Dysregulation of Histone Deacetylases Inhibits Trophoblast Growth during Early Placental Development Partially through TFEB-Dependent Autophagy-Lysosomal Pathway
Abstract
1. Introduction
2. Result
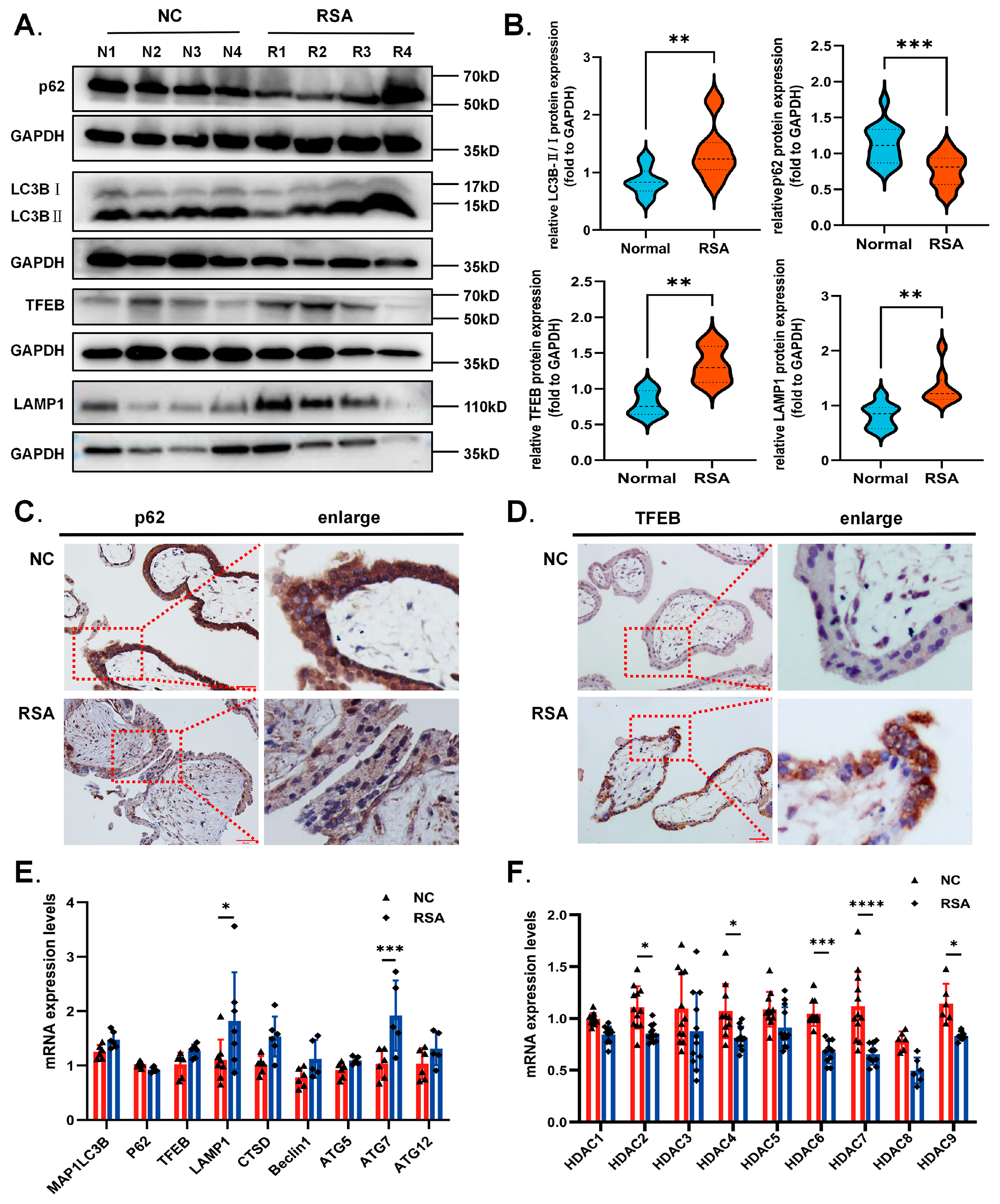
2.1. Decreased HDAC Expression and Excessive Autophagy Levels in the Villi of RSA Patients during Early Gestation
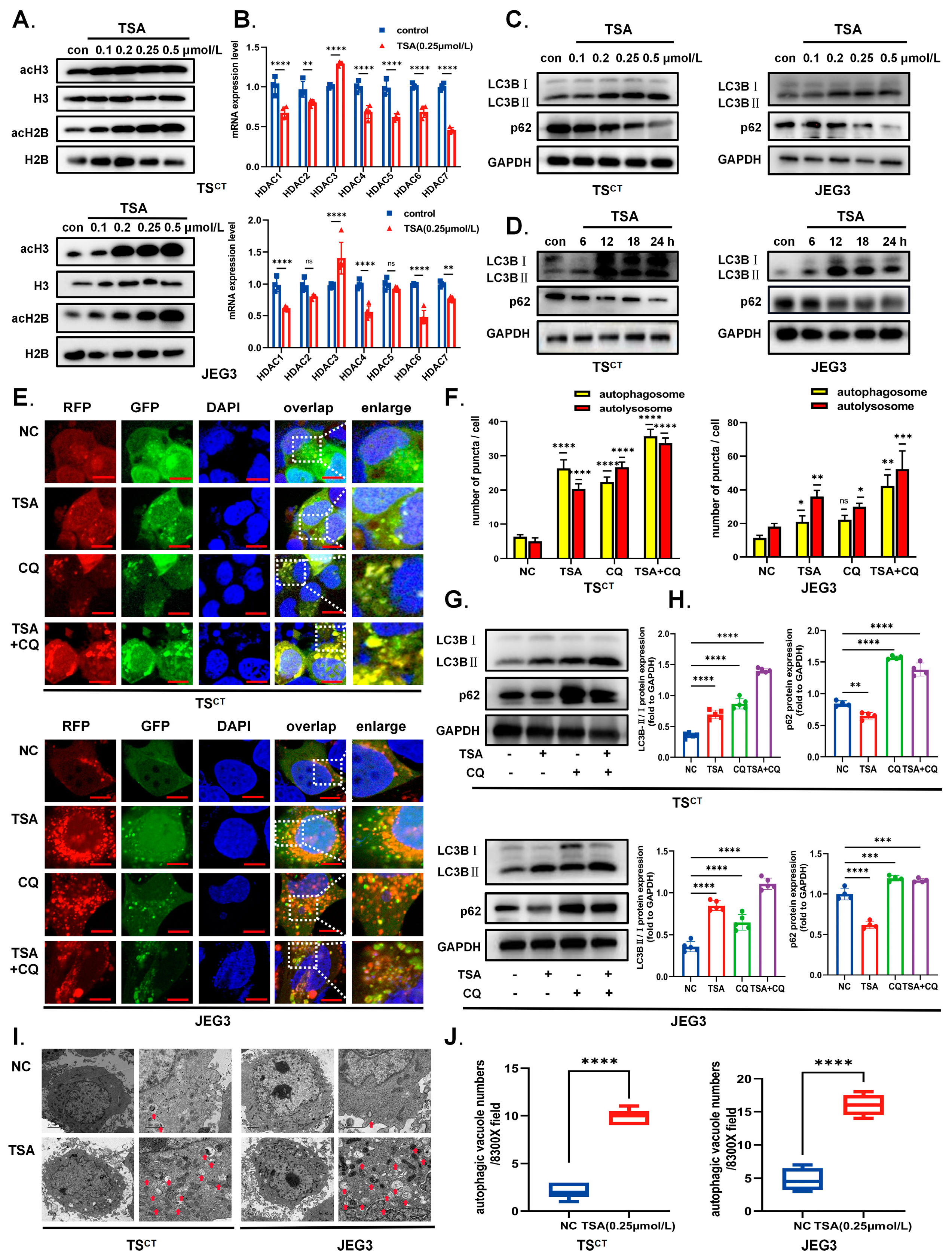
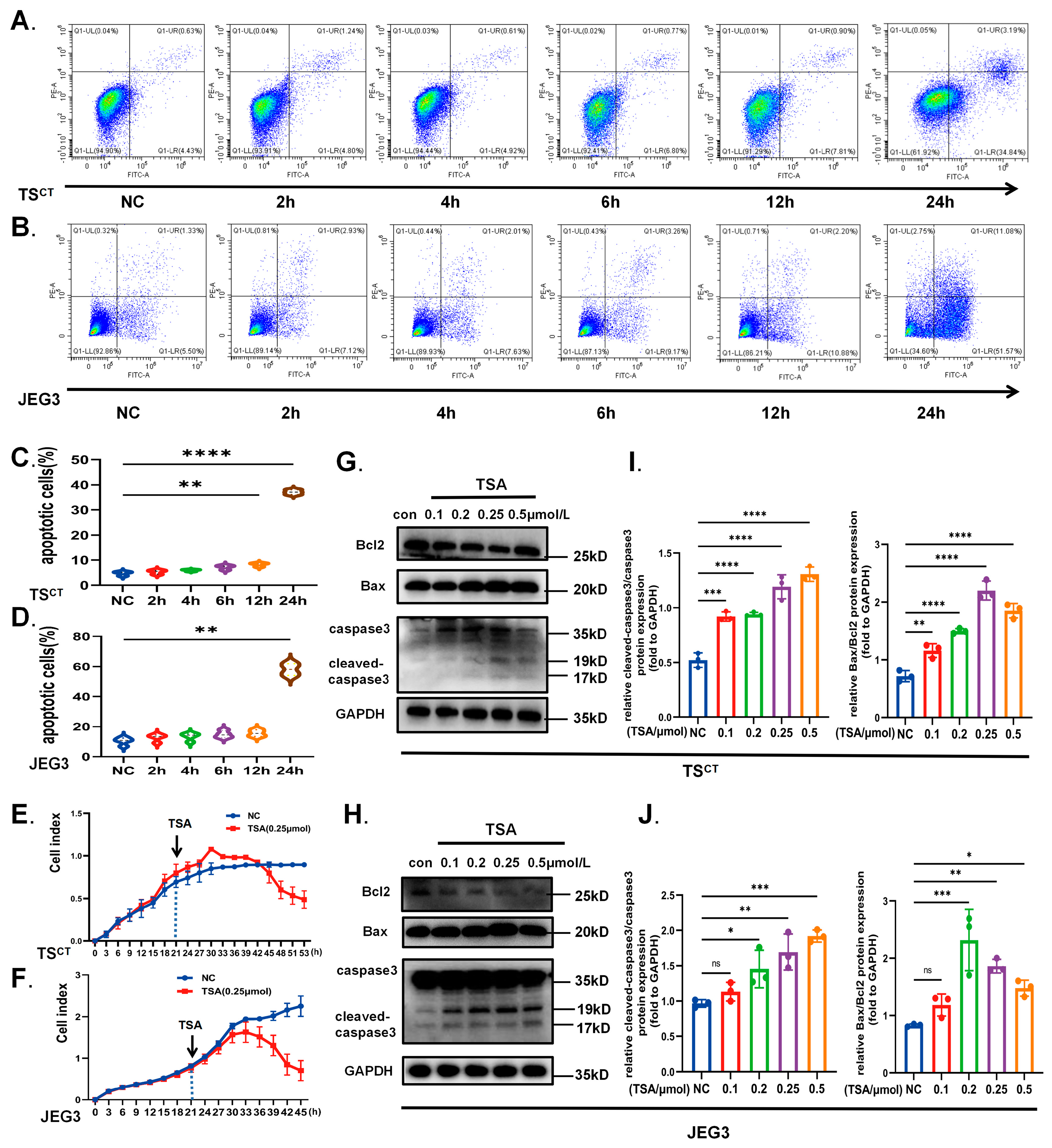
2.2. HDAC Inhibitor TSA Induces Acetylation and Autophagy in Human Trophoblast Cells
2.3. HDAC Inhibitor TSA Promotes Nuclear Translocation and Transcriptional Activity of TFEB in Human Trophoblast Cells
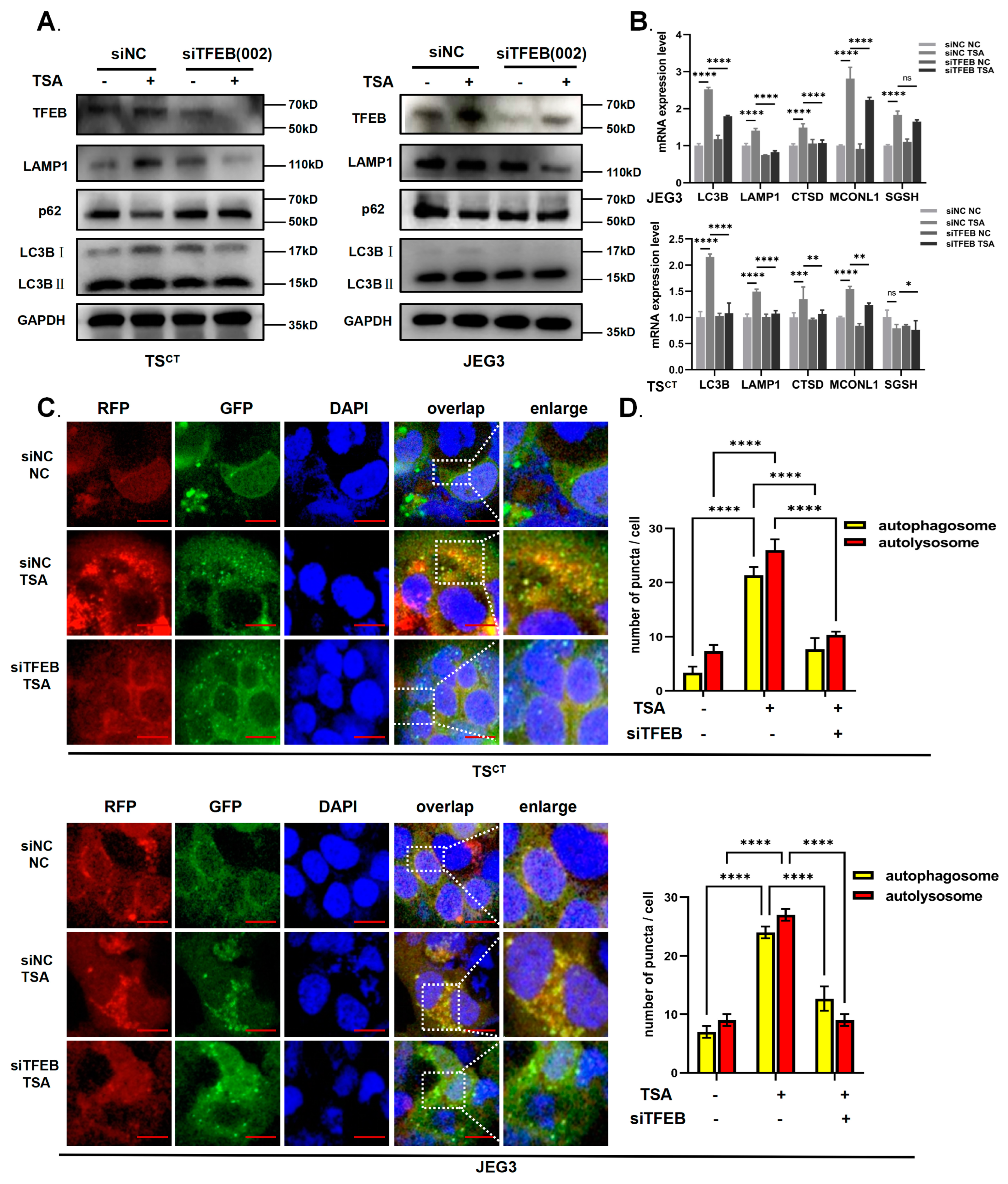
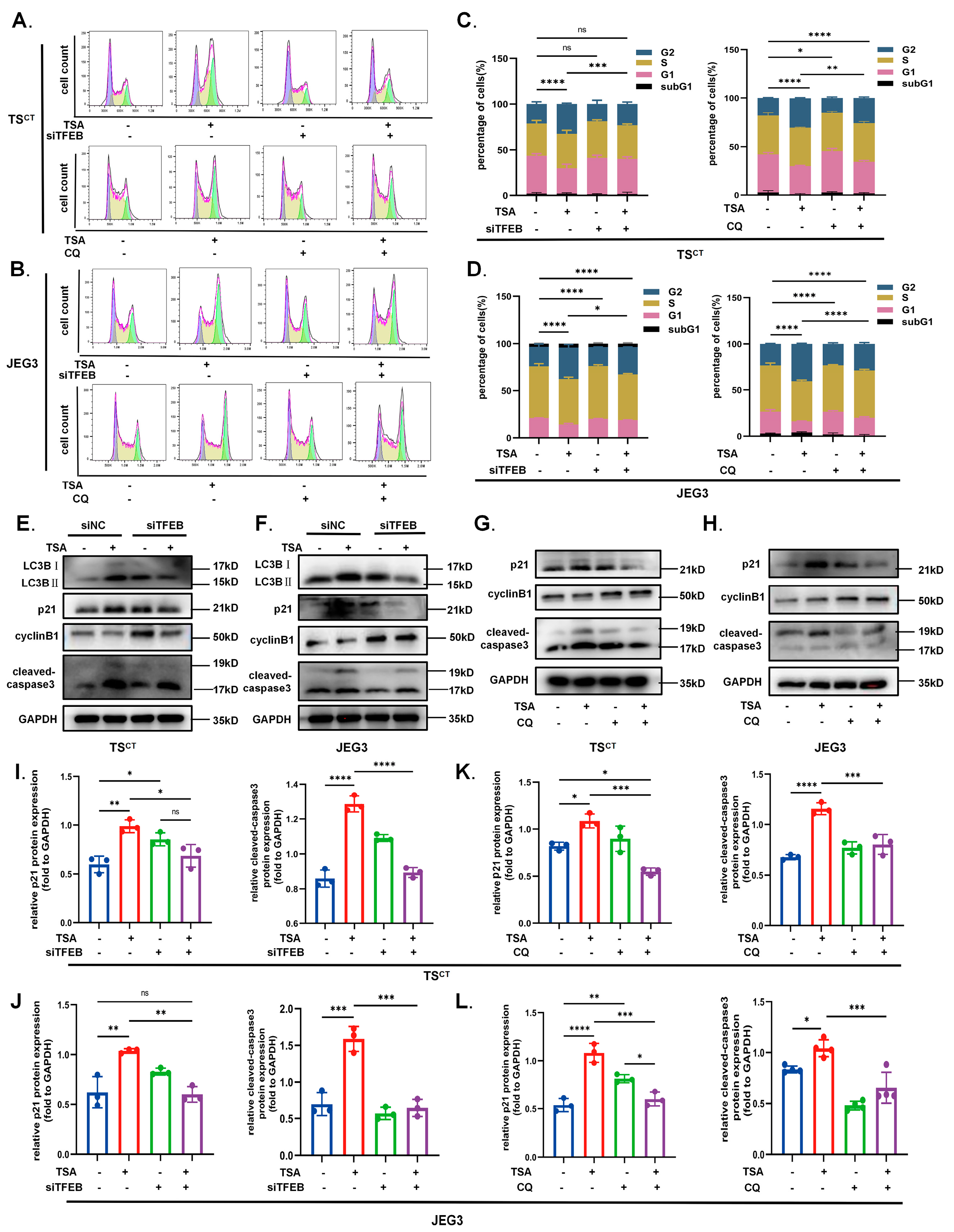
2.4. TFEB Is Involved in TSA-Induced Autophagy and Lysosome Biosynthesis in Human Trophoblast Cells
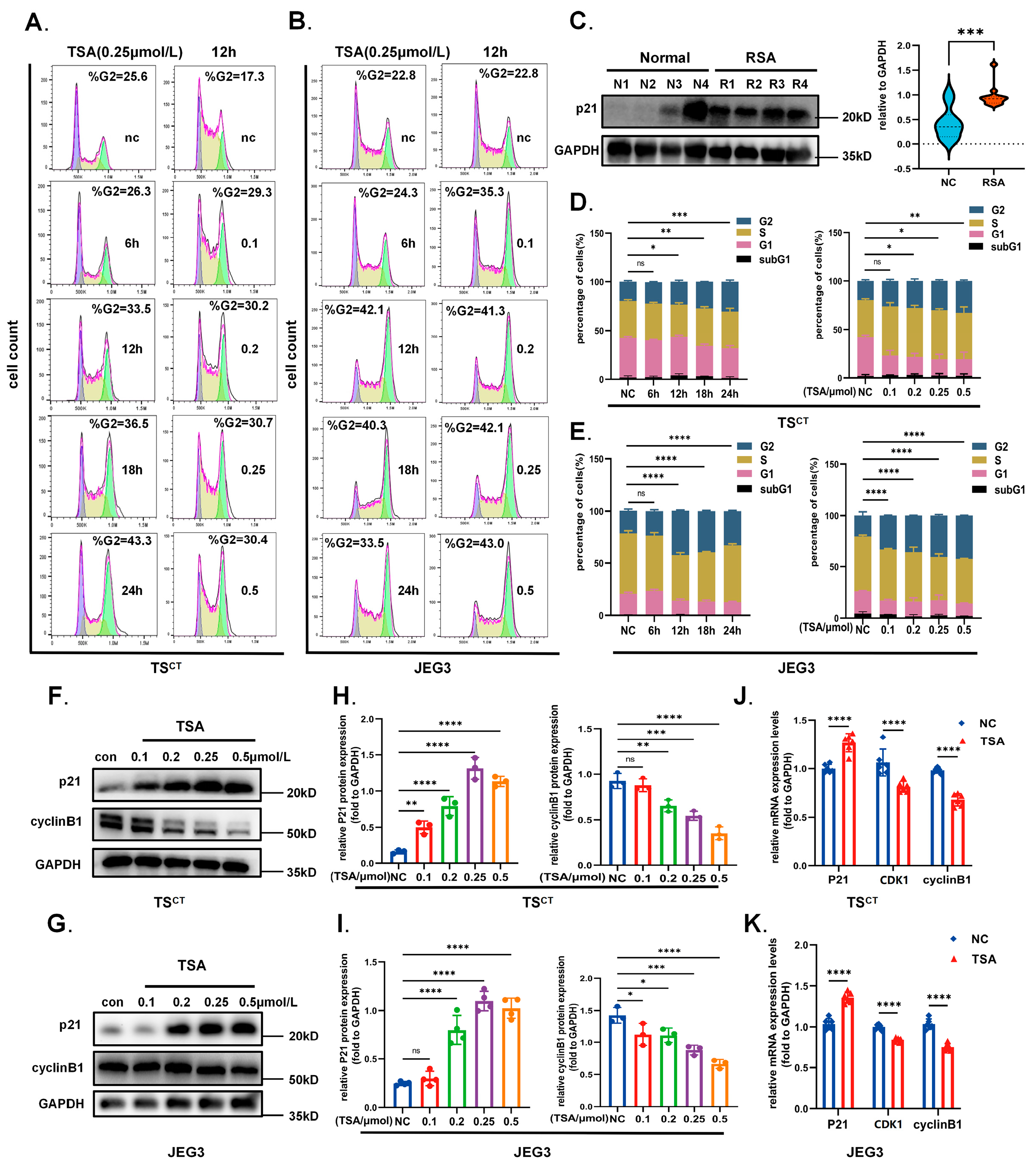
2.5. HDAC Inhibitor TSA Interferes with Trophoblast Growth
2.6. TFEB-Dependent Autophagy Contributes to the Growth Inhibition Effect of HDAC Inhibitor TSA on Trophoblast Cells
2.7. Vivo Experiments: Autophagy Inhibition Partially Reverses the Effects of the TSA-Mediated Acetylation on Murine Abortion Rate during Early Pregnancy
3. Discussion
4. Materials and Methods
4.1. Tissue Collection and Preparation
- (1)
- Normal pregnancy: 20 healthy women who asked for legal termination of pregnancy (years of age range from 20 to 35; gestational ages range from 6 to 8 weeks). Each sample of gestational villus was gathered in sterile conditions and went through several washes in sterile pre-chilled PBS. Part of the tissue collected was fixed in 10% formalin solution for immunohistochemicals, while the other part was frozen in liquid nitrogen and stored at −80 °C for Western blot and RT-qPCR extraction.
- (2)
- Recurrent spontaneous abortion (RSA): All cases were unexplained recurrent spontaneous abortions but had normal karyotypes. They were comparable to normal pregnancy samples in terms of gestational age. The villus samples were taken within one week after the embryo was confirmed to be terminated. Confounding factors were excluded such as anatomical abnormalities, metabolic disorders or other autoimmune diseases.
4.2. Human Trophoblast Stem (TS) Cell Isolation and Cell Culture
4.3. Reagents and Antibodies
4.4. RNA Extraction and Real-Time Quantitative PCR
4.5. Western Blot Analysis
4.6. Immunofluorescence Staining
4.7. Immunohistochemistry of Paraffin Sections
4.8. RNA Interference (Transient Transfection of siRNAs)
4.9. Autophagy Adenovirus Transfection
4.10. Examination of Lysosomal Acidification
4.11. Electron Microscopy
4.12. Nucleoplasmic Separation
4.13. Apoptosis and Necrosis Analysis
4.14. Cell Cycle Detection
4.15. Mice
4.16. Mouse Experiments
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, M.; Bai, L.; Wan, Z.; Wan, S.; Xiang, Y.; Qian, Y.; Cui, L.; You, J.; Hu, X.; Qu, F.; et al. circRNA-DURSA regulates trophoblast apoptosis via miR-760-HIST1H2BE axis in unexplained recurrent spontaneous abortion. Mol. Ther. Nucleic Acids 2021, 26, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.J.; Azari, S.; Webb, A.; Zhang, X.; Gavrilin, M.A.; Marshall, J.M.; Rood, K.; Seveau, S. Human Placental Trophoblasts Infected by Listeria Monocytogenes Undergo a Pro-Inflammatory Switch Associated with Poor Pregnancy Outcomes. Front. Immunol. 2021, 12, 709466. [Google Scholar] [CrossRef] [PubMed]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 2018, 22, 50–63.e6. [Google Scholar] [CrossRef]
- Sun, X.; Tong, X.; Hao, Y.; Li, C.; Zhang, Y.; Pan, Y.; Dai, Y.; Liu, L.; Zhang, T.; Zhang, S. Abnormal Cullin1 neddylation-mediated p21 accumulation participates in the pathogenesis of recurrent spontaneous abortion by regulating trophoblast cell proliferation and differentiation. Mol. Hum. Reprod. 2020, 26, 327–339. [Google Scholar] [CrossRef]
- Zong, S.; Li, C.; Luo, C.; Zhao, X.; Liu, C.; Wang, K.; Jia, W.; Bai, M.; Yin, M.; Bao, S.; et al. Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration. Sci. Rep. 2016, 6, 19916. [Google Scholar] [CrossRef]
- Zhou, H.; Pan, Y.; Yang, W.; Zhao, C.; Sun, X.; Hong, B.; Jin, X.; Zhang, T.; Zhang, Y.; Liu, N.; et al. S100P promotes trophoblast syncytialization during early placenta development by regulating YAP1. Front. Endocrinol. 2022, 13, 860261. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Shen, H.H.; Zhou, W.J.; Mei, J.; Lu, H.; Tan, X.F.; Zhu, R.; Zhou, W.H.; Li, D.J.; Zhang, T.; et al. Insight of Autophagy in Spontaneous Miscarriage. Int. J. Biol. Sci. 2022, 18, 1150–1170. [Google Scholar] [CrossRef]
- Nakashima, A.; Tsuda, S.; Kusabiraki, T.; Aoki, A.; Ushijima, A.; Shima, T.; Cheng, S.B.; Sharma, S.; Saito, S. Current Understanding of Autophagy in Pregnancy. Int. J. Mol. Sci. 2019, 20, 2342. [Google Scholar] [CrossRef]
- Wei, C.; Pan, Y.; Zhang, Y.; Dai, Y.; Jiang, L.; Shi, L.; Yang, W.; Xu, S.; Zhang, Y.; Xu, W.; et al. Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells. Cell Death Dis. 2020, 11, 755. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Xu, G.; Guo, Y.; Sun, L.; Zhang, C.; Li, X.; Li, B. Arsenic induces mTOR-dependent autophagy, whereas it impairs the autophagy-lysosome pathway and the potential role of TFEB in cultured dendritic cells. Metallomics 2020, 12, 1230–1245. [Google Scholar] [CrossRef]
- Yeganeh, B.; Lee, J.; Ermini, L.; Lok, I.; Ackerley, C.; Post, M. Autophagy is required for lung development and morphogenesis. J. Clin. Investig. 2019, 129, 2904–2919. [Google Scholar] [CrossRef]
- Füllgrabe, J.; Klionsky, D.J.; Joseph, B. Histone post-translational modifications regulate autophagy flux and outcome. Autophagy 2013, 9, 1621–1623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.; Chen, X.; Qiu, Y.; Jin, M.; Gong, M.; Kong, D. Stellettin B Induces G1 Arrest, Apoptosis and Autophagy in Human Non-small Cell Lung Cancer A549 Cells via Blocking PI3K/Akt/mTOR Pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef] [PubMed]
- Kohan-Ghadr, H.R.; Kadam, L.; Jain, C.; Armant, D.R.; Drewlo, S. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adhes. Migr. 2016, 10, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, H.; Zhuang, S. Histone deacetylases as targets for treatment of multiple diseases. Clin. Sci. Lond. Engl. 2013, 124, 651–662. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Lehrmann, H.; Pritchard, L.L.; Harel-Bellan, A. Histone acetyltransferases and deacetylases in the control of cell proliferation and differentiation. Adv. Cancer Res. 2002, 86, 41–65. [Google Scholar]
- Zhang, B.; Kim, M.Y.; Elliot, G.; Zhou, Y.; Zhao, G.; Li, D.; Lowdon, R.F.; Gormley, M.; Kapidzic, M.; Robinson, J.F.; et al. Human placental cytotrophoblast epigenome dynamics over gestation and alterations in placental disease. Dev. Cell 2021, 56, 1238–1252.e5. [Google Scholar] [CrossRef]
- Xie, D.; Zhu, J.; Liu, Q.; Li, J.; Song, M.; Wang, K.; Zhou, Q.; Jia, Y.; Li, T. Dysregulation of HDAC9 Represses Trophoblast Cell Migration and Invasion Through TIMP3 Activation in Preeclampsia. Am. J. Hypertens. 2019, 32, 515–523. [Google Scholar] [CrossRef]
- Togher, K.L.; Kenny, L.C.; O’Keeffe, G.W. Class-Specific Histone Deacetylase Inhibitors Promote 11-Beta Hydroxysteroid Dehydrogenase Type 2 Expression in JEG-3 Cells. Int. J. Cell Biol. 2017, 2017, 6169310. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; She, R.; Wang, Q.; Li, Y.; Zhang, H. Up-regulation of miR-299 suppressed the invasion and migration of HTR-8/SVneo trophoblast cells partly via targeting HDAC2 in pre-eclampsia. Biomed. Pharmacother. 2018, 97, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yu, H.; Jia, B.; Yu, X.; Cui, Q.; Liu, Z.; Sun, C.; Chu, Y. Association of downregulated HDAC 2 with the impaired mitochondrial function and cytokine secretion in the monocytes/macrophages from gestational diabetes mellitus patients. Cell Biol. Int. 2016, 40, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Mrakovcic, M.; Fröhlich, L.F. Molecular Determinants of Cancer Therapy Resistance to HDAC Inhibitor-Induced Autophagy. Cancers 2019, 12, 109. [Google Scholar] [CrossRef]
- Gilardini Montani, M.S.; Granato, M.; Santoni, C.; Del Porto, P.; Merendino, N.; D’Orazi, G.; Faggioni, A.; Cirone, M. Histone deacetylase inhibitors VPA and TSA induce apoptosis and autophagy in pancreatic cancer cells. Cell. Oncol. 2017, 40, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Mishra, S.R.; Behera, B.P.; Jena, M.; Bhutia, S.K. Dysregulation of histone deacetylases in carcinogenesis and tumor progression: A possible link to apoptosis and autophagy. Cell. Mol. Life Sci. CMLS 2019, 76, 3263–3282. [Google Scholar] [CrossRef]
- Wagner, G.P.; Kshitiz Dighe, A.; Levchenko, A. The Coevolution of Placentation and Cancer. Annu. Rev. Anim. Biosci. 2022, 10, 259–279. [Google Scholar] [CrossRef]
- Costanzo, V.; Bardelli, A.; Siena, S.; Abrignani, S. Exploring the links between cancer and placenta development. Open Biol. 2018, 8, 180081. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Liu, J.; Zhang, J.; Xu, M.; You, Z.; Peng, C.; Gong, Z.; Liu, W. Acetyltransferase GCN5 regulates autophagy and lysosome biogenesis by targeting TFEB. EMBO Rep. 2020, 21, e48335. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Kumsta, C.; Sandri, M.; Ballabio, A.; Hansen, M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 2015, 11, 867–880. [Google Scholar] [CrossRef]
- Li, T.; Yin, L.; Kang, X.; Xue, W.; Wang, N.; Zhang, J.; Yuan, P.; Lin, L.; Li, Y. TFEB acetylation promotes lysosome biogenesis and ameliorates Alzheimer’s disease-relevant phenotypes in mice. J. Biol. Chem. 2022, 298, 102649. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Jian, Y.; Xu, M.; Huang, X.; Wang, N.; Liu, Z.; Li, Q.; Li, J.; Zhou, H.; Xu, L.; et al. CDK4/6 regulate lysosome biogenesis through TFEB/TFE3. J. Cell Biol. 2020, 219, e201911036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhou, Z.; Park, J.E.; Wang, L.; Wu, S.; Sun, X.; Lu, L.; Wang, T.; Lin, Q.; et al. Importance of TFEB acetylation in control of its transcriptional activity and lysosomal function in response to histone deacetylase inhibitors. Autophagy 2018, 14, 1043–1059. [Google Scholar] [CrossRef]
- Medina, D.L.; Ballabio, A. Lysosomal calcium regulates autophagy. Autophagy 2015, 11, 970–971. [Google Scholar] [CrossRef]
- Nnah, I.C.; Wang, B.; Saqcena, C.; Weber, G.F.; Bonder, E.M.; Bagley, D.; De Cegli, R.; Napolitano, G.; Medina, D.L.; Ballabio, A.; et al. TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy 2019, 15, 151–164. [Google Scholar] [CrossRef]
- Fang, S.; Wan, X.; Zou, X.; Sun, S.; Hao, X.; Liang, C.; Zhang, Z.; Zhang, F.; Sun, B.; Li, H.; et al. Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death Dis. 2021, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Wang, S.; Zhao, K.; Li, Y.; Williams, J.A.; Li, T.; Chavan, H.; Krishnamurthy, P.; He, X.C.; Li, L.; et al. Impaired TFEB-Mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-Induced Liver Injury and Steatosis in Mice. Gastroenterology 2018, 155, 865–879.e12. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Lv, S.; Wang, N.; Lv, H.; Yang, J.; Liu, J.; Li, W.P.; Zhang, C.; Chen, Z.J. The Attenuation of Trophoblast Invasion Caused by the Downregulation of EZH2 Is Involved in the Pathogenesis of Human Recurrent Miscarriage. Mol. Ther. Nucleic Acids 2019, 14, 377–387. [Google Scholar] [CrossRef]
- Andrawus, M.; Sharvit, L.; Atzmon, G. Epigenetics and Pregnancy: Conditional Snapshot or Rolling Event. Int. J. Mol. Sci. 2022, 23, 12698. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Park, C.H.; Kim, H.S.; Jeong, Y.W.; Lee, J.Y.; Park, S.W.; Lee, S.Y.; Hyun, S.H.; Kim, Y.W.; Shin, T.; et al. Effects of Trichostatin A on In Vitro Development of Porcine Embryos Derived from Somatic Cell Nuclear Transfer. Asian-Australas. J. Anim. Sci. 2013, 26, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Cai, S.; Wang, S.; Zeng, X.; Ye, C.; Chen, M.; Zeng, X.; Qiao, S. Maternal short and medium chain fatty acids supply during early pregnancy improves embryo survival through enhancing progesterone synthesis in rats. J. Nutr. Biochem. 2019, 69, 98–107. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis via Suboptimal Mitochondrial Function: Assessment, Treatment and Classification Implications. Curr. Top. Med. Chem. 2020, 20, 524–539. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Bienvenue-Pariseault, J.; Vaillancourt, C. Melatonin: The smart molecule that differentially modulates autophagy in tumor and normal placental cells. PLoS ONE 2019, 14, e0202458. [Google Scholar] [CrossRef] [PubMed]
- Paauw, N.D.; Lely, A.T.; Joles, J.A.; Franx, A.; Nikkels, P.G.; Mokry, M.; van Rijn, B.B. H3K27 acetylation and gene expression analysis reveals differences in placental chromatin activity in fetal growth restriction. Clin. Epigenetics 2018, 10, 85. [Google Scholar] [CrossRef]
- Hepp, P.; Hutter, S.; Knabl, J.; Hofmann, S.; Kuhn, C.; Mahner, S.; Jeschke, U. Histone H3 lysine 9 acetylation is downregulated in GDM Placentas and Calcitriol supplementation enhanced this effect. Int. J. Mol. Sci. 2018, 19, 4061. [Google Scholar] [CrossRef]
- Carvajal, L.; Gutiérrez, J.; Morselli, E.; Leiva, A. Autophagy Process in Trophoblast Cells Invasion and Differentiation: Similitude and Differences with Cancer Cells. Front. Oncol. 2021, 11, 637594. [Google Scholar] [CrossRef]
- Oh, S.Y.; Choi, S.J.; Kim, K.H.; Cho, E.Y.; Kim, J.H.; Roh, C.R. Autophagy-related proteins, LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod. Sci. 2008, 15, 912–920. [Google Scholar] [CrossRef]
- Melland-Smith, M.; Ermini, L.; Chauvin, S.; Craig-Barnes, H.; Tagliaferro, A.; Todros, T.; Post, M.; Caniggia, I. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015, 11, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Cheng, S.B.; Ikawa, M.; Yoshimori, T.; Huber, W.J.; Menon, R.; Huang, Z.; Fierce, J.; Padbury, J.F.; Sadovsky, Y.; et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy 2020, 16, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Spampanato, C.; Feeney, E.; Li, L.; Cardone, M.; Lim, J.A.; Annunziata, F.; Zare, H.; Polishchuk, R.; Puertollano, R.; Parenti, G.; et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol. Med. 2013, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Normal (n = 20) | RSA (n = 15) | p Value (Normal vs. RSA) |
|---|---|---|---|
| Sample size (years) | 20 | 15 | - |
| Maternal age (years) | 29.85 ± 5.17 | 31.33 ± 4.7 | 0.2421 (ns) |
| Gestational age (days) | 50.15 ± 6.15 | 47.2 ± 4.44 | 0.1358 (ns) |
| TS Line | Maternal Age (Years) | Gestational Age at D&C | Karyotype |
|---|---|---|---|
| TSCT #1 | 31 | 8 weeks | 46, XY |
| TSCT #2 | 24 | 6 weeks | 46, XX |
| TSCT #3 | 32 | 7 weeks | 46, XX |
| Forward Primer | Reverse Primer | |
|---|---|---|
| HDAC1 | CTACTACGACGGGGATGTTGG | GAGTCATGCGGATTCGGTGAG |
| HDAC2 | ATGGCGTACAGTCAAGGAGG | TGCGGATTCTATGAGGCTTC |
| HDAC3 | CCTGGCATTGACCCATAGCC | CTCTTGGTGAAGCCTTGCATA |
| HDAC4 | GGCCCACCGGAATCTGAAC | GAACTCTGGTCAAGGGAACTG |
| HDAC5 | TCTTGTCGAAGTCAAAGGAGC | GAGGGGAACTCTGGTCCAAAG |
| HDAC6 | AAGAAGACCTAATCGTGGGACT | GCTGTGAACCAACATCAGCTC |
| HDAC7 | GGCGGCCCTAGAAAGAACAG | CTTGGGCTTATAGCGCAGCTT |
| HDAC8 | TCGCTGGTCCCGGTTTATATC | TACTGGCCCGTTTGGGGAT |
| HDAC9 | AGTAGAGAGGCATCGCAGAGA | GGAGTGTCTTTCGTTGCTGAT |
| HDAC10 | CAGTTCGACGCCATCTACTTC | CAAGCCCATTTTGCACAGCTC |
| LAMP1 | TCTCAGTGAACTACGACACCA | AGTGTATGTCCTCTTCCAAAAGC |
| CTSD | TGCTCAAGAACTACATGGACGC | CGAAGACGACTGTGAAGCACT |
| MAP1LC3B | GATGTCCGACTTATTCGAGAGC | TTGAGCTGTAAGCGCCTTCTA |
| BECLIN1 | CCATGCAGGTGAGCTTCGT | GAATCTGCGAGAGACACCATC |
| ATG5 | AAAGATGTGCTTCGAGATGTGT | CACTTTGTCAGTTACCAACGTCA |
| ATG7 | CAGTTTGCCCCTTTTAGTAGTGC | CCAGCCGATACTCGTTCAGC |
| ATG12 | CTGCTGGCGACACCAAGAAA | CGTGTTCGCTCTACTGCCC |
| MCOLN1 | TTCGCCGTCGTCTCAAATACT | CTCTTCCCGGAATGTCACAGC |
| SGSH | ACGGAGGCTTTGAGAGTGG | GCATTGCGAAAGAGGAGGCT |
| P21 | GATTCTGCCATACAAGGCTACAA | TGCCCTGGGTATAACTCCCAA |
| cyclinB1 | AATAAGGCGAAGATCAACATGGC | TTTGTTACCAATGTCCCCAAGAG |
| CDK1 | AAACTACAGGTCAAGTGGTAGCC | TCCTGCATAAGCACATCCTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zhao, C.; Zhou, H.; Huang, X.; Ying, H.; Zhang, S.; Pan, Y.; Zhu, H. Dysregulation of Histone Deacetylases Inhibits Trophoblast Growth during Early Placental Development Partially through TFEB-Dependent Autophagy-Lysosomal Pathway. Int. J. Mol. Sci. 2023, 24, 11899. https://doi.org/10.3390/ijms241511899
Wang P, Zhao C, Zhou H, Huang X, Ying H, Zhang S, Pan Y, Zhu H. Dysregulation of Histone Deacetylases Inhibits Trophoblast Growth during Early Placental Development Partially through TFEB-Dependent Autophagy-Lysosomal Pathway. International Journal of Molecular Sciences. 2023; 24(15):11899. https://doi.org/10.3390/ijms241511899
Chicago/Turabian StyleWang, Peixin, Chenqiong Zhao, Hanjing Zhou, Xiaona Huang, Hanqi Ying, Songying Zhang, Yibin Pan, and Haiyan Zhu. 2023. "Dysregulation of Histone Deacetylases Inhibits Trophoblast Growth during Early Placental Development Partially through TFEB-Dependent Autophagy-Lysosomal Pathway" International Journal of Molecular Sciences 24, no. 15: 11899. https://doi.org/10.3390/ijms241511899
APA StyleWang, P., Zhao, C., Zhou, H., Huang, X., Ying, H., Zhang, S., Pan, Y., & Zhu, H. (2023). Dysregulation of Histone Deacetylases Inhibits Trophoblast Growth during Early Placental Development Partially through TFEB-Dependent Autophagy-Lysosomal Pathway. International Journal of Molecular Sciences, 24(15), 11899. https://doi.org/10.3390/ijms241511899







