Decoding of miR-7-5p in Colony Forming Unit–Hill Colonies as a Biomarker of Subclinical Cardiovascular Disease—A MERIT Study †
Abstract
1. Introduction
2. Results
2.1. Subjects’ Clinical and Metabolic Characteristics
2.2. Comparisons of Inflammatory and Vascular Health Markers between T1DM and HC
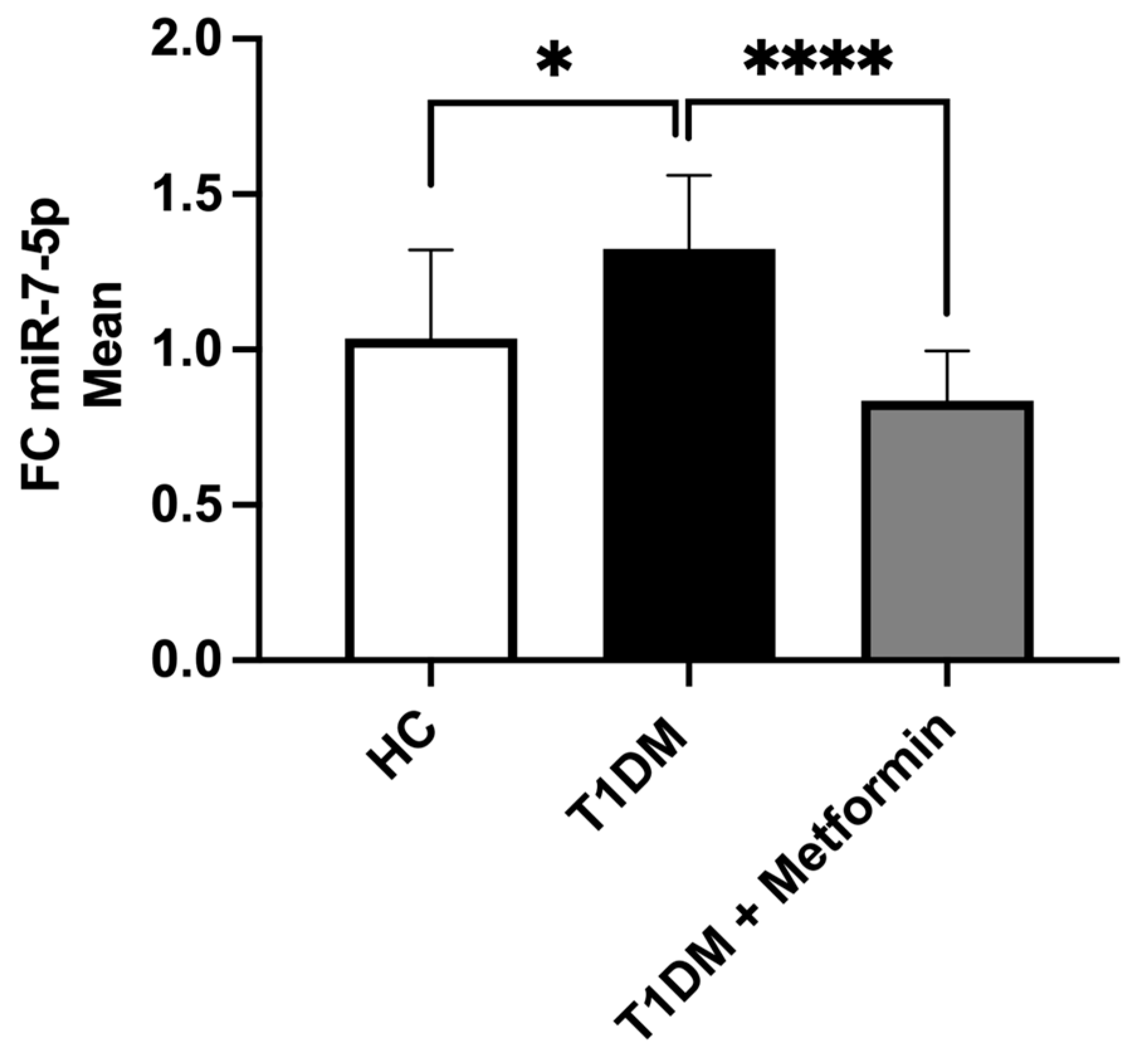
2.3. The Expression of miR-7-5p in T1DM and HC
2.4. Correlation of miR-7-5p with Inflammatory Markers
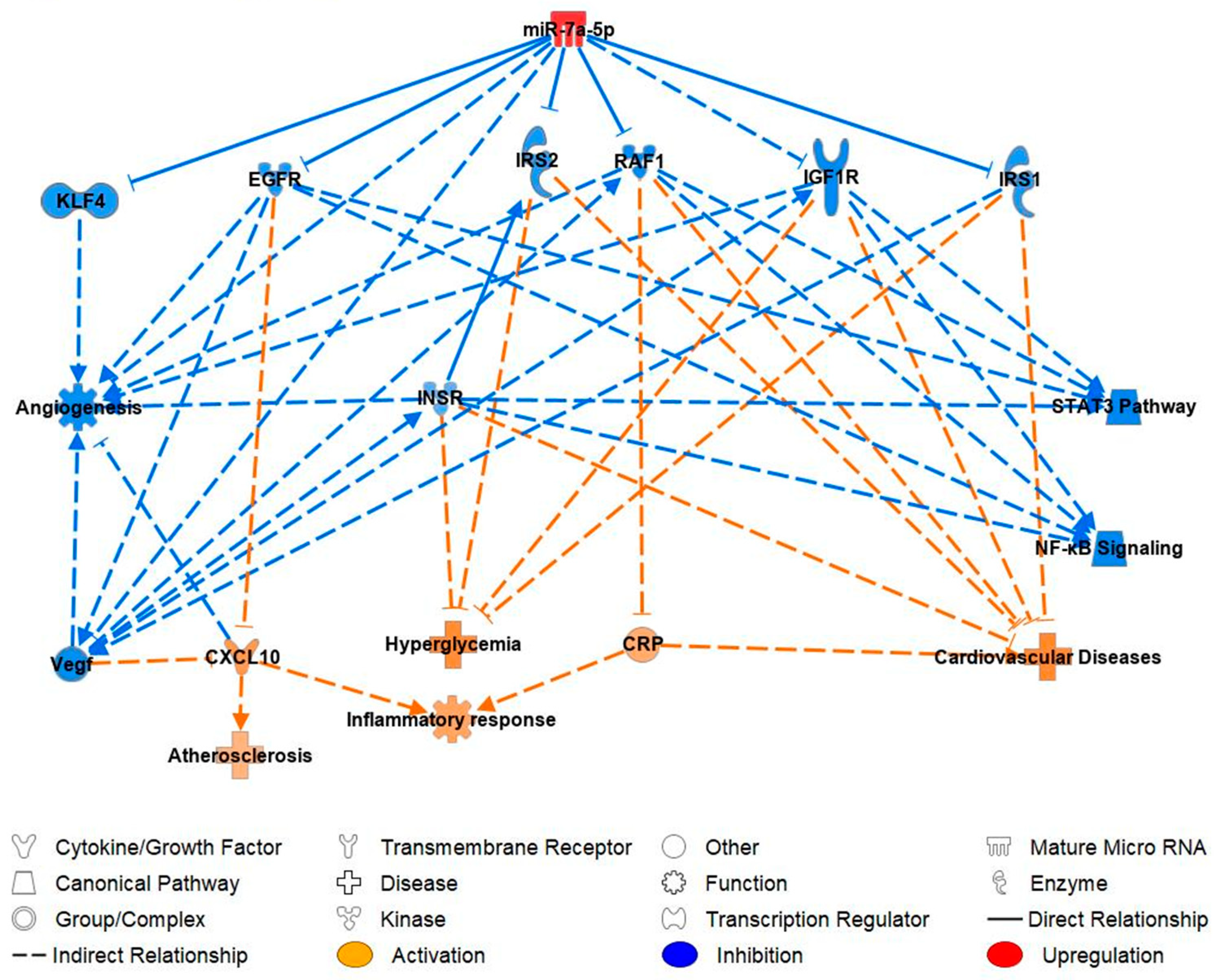
2.5. miR-7-5p Functional Pathway and Molecular Targets
3. Discussion
3.1. miR-7-5p Expression in T1DM Patients
3.2. Positive Association between miR-7-5p and Inflammatory Markers
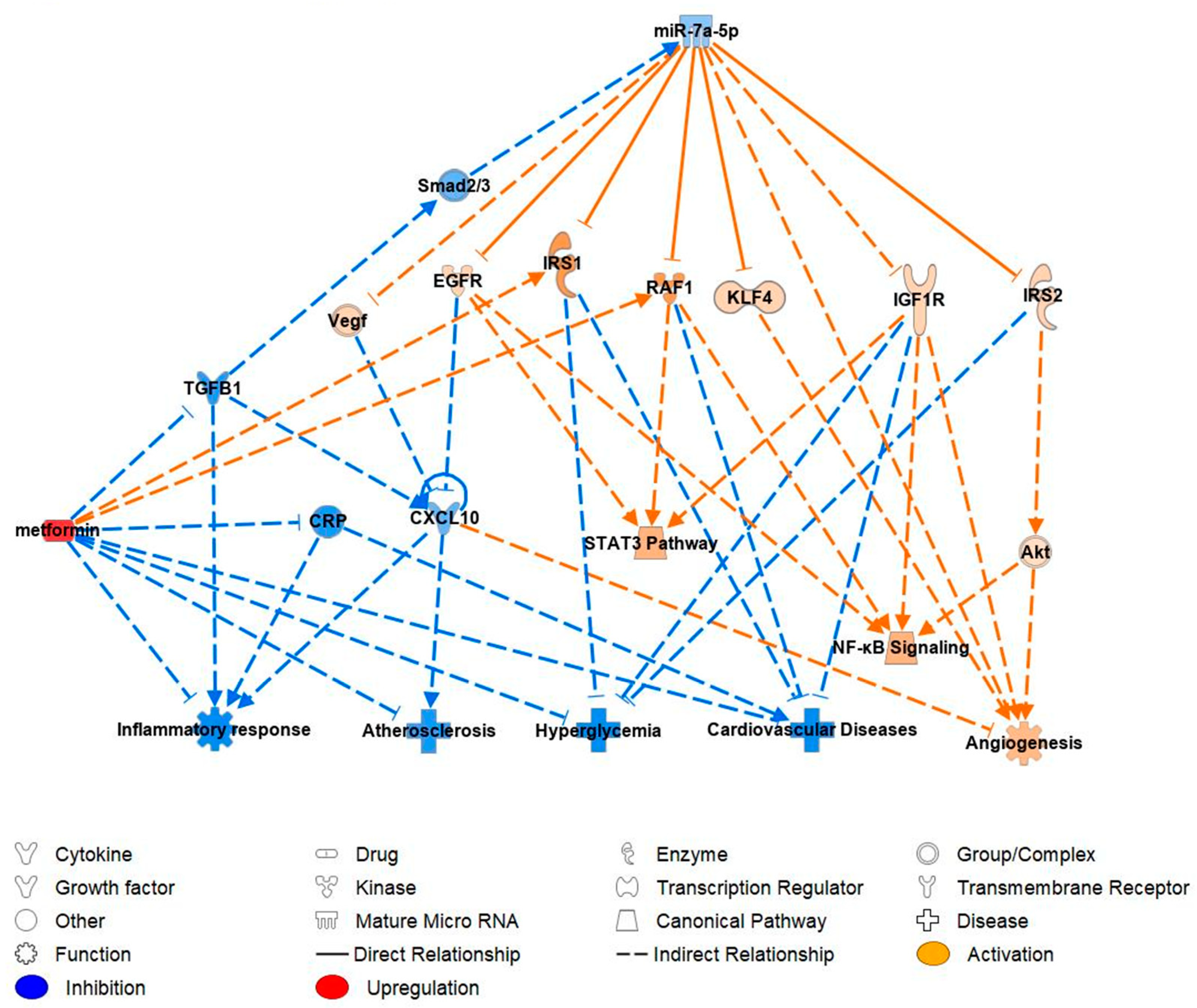
3.3. Downregulation of miR-7-5p by Metformin
3.4. Ingenuity Prediction Model: Pathway Analysis of miR-7-5p in Reference to Cardiovascular Function
3.5. Clinical Applications for Future CVD Research
3.5.1. Epidermal Growth Factor Receptor (EGFR)
3.5.2. Insulin Receptor Substrate 1 and 2 (IRS1/IRS2)
3.5.3. Raf-1 Proto-Oncogene (RAF1)
3.5.4. Insulin-like Growth Factor 1 Receptor (IGF-1R)
3.5.5. Kruppel-like Factors-4 (KLF4)
3.6. Contribution/Causation
4. Materials and Methods
4.1. Study
4.2. Cytokine Analysis
4.3. Evaluation of Circulating Endothelial Progenitor Cells by Flow Cytometry
4.4. Culture and Quantification of CFU–Hill Colonies
4.5. Plasma and Hill Colonies miRNA Extraction
4.6. Quantitative Real–Time Polymerase Chain Reaction for miRNA
4.7. Ingenuity Pathway Analysis (IPA) of miR-7-5p
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023; Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 8 July 2023).
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Kahanovitz, L.; Sluss, P.M.; Russell, S.J. Type 1 Diabetes—A Clinical Perspective. Point Care 2017, 16, 37–40. [Google Scholar] [CrossRef]
- Livingstone, S.J.; Looker, H.C.; Hothersall, E.J.; Wild, S.H.; Lindsay, R.S.; Chalmers, J.; Cleland, S.; Leese, G.P.; McKnight, J.; Morris, A.D.; et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012, 9, e1001321. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Sattar, N.; Franzen, S.; McGuire, D.K.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; Rosengren, A.; et al. Relative Prognostic Importance and Optimal Levels of Risk Factors for Mortality and Cardiovascular Outcomes in Type 1 Diabetes Mellitus. Circulation 2019, 139, 1900–1912. [Google Scholar] [CrossRef]
- Mazzone, T.; Chait, A.; Plutzky, J. Cardiovascular disease risk in type 2 diabetes mellitus: Insights from mechanistic studies. Lancet 2008, 371, 1800–1809. [Google Scholar] [CrossRef]
- Sibal, L.; Aldibbiat, A.; Agarwal, S.C.; Mitchell, G.; Oates, C.; Razvi, S.; Weaver, J.U.; Shaw, J.A.; Home, P.D. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia 2009, 52, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Law, H.N.; Gebbie, J.; Dashora, U.K.; Agarwal, S.C.; Home, P. Predicting the development of macrovascular disease in people with type 1 diabetes: A 9-year follow-up study. Ann. N. Y. Acad. Sci. 2006, 1084, 191–207. [Google Scholar] [CrossRef]
- Ahmed, F.W.; Rider, R.; Glanville, M.; Narayanan, K.; Razvi, S.; Weaver, J.U. Metformin improves circulating endothelial cells and endothelial progenitor cells in type 1 diabetes: MERIT study. Cardiovasc. Diabetol. 2016, 15, 116. [Google Scholar] [CrossRef]
- Asicioglu, E.; Gogas Yavuz, D.; Koc, M.; Ozben, B.; Yazici, D.; Deyneli, O.; Akalin, S. Circulating endothelial cells are elevated in patients with type 1 diabetes mellitus. Eur. J. Endocrinol. 2010, 162, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Snell-Bergeon, J.K.; West, N.A.; Mayer-Davis, E.J.; Liese, A.D.; Marcovina, S.M.; D’Agostino, R.B., Jr.; Hamman, R.F.; Dabelea, D. Inflammatory markers are increased in youth with type 1 diabetes: The SEARCH Case-Control study. J. Clin. Endocrinol. Metab. 2010, 95, 2868–2876. [Google Scholar] [CrossRef]
- West, D.J.; Campbell, M.D.; Gonzalez, J.T.; Walker, M.; Stevenson, E.J.; Ahmed, F.W.; Wijaya, S.; Shaw, J.A.; Weaver, J.U. The inflammation, vascular repair and injury responses to exercise in fit males with and without Type 1 diabetes: An observational study. Cardiovasc. Diabetol. 2015, 14, 71. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.H.; Kim, H.S.; Choi, J.H.; Kang, H.J.; Hwang, K.K.; Oh, B.H.; Lee, M.M.; Park, Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ingram, D.A.; Murphy, M.P.; Saadatzadeh, M.R.; Mead, L.E.; Prater, D.N.; Rehman, J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1675–H1682. [Google Scholar] [CrossRef]
- Yang, Z.; von Ballmoos, M.W.; Faessler, D.; Voelzmann, J.; Ortmann, J.; Diehm, N.; Kalka-Moll, W.; Baumgartner, I.; Di Santo, S.; Kalka, C. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis 2010, 211, 103–109. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Rossig, L.; Fichtlscherer, S.; Vasa, M.; Britten, M.; Kamper, U.; Dimmeler, S.; Zeiher, A.M. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005, 111, 2981–2987. [Google Scholar] [CrossRef]
- Werner, N.; Kosiol, S.; Schiegl, T.; Ahlers, P.; Walenta, K.; Link, A.; Bohm, M.; Nickenig, G. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005, 353, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Ghani, U.; Shuaib, A.; Salam, A.; Nasir, A.; Shuaib, U.; Jeerakathil, T.; Sher, F.; O’Rourke, F.; Nasser, A.M.; Schwindt, B.; et al. Endothelial progenitor cells during cerebrovascular disease. Stroke 2005, 36, 151–153. [Google Scholar] [CrossRef]
- Chu, K.; Jung, K.H.; Lee, S.T.; Park, H.K.; Sinn, D.I.; Kim, J.M.; Kim, D.H.; Kim, J.H.; Kim, S.J.; Song, E.C.; et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 2008, 39, 1441–1447. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Wang, B. MiR-7-5p Enhances Cerebral Ischemia-Reperfusion Injury by Degrading sirt1 mRNA. J. Cardiovasc. Pharmacol. 2020, 76, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Wen, L.; Wei, X.; Wang, Q.R.; Xu, L.W.; Zhang, H.M.; Liu, W.C. Inhibition of miR-7 promotes angiogenesis in human umbilical vein endothelial cells by upregulating VEGF via KLF4. Oncol. Rep. 2016, 36, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Sahu, A.; Sun, Y.; Mohan, M.L.; Kumar, A.; Zalavadia, A.; Wang, X.; Martelli, E.E.; Stenson, K.; Witherow, C.P.; et al. Cardiac expression of microRNA-7 is associated with adverse cardiac remodeling. Sci. Rep. 2021, 11, 22018. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Yin, M.; van der Horst, I.C.; van Melle, J.P.; Qian, C.; van Gilst, W.H.; Sillje, H.H.; de Boer, R.A. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H459–H468. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.W.C.; de Sousa, G.J.; Birocale, A.M.; Gouvea, S.A.; de Figueiredo, S.G.; de Abreu, G.R.; Bissoli, N.S. Chronic metformin reduces systemic and local inflammatory proteins and improves hypertension-related cardiac autonomic dysfunction. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 274–281. [Google Scholar] [CrossRef]
- Li, S.N.; Wang, X.; Zeng, Q.T.; Feng, Y.B.; Cheng, X.; Mao, X.B.; Wang, T.H.; Deng, H.P. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessel. 2009, 24, 446–453. [Google Scholar] [CrossRef]
- Liang, W.J.; Zhou, S.N.; Shan, M.R.; Wang, X.Q.; Zhang, M.; Chen, Y.; Zhang, Y.; Wang, S.X.; Guo, T. AMPKalpha inactivation destabilizes atherosclerotic plaque in streptozotocin-induced diabetic mice through AP-2alpha/miRNA-124 axis. J. Mol. Med. 2018, 96, 403–412. [Google Scholar] [CrossRef]
- Tamara, A.; Coulson, D.J.; Latief, J.S.; Bakhashab, S.; Weaver, J.U. Upregulated anti-angiogenic miR-424-5p in type 1 diabetes (model of subclinical cardiovascular disease) correlates with endothelial progenitor cells, CXCR1/2 and other parameters of vascular health. Stem Cell Res. Ther. 2021, 12, 249. [Google Scholar] [CrossRef]
- Phowira, J.; Ahmed, F.W.; Bakhashab, S.; Weaver, J.U. Upregulated miR-18a-5p in Colony Forming Unit-Hill’s in Subclinical Cardiovascular Disease and Metformin Therapy; MERIT Study. Biomedicines 2022, 10, 2136. [Google Scholar] [CrossRef]
- Paul, A.; Ko, K.W.; Li, L.; Yechoor, V.; McCrory, M.A.; Szalai, A.J.; Chan, L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004, 109, 647–655. [Google Scholar] [CrossRef]
- Venugopal, S.K.; Devaraj, S.; Yuhanna, I.; Shaul, P.; Jialal, I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 2002, 106, 1439–1441. [Google Scholar] [CrossRef]
- Verma, S.; Li, S.H.; Badiwala, M.V.; Weisel, R.D.; Fedak, P.W.; Li, R.K.; Dhillon, B.; Mickle, D.A. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 2002, 105, 1890–1896. [Google Scholar] [CrossRef]
- Cao, Y.L.; Liu, D.J.; Zhang, H.G. MiR-7 regulates the PI3K/AKT/VEGF pathway of retinal capillary endothelial cell and retinal pericytes in diabetic rat model through IRS-1 and inhibits cell proliferation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4427–4430. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, W.; Sun, Y.; Wang, Z.; Yuan, W.; Du, Z. Functional Network Analysis Reveals Versatile MicroRNAs in Human Heart. Cell Physiol. Biochem. 2015, 36, 1628–1643. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, C.M.; Nascimento, J.S.; Moreira, M.C.R.; Ludovico, N.D.; Santana, A.P.; Silva, R.A.A.; Silva-Jardim, I.; Santos, J.L.; Sousa, S.M.B.; Lima, P.S.P. MicroRNA profiling identifies miR-7-5p and miR-26b-5p as differentially expressed in hypertensive patients with left ventricular hypertrophy. Braz. J. Med. Biol. Res. 2017, 50, e6211. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Wang, J.; Wang, J.; Wu, J.; Song, J.; Zhang, C.Y.; Zhang, C.; Wang, C.; Wang, J.J. Increased serum miR-7 is a promising biomarker for type 2 diabetes mellitus and its microvascular complications. Diabetes Res. Clin. Pract. 2017, 130, 171–179. [Google Scholar] [CrossRef]
- Latreille, M.; Hausser, J.; Stutzer, I.; Zhang, Q.; Hastoy, B.; Gargani, S.; Kerr-Conte, J.; Pattou, F.; Zavolan, M.; Esguerra, J.L.; et al. MicroRNA-7a regulates pancreatic beta cell function. J. Clin. Investig. 2014, 124, 2722–2735. [Google Scholar] [CrossRef]
- Niki, T.; Soeki, T.; Yamaguchi, K.; Taketani, Y.; Yagi, S.; Iwase, T.; Yamada, H.; Wakatsuki, T.; Shimabukuro, M.; Sata, M. Elevated Concentration of Interferon-Inducible Protein of 10 kD (IP-10) Is Associated With Coronary Atherosclerosis. Int. Heart J. 2015, 56, 269–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heller, E.A.; Liu, E.; Tager, A.M.; Yuan, Q.; Lin, A.Y.; Ahluwalia, N.; Jones, K.; Koehn, S.L.; Lok, V.M.; Aikawa, E.; et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 2006, 113, 2301–2312. [Google Scholar] [CrossRef]
- Segers, D.; Lipton, J.A.; Leenen, P.J.; Cheng, C.; Tempel, D.; Pasterkamp, G.; Moll, F.L.; de Crom, R.; Krams, R. Atherosclerotic Plaque Stability Is Affected by the Chemokine CXCL10 in Both Mice and Humans. Int. J. Inflam. 2011, 2011, 936109. [Google Scholar] [CrossRef][Green Version]
- Tavakolian Ferdousie, V.; Mohammadi, M.; Hassanshahi, G.; Khorramdelazad, H.; Khanamani Falahati-Pour, S.; Mirzaei, M.; Allah Tavakoli, M.; Kamiab, Z.; Ahmadi, Z.; Vazirinejad, R.; et al. Serum CXCL10 and CXCL12 chemokine levels are associated with the severity of coronary artery disease and coronary artery occlusion. Int. J. Cardiol. 2017, 233, 23–28. [Google Scholar] [CrossRef]
- Ardigo, D.; Assimes, T.L.; Fortmann, S.P.; Go, A.S.; Hlatky, M.; Hytopoulos, E.; Iribarren, C.; Tsao, P.S.; Tabibiazar, R.; Quertermous, T.; et al. Circulating chemokines accurately identify individuals with clinically significant atherosclerotic heart disease. Physiol. Genom. 2007, 31, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Pupilli, C.; d’Annunzio, G.; Lorini, R.; Vanelli, M.; Ferrannini, E. Serum Th1 (CXCL10) and Th2 (CCL2) chemokine levels in children with newly diagnosed Type 1 diabetes: A longitudinal study. Diabet. Med. 2008, 25, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Conget, I.; Di Mauro, M.; Di Marco, R.; Mazzarino, M.C.; Bendtzen, K.; Messina, A.; Gomis, R. Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia 2002, 45, 1107–1110. [Google Scholar] [CrossRef]
- Mascia, F.; Mariani, V.; Girolomoni, G.; Pastore, S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003, 163, 303–312. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yao, Y.M. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, F.; Wu, J.; Wang, C.; Yan, J.; Li, Z.L.; Song, J.X.; Wang, J.J. Elevated Serum miR-7, miR-9, miR-122, and miR-141 Are Noninvasive Biomarkers of Acute Pancreatitis. Dis. Markers 2017, 2017, 7293459. [Google Scholar] [CrossRef]
- Emerging Risk Factors, C.; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Poland, D.C.; van Dijk, W.; Kok, A.; Emeis, J.J.; Drager, A.M.; Doni, A.; van Hinsbergh, V.W.; Stehouwer, C.D. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: Evidence for chronic inflammation. Diabetologia 1999, 42, 351–357. [Google Scholar] [CrossRef]
- Ray, S.L.; Coulson, D.J.; Yeoh, M.L.Y.; Tamara, A.; Latief, J.S.; Bakhashab, S.; Weaver, J.U. The Role of miR-342 in Vascular Health. Study in Subclinical Cardiovascular Disease in Mononuclear Cells, Plasma, Inflammatory Cytokines and PANX2. Int. J. Mol. Sci. 2020, 21, 7212. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Pena, E.; Arderiu, G.; Padro, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Frangogiannis, N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007, 74, 184–195. [Google Scholar] [CrossRef]
- Kuwahara, F.; Kai, H.; Tokuda, K.; Kai, M.; Takeshita, A.; Egashira, K.; Imaizumi, T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 2002, 106, 130–135. [Google Scholar] [CrossRef]
- Xiao, H.; Ma, X.; Feng, W.; Fu, Y.; Lu, Z.; Xu, M.; Shen, Q.; Zhu, Y.; Zhang, Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc. Res. 2010, 87, 504–513. [Google Scholar] [CrossRef]
- Han, F.; Shu, J.; Wang, S.; Tang, C.E.; Luo, F. Metformin Inhibits the Expression of Biomarkers of Fibrosis of EPCs In Vitro. Stem Cells Int. 2019, 2019, 9019648. [Google Scholar] [CrossRef]
- Makki, N.; Thiel, K.W.; Miller, F.J., Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int. J. Mol. Sci. 2013, 14, 20597–20613. [Google Scholar] [CrossRef]
- Nanney, L.B.; Stoscheck, C.M.; King, L.E. Characterization of binding and receptors for epidermal growth factor in smooth muscle. Cell Tissue Res. 1988, 254, 125–132. [Google Scholar] [CrossRef]
- Schreier, B.; Rabe, S.; Schneider, B.; Bretschneider, M.; Rupp, S.; Ruhs, S.; Neumann, J.; Rueckschloss, U.; Sibilia, M.; Gotthardt, M.; et al. Loss of epidermal growth factor receptor in vascular smooth muscle cells and cardiomyocytes causes arterial hypotension and cardiac hypertrophy. Hypertension 2013, 61, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Pareja, M.; Sanchez, O.; Lorita, J.; Soley, M.; Ramirez, I. Activated epidermal growth factor receptor (ErbB1) protects the heart against stress-induced injury in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R455–R462. [Google Scholar] [CrossRef] [PubMed]
- Barrick, C.J.; Yu, M.; Chao, H.H.; Threadgill, D.W. Chronic pharmacologic inhibition of EGFR leads to cardiac dysfunction in C57BL/6J mice. Toxicol. Appl. Pharmacol. 2008, 228, 315–325. [Google Scholar] [CrossRef]
- Webster, R.J.; Giles, K.M.; Price, K.J.; Zhang, P.M.; Mattick, J.S.; Leedman, P.J. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J. Biol. Chem. 2009, 284, 5731–5741. [Google Scholar] [CrossRef] [PubMed]
- Lavin, D.P.; White, M.F.; Brazil, D.P. IRS proteins and diabetic complications. Diabetologia 2016, 59, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, Z.; Zhu, Q.; Thomas, C.; Kumar, R.; Feng, H.; Dostal, D.E.; White, M.F.; Baker, K.M.; Guo, S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38alpha MAPK during insulin resistance. Diabetes 2013, 62, 3887–3900. [Google Scholar] [CrossRef]
- Galkina, E.V.; Butcher, M.; Keller, S.R.; Goff, M.; Bruce, A.; Pei, H.; Sarembock, I.J.; Sanders, J.M.; Nagelin, M.H.; Srinivasan, S.; et al. Accelerated atherosclerosis in Apoe−/− mice heterozygous for the insulin receptor and the insulin receptor substrate-1. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 247–256. [Google Scholar] [CrossRef]
- Liu, J.; Yi, X.; Tao, Y.; Wang, Y.; Xu, Z. Insulin-receptor substrate 1 protects against injury in endothelial cell models of ox-LDL-induced atherosclerosis by inhibiting ER stress/oxidative stress-mediated apoptosis and activating the Akt/FoxO1 signaling pathway. Int. J. Mol. Med. 2020, 46, 1671–1682. [Google Scholar] [CrossRef]
- Kubota, T.; Kubota, N.; Moroi, M.; Terauchi, Y.; Kobayashi, T.; Kamata, K.; Suzuki, R.; Tobe, K.; Namiki, A.; Aizawa, S.; et al. Lack of insulin receptor substrate-2 causes progressive neointima formation in response to vessel injury. Circulation 2003, 107, 3073–3080. [Google Scholar] [CrossRef]
- Muslin, A.J. Role of raf proteins in cardiac hypertrophy and cardiomyocyte survival. Trends Cardiovasc. Med. 2005, 15, 225–229. [Google Scholar] [CrossRef]
- Harris, I.S.; Zhang, S.; Treskov, I.; Kovacs, A.; Weinheimer, C.; Muslin, A.J. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation 2004, 110, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, O.; Watanabe, T.; Nishida, K.; Kashiwase, K.; Higuchi, Y.; Takeda, T.; Hikoso, S.; Hirotani, S.; Asahi, M.; Taniike, M.; et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J. Clin. Investig. 2004, 114, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Li, L.; Xu, Z.; Bi, B.; Wang, Y.; Li, J.Y. MiR-7-5p is frequently downregulated in glioblastoma microvasculature and inhibits vascular endothelial cell proliferation by targeting RAF1. Tumour Biol. 2014, 35, 10177–10184. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Maki, T.; Kishimoto, I.; Tokudome, T.; Okumura, H.; Yoshihara, F.; Suga, S.; Takeo, S.; Kawano, Y.; Kangawa, K. Production and autocrine/paracrine effects of endogenous insulin-like growth factor-1 in rat cardiac fibroblasts. Regul. Pept. 2005, 124, 65–72. [Google Scholar] [CrossRef]
- Tejada, T.; Tan, L.; Torres, R.A.; Calvert, J.W.; Lambert, J.P.; Zaidi, M.; Husain, M.; Berce, M.D.; Naib, H.; Pejler, G.; et al. IGF-1 degradation by mouse mast cell protease 4 promotes cell death and adverse cardiac remodeling days after a myocardial infarction. Proc. Natl. Acad. Sci. USA 2016, 113, 6949–6954. [Google Scholar] [CrossRef]
- Laustsen, P.G.; Russell, S.J.; Cui, L.; Entingh-Pearsall, A.; Holzenberger, M.; Liao, R.; Kahn, C.R. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol. Cell. Biol. 2007, 27, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, X.; Chen, Z.; Jin, Y.; Heidbreder, C.E.; Kolokythas, A.; Wang, A.; Dai, Y.; Zhou, X. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem. J. 2010, 432, 199–205. [Google Scholar] [CrossRef]
- Liao, X.; Shen, Y.; Zhang, R.; Sugi, K.; Vasudevan, N.T.; Alaiti, M.A.; Sweet, D.R.; Zhou, L.; Qing, Y.; Gerson, S.L.; et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc. Natl. Acad. Sci. USA 2018, 115, E4661–E4669. [Google Scholar] [CrossRef]
- Liao, X.; Haldar, S.M.; Lu, Y.; Jeyaraj, D.; Paruchuri, K.; Nahori, M.; Cui, Y.; Kaestner, K.H.; Jain, M.K. Kruppel-like factor 4 regulates pressure-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2010, 49, 334–338. [Google Scholar] [CrossRef]


| Target Gene | Representative Transcript | Gene Name | Transcript Position | Predicted Consequential Pairing of Target Region. Transcript (Top) and miRNA (Bottom) | Site Type |
|---|---|---|---|---|---|
| KLF4 | ENST00000374672. 4 | Kruppel-like Factor 4 | 66–72 3′ UTR | (Transcript) 5′…UUUACUUUUCACACUGUCUUCCC… (miRNA) 3′UGUUGUUUUAGUGAU–CAGAAGGU | 7mer– m8 |
| 574–580 3′ UTR | (Transcript) 5′…GGAAAAUCUAUAUUUGUCUUCC G… (miRNA) 3′ UGUUGUUUUAGUGAU–––CAGAAGGU | 7mer– m8 | |||
| IRS1 | ENST00000305123. 5 | Insulin Receptor Substrate 1 | 908–915 3′ UTR | (Transcript) 5′ …GAAGAGGAAAUUAAA– GUCUUCCA… (miRNA) 3′ UGUUGUUUUAGUGAU– CAGAAGGU | 8mer |
| IRS2 | ENST00000375856. 3 | Insulin Receptor Substrate 2 | 1588–1594 3′ UTR | (Transcript) 5′…AAUGGCAAUGCAAAAGUCUUCCU.. (miRNA) 3′ UGUUGUUUUAGUGAU– CAGAAGGU | 7mer– m8 |
| 2307–2314 3′ UTR | (Transcript) 5′…AACUUAUCUUGCUCUGUCUUCCA.. (miRNA) 3′UGUUGUUUUAGUGAU–CAGAAGGU | 8mer | |||
| EGFR | ENST00000275493. 2 | Epidermal Growth Factor Receptor | 457–464 3′ UTR | (Transcript) 5′…GAGCACAAGCCACAAGUCUUCCA… (miRNA) 3′UGUUGUUUUAGUGAU–CAGAAGGU | 8mer |
| RAF1 | ENST00000251849. 4 | Raf-1 Proto–Oncogene, Serine/ Threonine Kinase | 674–681 3′ UTR | (Transcript) 5′ …AUCAUGCUGAAUUUU––GUCUUCCA… (miRNA) 3′ UGUUGUUUUAGUGAU– CAGAAGGU | 8mer |
| IGF1R | ENST00000268035. 6 | Insulin-Like Growth Factor 1 Receptor | 5950–5957 3′ UTR | (Transcript) 5′…AUCUUCAGUAUCUUGGUCUUCCA… (miRNA) 3′UGUUGUUUUAGUGAUCAGAAGGU | 8mer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhashab, S.; Megantara, H.P.; Mahaputra, D.K.; O’Neill, J.; Phowira, J.; Weaver, J.U. Decoding of miR-7-5p in Colony Forming Unit–Hill Colonies as a Biomarker of Subclinical Cardiovascular Disease—A MERIT Study. Int. J. Mol. Sci. 2023, 24, 11977. https://doi.org/10.3390/ijms241511977
Bakhashab S, Megantara HP, Mahaputra DK, O’Neill J, Phowira J, Weaver JU. Decoding of miR-7-5p in Colony Forming Unit–Hill Colonies as a Biomarker of Subclinical Cardiovascular Disease—A MERIT Study. International Journal of Molecular Sciences. 2023; 24(15):11977. https://doi.org/10.3390/ijms241511977
Chicago/Turabian StyleBakhashab, Sherin, Hamzah Pratama Megantara, Dimas Kirana Mahaputra, Josie O’Neill, Jason Phowira, and Jolanta U. Weaver. 2023. "Decoding of miR-7-5p in Colony Forming Unit–Hill Colonies as a Biomarker of Subclinical Cardiovascular Disease—A MERIT Study" International Journal of Molecular Sciences 24, no. 15: 11977. https://doi.org/10.3390/ijms241511977
APA StyleBakhashab, S., Megantara, H. P., Mahaputra, D. K., O’Neill, J., Phowira, J., & Weaver, J. U. (2023). Decoding of miR-7-5p in Colony Forming Unit–Hill Colonies as a Biomarker of Subclinical Cardiovascular Disease—A MERIT Study. International Journal of Molecular Sciences, 24(15), 11977. https://doi.org/10.3390/ijms241511977





