.; Zhao, Y.; Lv, H.; Hou, F.; Yi, B.; et al. Supplementation With Chinese Medicinal Plant Extracts From Lonicera hypoglauca and Scutellaria baicalensis Mitigates Colonic Inflammation by Regulating Oxidative Stress and Gut Microbiota in a Colitis Mouse Model. Front. Cell. Infect. Microbiol. 2022, 11, 798052. [Google Scholar] [CrossRef]
- Bojko, N.N.; Pisarev, D.I.; Zhiljakova, E.T.; Maljutina, A.J.; Novikov, O.O.; Bocharnikova, M.A. Izuchenie kinetiki gidroliza bajkalina pri ego jekstrakcii iz kornej shlemnika bajkal’skogo. [Study of the kinetics of baicalin hydrolysis during its extraction from the roots of Scutellaria baicalensis]. Pharm. Pharmacol. 2019, 7, 129–137. (In Russian) [Google Scholar] [CrossRef]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Victoria Moreno-Arribas, M.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef]
- Park, H.S.; Wijerathne, C.; Jeong, H.Y.; Seo, C.S.; Ha, H.; Kwun, H.J. Gastroprotective effects of Hwanglyeonhaedok-tang against Helicobacter pylori-induced gastric cell injury. J. Ethnopharmacol. 2018, 216, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, H.U.; Delsoglio, M.; Brix, S.; Pessione, E.; Svensson, B. Plant Polyphenols Stimulate Adhesion to Intestinal Mucosa and Induce Proteome Changes in the Probiotic Lactobacillus acidophilus NCFM. Mol. Nutr. Food Res. 2018, 62, 1700638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, T.H.; Castillo, A.; Lucia, L.M.; Acuff, G.R. Growth of Helicobacter pylori in various liquid and plating media. Lett. Appl. Microbiol. 2000, 30, 192–196. [Google Scholar] [CrossRef] [PubMed]
- García, C.A.; Henríquez, A.P.; Retamal, R.C.; Pineda, C.S.; Delgado Sen, C.; González, C.C. Propiedades probióticas de Lactobacillus spp. aislados de biopsias gástricas de pacientes con y sin infección por Helicobacter pylori [Probiotic properties of Lactobacillus spp. isolated from gastric biopsies of Helicobacter pylori infected and non-infected individuals]. Rev. Med. Chile 2009, 137, 369–376. [Google Scholar]



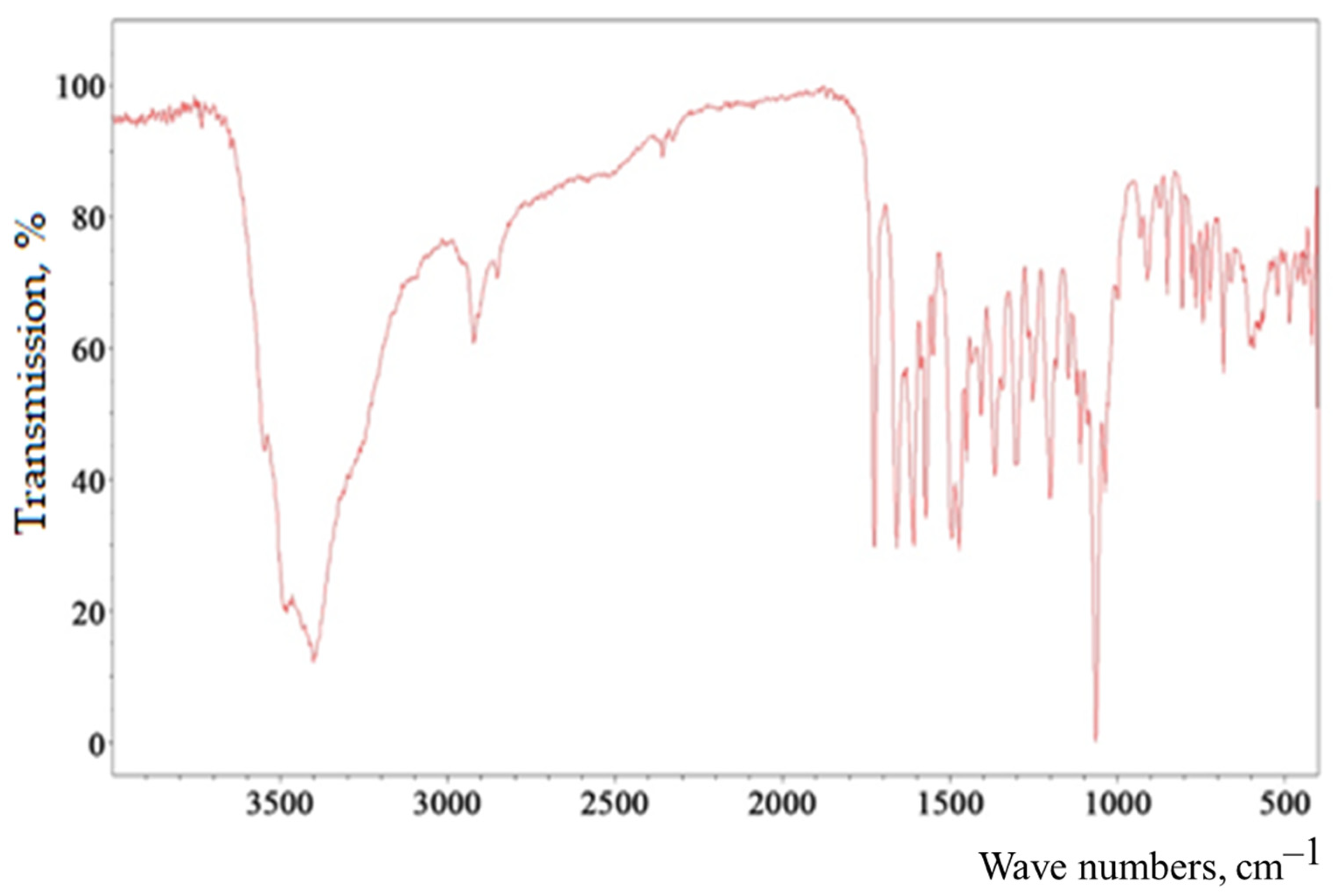

| Spike Number | BAS | Molecular Formula | Structural Formula | Retention Time, min | Content, mg/g |
|---|---|---|---|---|---|
| 1 | 3,4-Dihydroxycinnamic acid | C9H8O4 | 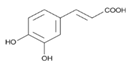 | 16.28 | 3.990 ± 0.003 |
| 2 | Luteolin | C15H10O6 |  | 20.03 | 1.710 ± 0.001 |
| 3 | Rutin | C27H30O16 | 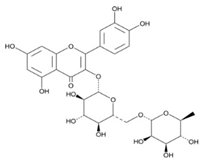 | 28.35 | 1.140 ± 0.002 |
| 4 | Scutellarin | C15H10O6 | 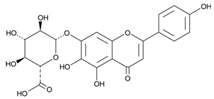 | 39.18 | 1.430 ± 0.003 |
| 5 | Chrysin | C18H12 | 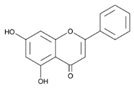 | 41.10 | 0.860 ± 0.001 |
| 6 | Baicalin | C21H18O11 | 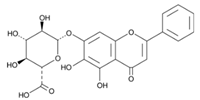 | 58.53 | 7.130 ± 0.004 |
| Substance | Concentration, mg/mL | ||
|---|---|---|---|
| 0.25 | 0.5 | 1.0 | |
| Baicalin | 13.40 ± 0.05 * | 15.10 ± 0.05 * | 18.90 ± 0.05 * |
| Clarithromycin | 19.60 ± 0.05 | 29.30 ± 0.05 | 37.10 ± 0.05 |
| Amoxicillin | 24.60 ± 0.05 | 32.20 ± 0.05 | 43.50 ± 0.05 |
| Sample | Baicalin, mg/mL | pH | Test Culture | ||||
|---|---|---|---|---|---|---|---|
| H. pylori (A) | L. casei (B) | L. brevis (C) | B. longum (D) | B. adolescentis (E) | |||
| Y0 | Control | 0.5803 ± 0.0135 | 0.7498 ± 0.0303 | 0.7996 ± 0.0330 | 0.5621 ± 0.0236 | 0.5655 ± 0.0213 | |
| Y1 | 0.25 | 1.5–2.0 | 0.2288 ± 0.0104 | 0.8736 ± 0.0386 | 0.9467 ± 0.0455 | 0.6211 ± 0.0281 | 0.7000 ± 0.032 |
| Y2 | 0.25 | 5.5–6.0 | 0.3668 ± 0.1533 | 0.7521 ± 0.0337 | 0.7806 ± 0.0367 | 0.4030 ± 0.0204 | 0.2910 ± 0.0140 |
| Y3 | 0.25 | 7.5–8.0 | 0.4136 ± 0.0195 | 0.6198 ± 0.0301 | 0.7651 ± 0.0363 | 0.3714 ± 0.0122 | 0.2407 ± 0.0188 |
| Y4 | 0.50 | 1.5–2.0 | 0.1678 ± 0.0059 | 0.9214 ± 0.0391 | 0.9466 ± 0.0433 | 0.5982 ± 0.0205 | 0.7697 ± 0.0302 |
| Y5 | 0.50 | 5.5–6.0 | 0.4120 ± 0.0270 | 0.7564 ± 0.0352 | 0.8297 ± 0.0393 | 0.5010 ± 0.0206 | 0.7096 ± 0.0344 |
| Y6 | 0.50 | 7.5–8.0 | 0.3680 ± 0.0174 | 0.6966 ± 0.0342 | 0.8150 ± 0.0397 | 0.3935 ± 0.0159 | 0.5188 ± 0.0251 |
| Y7 | 1.00 | 1.5–2.0 | 0.1266 ± 0.0051 | 0.9464 ± 0.0434 | 0.9900 ± 0.0468 | 0.4183 ± 0.0205 | 0.7943 ± 0.0372 |
| Y8 | 1.00 | 5.5–6.0 | 0.1270 ± 0.0028 | 0.7066 ± 0.0360 | 0.9086 ± 0.0435 | 0.2887 ± 0.0132 | 1.0797 ± 0.0484 |
| Y9 | 1.00 | 7.5–8.0 | 0.3570 ± 0.0147 | 0.6600 ± 0.0303 | 0.8184 ± 0.0428 | 0.3091 ± 0.0152 | 0.6628 ± 0.0327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrieva, A.; Kozlova, O.; Atuchin, V.; Milentieva, I.; Vesnina, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Study of the Effect of Baicalin from Scutellaria baicalensis on the Gastrointestinal Tract Normoflora and Helicobacter pylori. Int. J. Mol. Sci. 2023, 24, 11906. https://doi.org/10.3390/ijms241511906
Dmitrieva A, Kozlova O, Atuchin V, Milentieva I, Vesnina A, Ivanova S, Asyakina L, Prosekov A. Study of the Effect of Baicalin from Scutellaria baicalensis on the Gastrointestinal Tract Normoflora and Helicobacter pylori. International Journal of Molecular Sciences. 2023; 24(15):11906. https://doi.org/10.3390/ijms241511906
Chicago/Turabian StyleDmitrieva, Anastasia, Oksana Kozlova, Victor Atuchin, Irina Milentieva, Anna Vesnina, Svetlana Ivanova, Lyudmila Asyakina, and Alexander Prosekov. 2023. "Study of the Effect of Baicalin from Scutellaria baicalensis on the Gastrointestinal Tract Normoflora and Helicobacter pylori" International Journal of Molecular Sciences 24, no. 15: 11906. https://doi.org/10.3390/ijms241511906








