1. Introduction
From the standpoint of conventional chemistry and biochemistry, by dilution, any activity ascribed to substances in the original solution should diminish proportionally to the level of dilution. However, a growing body of published research is coming to quite different conclusions, considering aqueous solutions subjected to high dilution and special physical treatment. Sometimes, they go even beyond Avogadro’s limit and further (i.e., ultra-high dilutions 10
−12 and more), where even theoretically, not a single molecule of the original substance(s) in aqueous solution should be left. These ultra-high dilutions will be denoted as UHD solutions. As to the physical treatment, most frequently, it represents mechanical vibrations, rotations, or electromagnetic field exposure during the dilution process or afterward [
1]. The subject of the present research report concerns such processed aqueous solutions (liquids), which may be called processed liquids (solutions) that allegedly possess molecular imprints of the biologically active compounds which were initially present in physiologically effective concentrations. These imprints will be named UHD signals or molecular information and their further processing UHD signal transfer.
The research methods that demonstrate the UHD signal via differences between processed solutions to equally treated water with no diluted substances (i.e., control) are mostly physicochemical, physical [
2,
3,
4,
5], and even biological [
6,
7,
8,
9]. In numerous instances, processed solutions may not contain even a single molecule of the original substance(s) in the water. As a result, the effects observed cannot be attributed to chemistry but rather to the ultra-high dilution (UHD) signal that remains stored in the processed water, whether in liquid or solution form. While the precise nature of this signal remains elusive, it is generally assumed to reside within specifically ordered water molecular clusters or coherent domains forming the so-called mesoscopic water phase [
10,
11,
12,
13,
14]. The most persuasive theoretical model is based on quantum electrodynamics (QED; [
15,
16,
17]), although alternative explanations, such as Meesen’s nano-pearls [
18] or clathrates [
19], are also worth considering. Despite the inability of QED to provide exact mathematical predictions for experimental results in processing the ultra-high dilution (UHD) signal, it offers models that can serve as a comprehensive theoretical foundation for understanding molecular imprinting (i.e., the creation of the UHD signal) and the storage of this signal in a fluid medium through the aforementioned coherent domains of various types. The QED theory sees that as a process analogous to that in materials consisting of domains with magnetic moments [
20,
21].
In the scientific literature, various physicochemical and physical methods have been used to study UHD signal transmission with varying degrees of success. The present study used well-established methods, such as electrical conductivity (χ), pH, oxidation-reduction potential (ORP), and UV/VIS spectrometry. According to the established research practice, χ speaks about the concentration of dissolved ions in water. However, in studies of ultra-highly diluted solutions, a higher χ frequently indicates the presence of the above physical signal, i.e., molecular imprints in water [
21,
22,
23,
24]. It is frequently used in studying interfacial water bordering hydrophilic surfaces, where the hydronium ion (H
3O
+) may play the leading role [
13,
18] or dynamically ordered mesoscopic water states, also known as thixotropic phenomena [
25,
26,
27] or structures such as nano-associates [
28], clusters [
29,
30,
31,
32], domains [
33,
34,
35], nanobubbles [
36,
37], nanoparticles [
38], or naneons [
39].
pH is frequently used in unconventional water research. Ryzhkina et al. found that with successive dilution and shaking, the pH in UHD solutions of 4-Aminopyridine decreases monotonically [
24]; in other studies, they have found the pH to show non-linear response in UHD solutions compared to control [
40], which was also confirmed in the systematic research report by Yablonskaya et al. [
41]. These studies have indicated that, via pH measurements, we can detect more complex aspects of the UHD signals while they are processed through serial dilutions. In line with our previous research, we expected pH values to come closer to 7 when exposed to the UHD signal.
Although ORP measurements are infrequently used in non-conventional water research, it has been demonstrated that ultra-high dilutions combined with shaking result in raising the electrical voltage [
42]. The separation of charges (rise of electrical tension) was also observed in specially structured water, also known as EZ water, adjacent to hydrophilic surfaces [
43,
44]. We, therefore, expected the UHD signal to increase the ORP at least slightly relative to the untreated (control) water.
As a review by Yinnon shows, UV/VIS spectrometry of serially diluted and vigorously shaken solutions is likewise a good marker of their ordered states [
45]. Here, three different wavelength bands maxima are given: 205–210 nm, 260–280 nm, and 300–310 nm. The first quoted band is reported for the serially diluted and vigorously shaken solutions [
24,
40]. The second listed band is explored in EZ water research, where a broad peak absorption is regularly seen at 270 nm [
46,
47]. The third quoted absorption band (around 300 nm) is mostly studied in specially treated and presumably highly ordered MiliQ water [
48]. In theoretical considerations, coherent domains, as postulated in QED, should be a major agent for a universally detected UV absorption of dynamically ordered UHD water solutions [
45]. The relative absorption is expected to rise.
As already indicated, the UHD signal transfer research does not stop with dynamically ordered water structures. Surprisingly, studies by several teams have shown that the UHD signal not only changes the water in which an original substance has been diluted but can also be transferred to other liquids without direct contact between the donor and recipient solutions [
49,
50,
51]. The nature of the UHD signal, therefore, appears to be complex and, at least at one of its levels, should go beyond dynamically ordered water structures; otherwise, it could not be transferred via glass, air, or magnetic field. Accordingly, we can assume that the signal in ordered water domains is a particular energy or field structure, which only in liquids uses mesoscopic structures (domains) for its residence. After certain physical conditions (such as shaking) are met, it can escape from its temporary abode and be taken up and stored by another, physically separated, liquid. Regarding the nature of the physical background of the UHD signal, most frequently, it is assumed to be electromagnetic in nature [
51,
52,
53]. However, while it may exist in the form of coherent EM oscillations in mesoscopic water domains, out of the water, it may represent a quantum phenomenon or involvement of an as yet unknown quantum field or the field of quasi-particles; for instance, Kernbach proposes spin transfer [
54]. In this case, roughly speaking, we should have a UHD signal in the donor solution resulting from dilution and shaking its transfer via a non-liquid medium (air, glass, EM field) and its reception in a fluid medium (receiver solution).
The above references clearly indicate that the UHD signal has been verified on multiple occasions. However, its specific physical nature has remained elusive for unequivocal confirmation. To better comprehend its nature and move closer to defining its physical properties, our team has conducted an extensive pilot study with three primary objectives:
- (a)
To detect the underlying UHD signal in original solutions;
- (b)
To identify the same signal in a further 100-fold dilution, along with simultaneous activation (C1 processing, see
Section 4.1.2):
- (c)
To detect the same UHD signal while attempting to transfer it from the donor solution to the recipient solution without an intermediate aqueous phase (physical transfer).
The overarching goal was:
- (1)
To determine the key relations among the same kind of UHD signals in their three forms (original, C1 dilution, and physical transfer);
- (2)
To find relations among the three chosen signals themselves, as they represent three distinct categories: a high and pure dilution of a biologically active compound, a mixed dilution of the latter, and just potentiated water.
For the biologically active compound that was subject to ultra-high dilution processes, we chose antibodies to IFNγ (marked as a-IFNγ) since the behavior of this specific active biological substance (and also a-IFNγ itself) has already been subject to extensive research with a noticeable outcome [
1,
55,
56]. In the overall study, we used the a-IFNγ diluted in water with no further protecting measures, like dilution in alcohol or application to sugar pellets. Given some of the claims made by researchers [
19] and our previous experience [
57,
58] that pure water is changeable and sensitive to new impressions coming from the surroundings, we did not expect the UHD signals to be very intense and clear, especially taking into account that the solutions have had to be transported across state borders. We used two different dilutions of a-IFNγ, namely a pure one (9 × 10
−2 diluted), a mixed one with three different simultaneous dilutions (see
Section 4.1.1 for more detail), and distilled water diluted in itself (the UHD signal of water; 9 × 10
−2 “dilution”).
According to the goals and following our experiences and other research in the already cited literature, our assumptions were as follows:
- (1)
We would successfully detect the UHD signal at all three processing stages by at least one measurement method;
- (2)
The water (W) signal would probably be weaker than the pure antigen signal, which in turn would be weaker than the dilution mixture. This is due to the more complex processing of the dilution mixture, which allows for possible signal superimposition;
- (3)
The physically transmitted signal would likely be weaker than either the original or C1-processed signal; however, a longer exposure time of the receiving liquid would result in its better detection;
- (4)
The C1-processed signal might vary from the original signal in terms of intensity, reliability, and sign; however, in principle, it would follow the nature of the UHD signal from the original solutions.
4. Materials and Methods
To minimize potential experimental errors, we consistently implemented the same procedure for all samples, both control and treated, within the same experimental setup.
In the scientific water research area of not yet generally accepted and theoretically well-established phenomena, research using various detecting and measuring methods abound; however, there is not yet generally accepted methodology. Moreover, there is no specific sensor for dynamically ordered states of mesoscopic water. In this case, we need to use a statistically well-founded measurement system. To ensure maximal reliability of measurements, the BION Institute water research team devoted a special effort to developing and calibrating physicochemical and UV/VIS spectroscopical measuring protocols. They were developed and tested in order to be simultaneously robust and sensitive enough to detect subtle investigated phenomena. The system developed so far and used in the present study includes physicochemical methods (pH, ORP, and conductivity) and UV-VIS spectrometry.
4.1. Liquids
4.1.1. Original (Received) Solutions and Waters
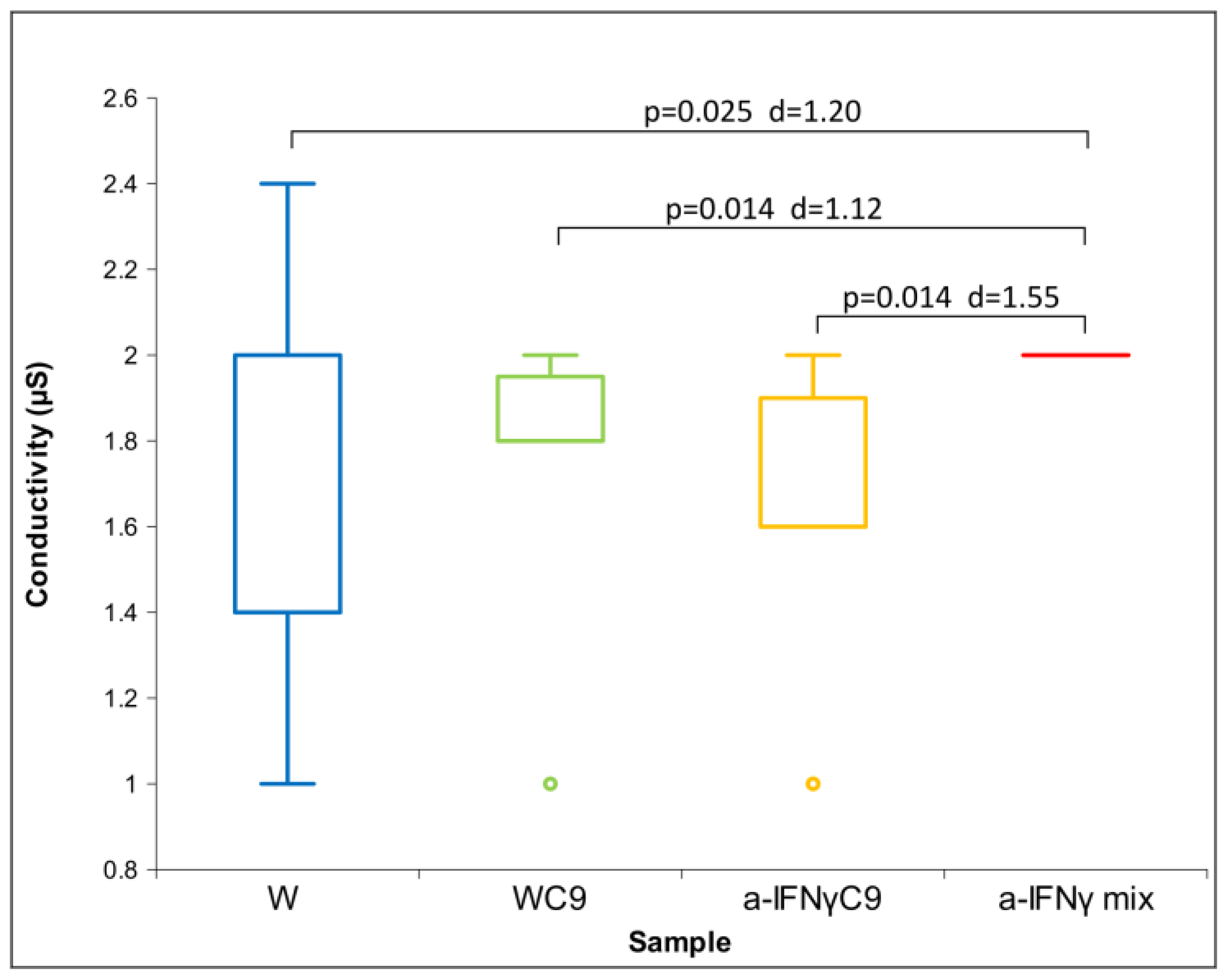
All original substances (water and solutions, four different liquids altogether) subject to research were obtained from OOO “NPF ”Materia Medica Holding”, Moscow, Russia. They were the unprocessed (not diluted or shaken) distilled water that played the role of control in all experiments. It was the basis for other dilutions and is marked as W. Another liquid was the same water processed as if diluted in itself by nine centesimal sequential dilutions (9 × 10−2). It was consecutively shaken between each “dilution” and is marked as WC9. The third sent liquid, prepared by Materia Medica, was a processed dilution of antibodies to interferon-gamma (a-IFNγ). It was processed in the same nine-fold centesimal manner as the second liquid (9 × 10−2) and is marked as a-IFNγC9. The fourth liquid is a multiple-processed dilution of a-IFNγ. The liquid represents the following mixture of successive centesimal dilutions (C) with intermediate shaking: C12, C30, and C50 (a-IFNγ C12, C30, C50) and is marked as the a-IFNγ mix.
The samples were supplied in polypropylene flasks, but before the experiment, they were poured from polypropylene into glass flasks (Duran® GL 45, UAB Santonika, Kaunas, Lithuania), protected from intense direct light, and stored at room temperature with closed lids (20–23 °C).
4.1.2. Further Centesimal (C1) Processing of Solution
In order to study the nature of the UHD signal after further dilution and activation, the original solutions were diluted 100 times (10−2) in one step, followed by 100 blows on the glass of a Duran® bottle with a wooden mallet (C1 treatment already defined). The estimated force exerted during the manual strokes is approximately 0.04 N, with a frequency of 2 Hz. In this case, the original solutions were not diluted in distilled water but in a special solution with the following composition, marked as RS1: 0.413 mL of 3% hydrogen peroxide (Lekarna Ljubljana Pharmacy, Ljubljana, Slovenia) per liter (12.39 mg/L of pure H2O2; i.e., 0.36 mM H2O2) and 0.2 g/L sodium hydrogen carbonate (Solvay Chimica Italia S.p.A, Rosignano, Italy; 0.01 M NaHCO3) diluted on distilled water provided by the Bion distiller (χ = 2 µS). Therefore, the original distilled water (W) became WC1, WC9 became WC10, a-IFNγC9 became a-IFNγC10, and the a-IFNγ mix became a-IFNγ mixC1.
4.1.3. Receiver Solutions
In the study of further diluting of the received substances as defined in
Section 4.1.1, we used various receiver solutions for investigating the contact transfer of molecular information from the donor (original) substances. Following our previous experiences and additional examination of optimizing possible liquids for the present investigation, as well as taking into consideration the profound study by Voeikov [
71], we used the already mentioned and defined RS1 containing traces of hydrogen peroxide and hydrogen carbonate.
4.2. Physical Transfer of UHD Signal via Glass
4.2.1. General Methodology and Distance
Principally, we performed a physical transfer of the UHD signal of the original substances (donor) via glass or air by applying strokes to the bottle with the donor liquid in the direction of the bottle with the receiver solution. This transfer method is called “with activation”; it means that the glass bottle with the donor substance is shaken 15 times with a mallet shaker while the vibrations are transmitted from the first bottle to the second bottle. The transfer with activation was performed via glass, where the donor bottle was touching the receiver bottle. While doing the air transfer through shaking, the donor bottle had no contact with the surface on which the bottle of the receiver was placed. We mark the transferred signals from the original substances as W-sig. (from W), WC9-sig. (from WC9), a-IFNγC9-sig. (from a-IFNγC9) and a-IFNγ mix-sig. (from the a-IFNγ mix).
4.2.2. Exposure and Separation Times
We also studied different times of exposure of the receiver solution to the UHD signal. In most cases, the measurements were taken immediately after activation. In addition, we also studied the reception and expression of the UHD signal after different exposure times and a one-hour separation of the donor and receiver bottles by moving the previously attached bottles away from each other. The purpose of this experiment was to repeat the measurement of the solutions’ parameters after one hour of separation of the bottles. These different time experiments will be presented in detail in another publication; here, we will disclose the following experiments:
- (a)
immediately after activation (mark: 00);
- (b)
one hour of exposure after activation (mark: 10);
- (c)
overnight exposure (mark: N0).
4.3. Measurement Methods
4.3.1. General
For measurement of the physicochemical parameters, we used The Vernier Go Direct® devices (Vernier Software & Technology, Beaverton, Oregon, United States): Temperature Probe, pH Sensor, Conductivity Probe, and ORP Sensor. We employed simultaneous temperature measurements as a control to ensure that the observed data differences did not result from temperature variations. Conductivity is known to have a strong correlation with temperature, and to a lesser extent, this also pertains to the other measurement methods used. Each individual measurement was conducted in a separate beaker. The measured range of accuracy (which deviates from the officially declared value and is not part of the observed, investigated, and considered measurement drift) for these three devices is as follows: 0.5 µS/cm for conductivity, 2 mV for ORP, and 0.02 for pH.
For UV/VIS absorption spectroscopy measurements, we used a Macherey–Nagel spectrophotometer, wavelength range 190–1100 nm, with a 50 mm quartz cuvette cell. The measured accuracy range here is 0.003. Here, too, each measurement was performed in a single glass beaker.
4.3.2. Measurement Protocol
For the physicochemical measurements of the original substances, the liquids obtained in the bottles were poured into beakers. All physicochemical parameters (conductivity, ORP, pH) and temperature were measured simultaneously by two sets of measuring devices. In order to prevent possible slight measurement errors due to the drift of measurements over time, we did not measure different solutions consecutively but alternately. Regarding the receiver water, the RS1 was found to be suitable for testing further processed solutions (C1 experiments, physicochemical method). Physicochemical methods such as conductivity and pH have also been used in various other studies to detect signals in ultra-high diluted solutions [
41,
72,
73].
For UV/VIS absorption measurements of the original substances, the liquid was poured into a 50 mm quartz cuvette cell, wherein measurements were performed alternately with different solutions. All the measurements were relative to the reference (W). Due to possible slight measurement drift, we performed the measurements according to the procedure explained for the physicochemical measurements above. According to some other authors, the strongest signal is detected at 260 nm in the original UV/VIS spectroscopy measurements [
24,
40,
43,
74]. Furthermore, we also obtained a statistical difference between the substances and the control at 260 nm.
For UV/VIS measurements of C1-processed solutions, initially, we used the previously explained dilution methodology (see
Section 4.1.2) and then poured the newly produced liquids into a 50 mm quartz cuvette cell that we used for comparison. Again, all measurements were relative to the reference W (centesimally (10
−2) diluted in RS1. We decided to compare the substances at 210 nm wavelength.
For analyses, we used the wavelength of 260 nm with the original substances and 210 nm with C1-processed samples due to a different liquid composition. Due to a larger variation in the UV absorption with the physical transfer of the UHD signal, we decided not to choose a particular wavelength but the average absorption values of the band spanning from 190–210 nm.
4.4. Experimental Procedures
The following
Table 4,
Table 5 and
Table 6 present the experimental procedures according to the number of individual measurements per substance and the method used: electrical conductivity (χ), pH, ORP, and UV/VIS. All tested situations in all experiments had the same treatment and exposure to (un)controlled environmental factors. Measurement drift and temperature were taken into account as well.
The cumulative number of replicate measurements made with the original substances and the three physicochemical methods is shown in
Table 4.
Table 5 below shows the number of replicate measurements performed for the C1-processed substances comprising all three physicochemical methods.
Table 6 shows the number of repeated UV/VIS measurements taken with both the original and C1 solutions. Due to the high sensitivity of the UV/VIS device, a lower external noise (from the measurements procedure) compared to physicochemical methods, and a better absorption rate in RS1, we decided to conduct fewer measurements for C1-processed liquids.
4.5. Experimental Plot
Measurements were performed with all four substances (W, WC9, a-IFNγC9, and a-IFNγ mix) using physicochemical methods and UV/VIS spectroscopy. In experiments applying further C1 processing, the liquids were subjected to the treatment described in
Section 4.1.2 (see
Table 7, second experimental group). We compared the results of all measured solutions with each other.
In the third experimental group (
Table 7), we performed various experiments on the physical transfer of the UHD signal. We compared the RS1 solution of the transferred control signal (W) with the RS1 solutions of other transferred substances at different distances: 0, 1, and 4 cm. However, in the present paper, we will report only about the experiments via glass (distance 0) that were conducted with physicochemical methods immediately after activation (marked as 00, all four substances), after one hour of exposure (marked as 10), and after overnight exposure (marked as N0).
4.6. Statistical Analysis of the Results
For estimating statistical significance, we applied appropriate tests regarding normality and the number of groups. In the case of the normal distribution, we used ANOVA; otherwise, we used the Friedman test. For post hoc analysis, we subjected the groups to a post hoc t-test (normal distribution) or/and Wilcoxon signed-rank test. All tests were performed via pairwise comparison.
Statistical data analysis was performed using XLSTAT statistical software (XLSTAT PREMIUM-Evaluation 2022.3.1) for Excel. For basic statistical parameters of groups, we calculated the average, standard deviation, standard error, and normality with the Shapiro–Wilcoxon test. We consider that the pairwise method of the Shapiro–Wilcoxon test is appropriate for two reasons: (a) the samples are not unrelated, as they have the same original distilled water, (b) there is often a slight drift in the measurements, which can lead to a systematic measurement error in the required precision range in detecting statistically significant differences. To estimate statistical significance in the data variation, we used Levene’s test or F-test for differences in variance and Cohen’s D for assessing standardized effect size.
Differences are considered statistically significant with p < 0.05 or if 0.1 < p < 0.05, while the absolute Cohen’s D value is higher than 0.5.
To make various comparisons, not only between the substances in their original or processed forms but also between the efficacy of the used physicochemical methods and the methods of UHD signal treatment, we resorted to the calculation of the average of absolute Cohen’s D values, where the latter were calculated vs. control (W). Thus, we could have made synthetic evaluations of the study results from different perspectives.
5. Conclusions
This extended pilot study confirmed the measurable presence of UHD signals stored in water. The phenomenon was not observed only in liquids where originally a biologically active substance was diluted but also in physically processed distilled water (WC9), although the detection here was less reliable.
C1 processing yielded comparable results to the original solutions, although two of them (of twelve altogether) went in the opposite direction. The best score of matching was achieved by the a-IFNγ mix signal.
After the physical transfer via glass, the signal’s detection was weaker than the one from the original samples. The results may have diverged from the original, although it is difficult to compare the two since the experimental setups (different times of exposure, the selection of original substances) do not quite fit together.
Regarding the methods, the most effective proved to be conductivity measurements, followed closely by UV/VIS.
Summarizing the measuring methods and the signal processing, the a-IFNγ mix showed the highest difference to the control (unprocessed water, W), the a-IFNγC9 was slightly lower; as expected, the processed water (WC9) presented the lowest overall difference to the control.