Changes in Adenosine Deaminase Activity and Endothelial Dysfunction after Mild Coronavirus Disease-2019
Abstract
1. Introduction
2. Results
2.1. Circulating Activities of Adenosine Deaminase Isoenzymes and CD26 Protein Level in Serum Are Deregulated in Post-COVID Patients
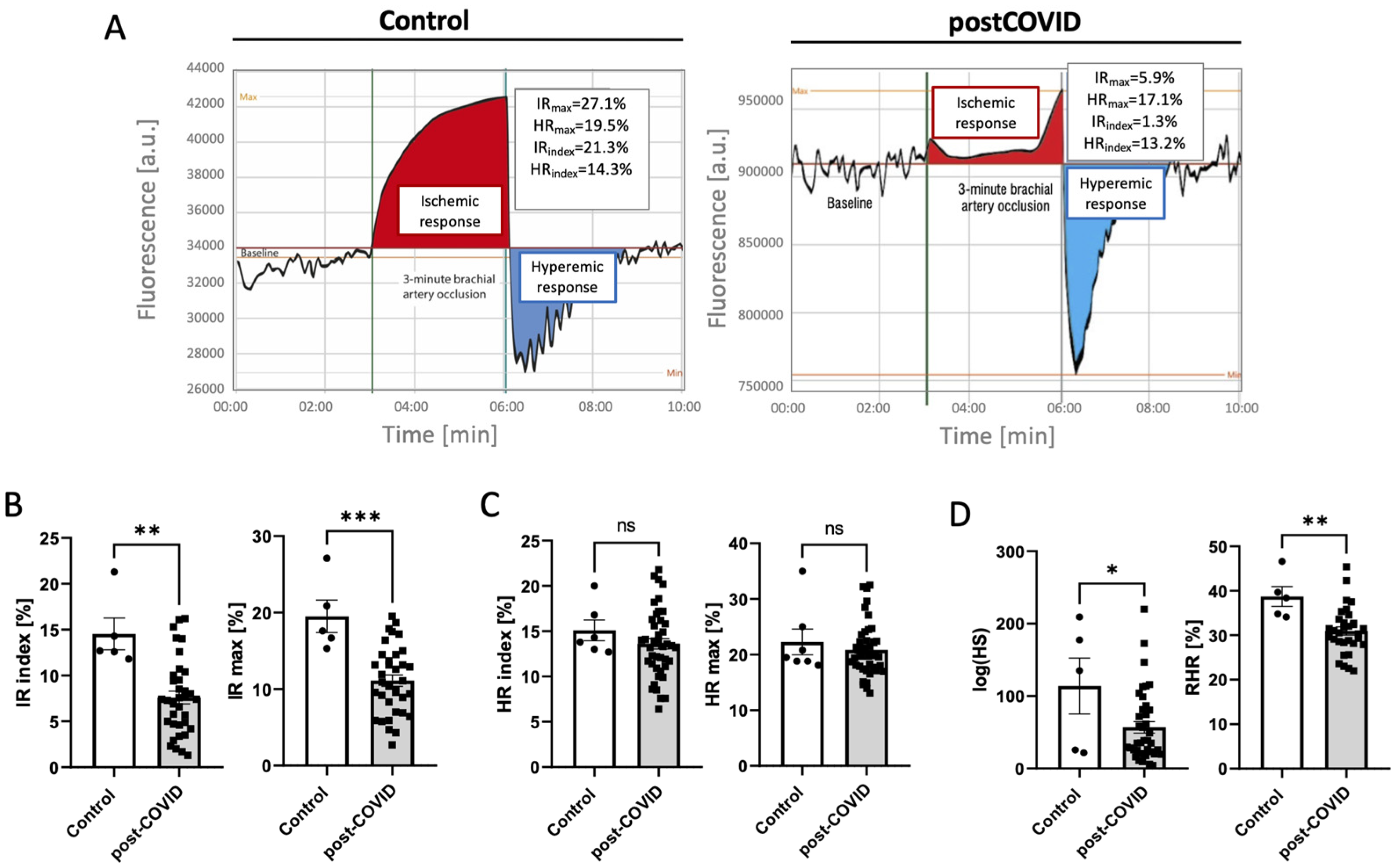
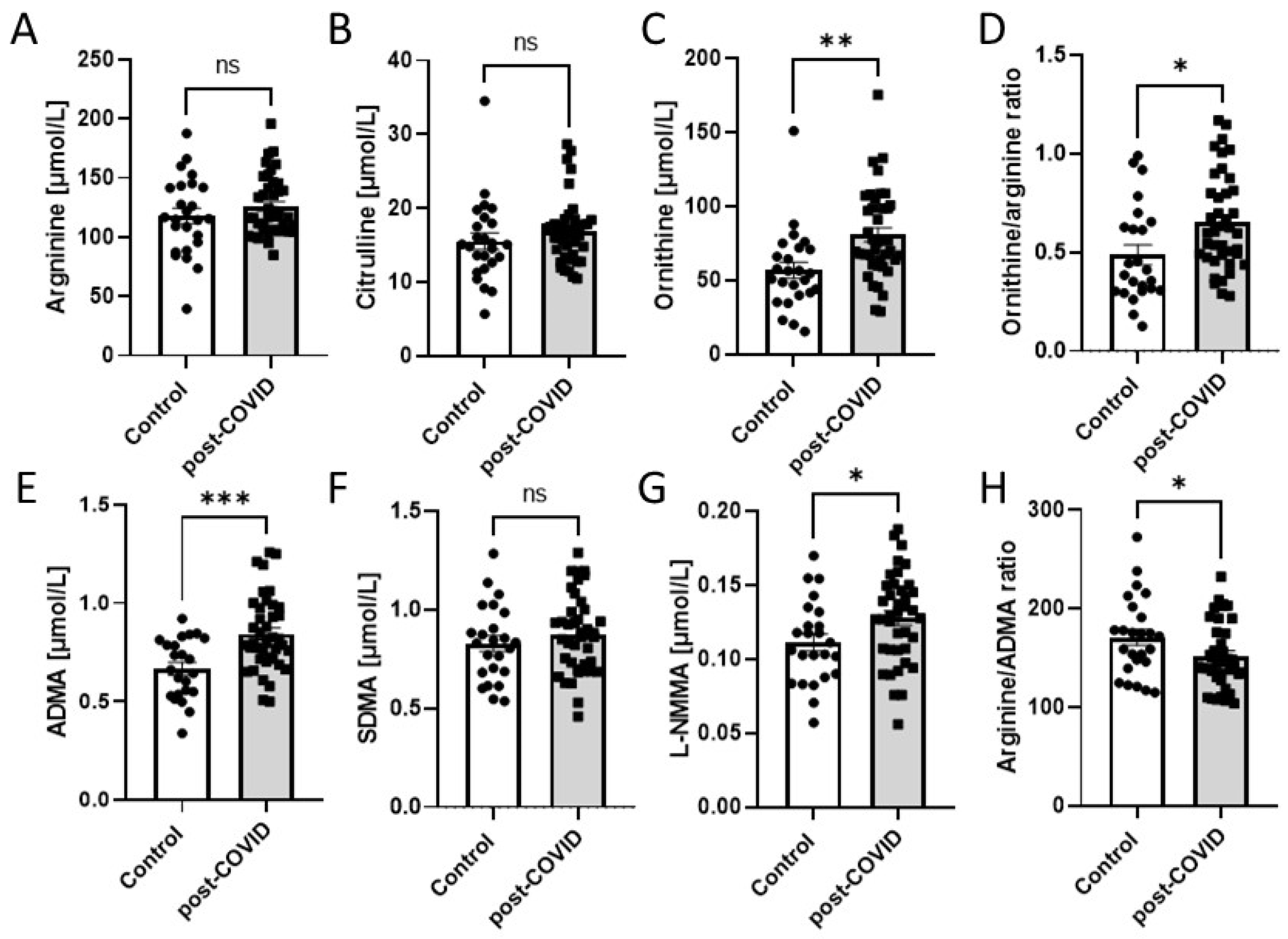
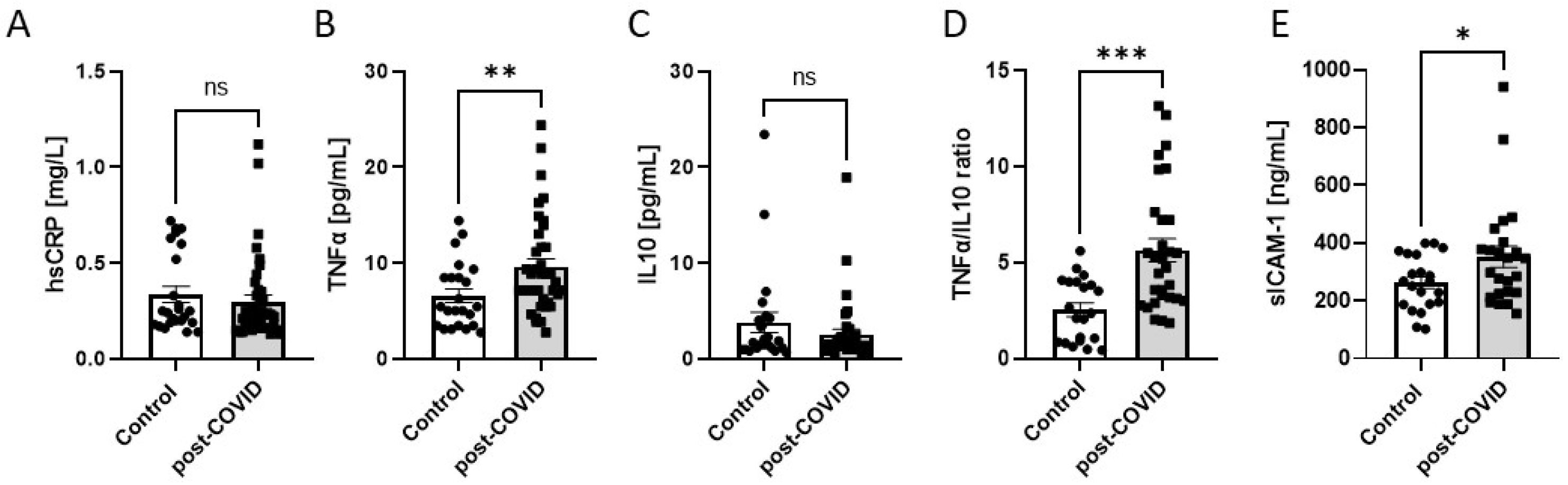
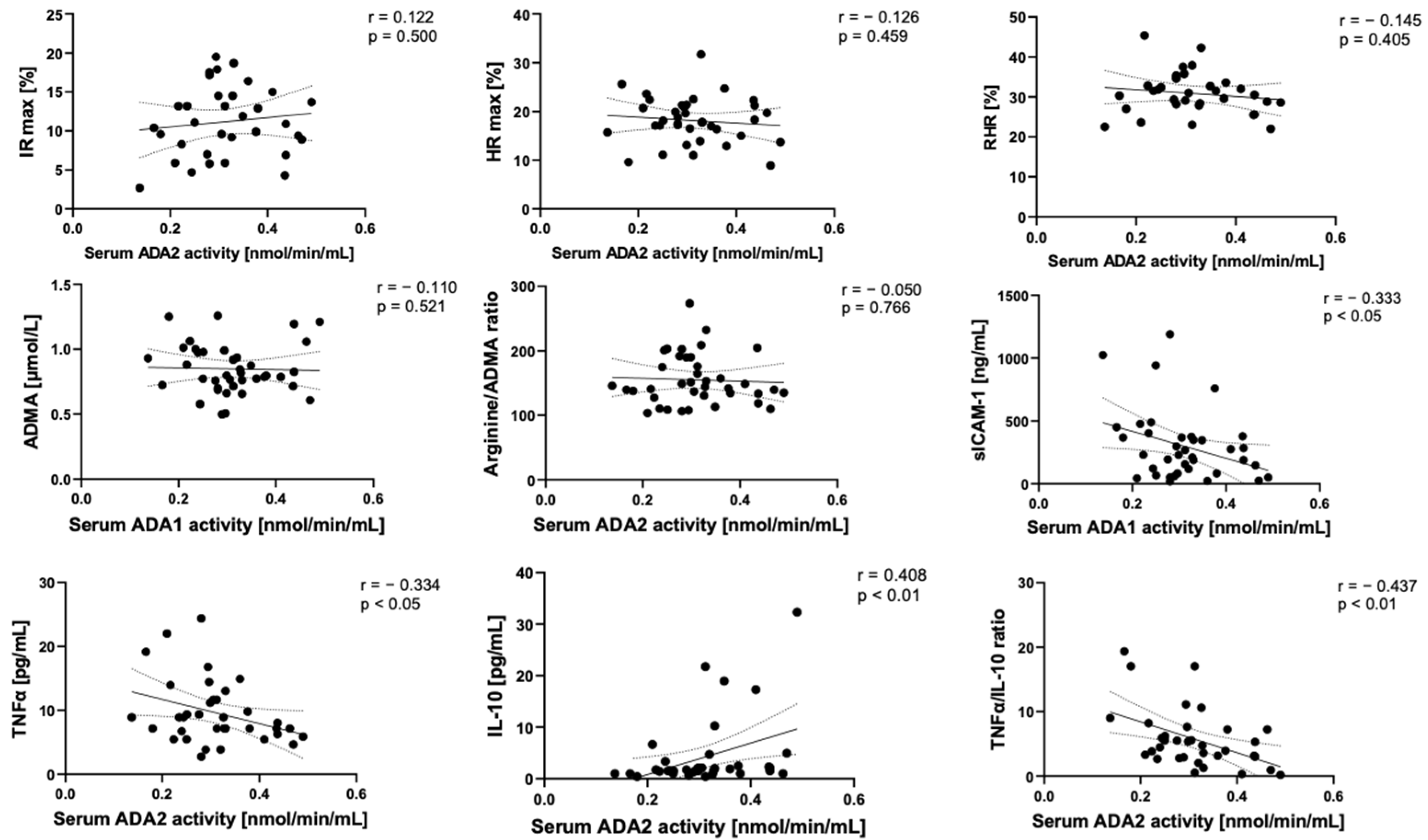
2.2. Microvascular and Endothelial Dysfunction with Proinflammatory Phenotype in Post-COVID Patients
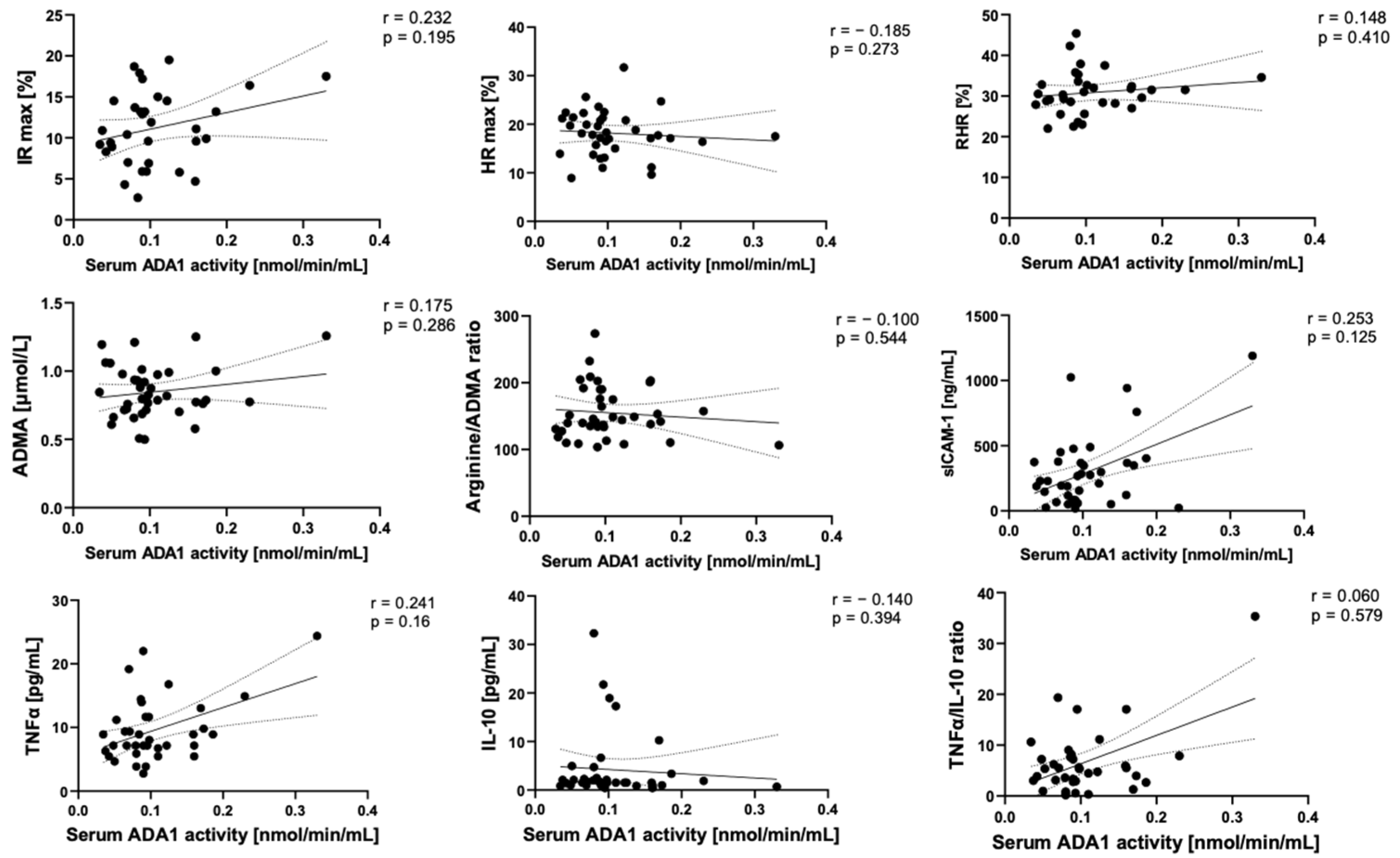
2.3. Adenosine Deaminase 1 Is Overactivated on the Surface of Endothelial Cells Stimulated with Post-COVID Patient Sera
3. Discussion
4. Materials and Methods
4.1. Human Participants
4.2. Measurement of Microvascular Function
4.3. Quantification of Amino Acids in Serum
4.4. Quantification of hsCRP, sICAM-1, CD26 Protein, and Cytokins’ Concentration in Serum and Peripheral Blood Morphology
4.5. Measurement of Adenosine Deaminase Activity in Serum
4.6. Cell Culture
4.7. Measurement of Adenosine Deaminase Activity on Endothelial Cell Surface
4.8. Immune Cell Adhesion Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; He, Q.; Qian, H.; Liu, J. Overview of the Pathogenesis of COVID-19 (Review). Exp. Ther. Med. 2021, 22, 1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, Q.; Chang, H. Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects. Front. Immunol. 2022, 13, 1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, L.; Li, M.; Xie, B.; He, L.; Wang, M.; Zhang, R.; Hou, N.; Zhang, Y.; Jia, F. Real-Word Effectiveness of Global COVID-19 Vaccines Against SARS-CoV-2 Variants: A Systematic Review and Meta-Analysis. Front. Med. (Lausanne) 2022, 9, 1189. [Google Scholar] [CrossRef]
- Sheleme, T.; Bekele, F.; Ayela, T. Clinical Presentation of Patients Infected with Coronavirus Disease: A Systematic Review. Infect. Dis. 2020, 13, 117863372095207. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.; Gundry, R.L.; Brett-Major, D.M.; Mahr, C.; Thiele, G.M.; Lindsey, M.L.; Anderson, D.R. COVID-19 and Cardiovascular Disease: What We Know, What We Think We Know, and What We Need to Know. J. Mol. Cell Cardiol. 2020, 144, 12–14. [Google Scholar] [CrossRef]
- Marik, P.E.; Iglesias, J.; Varon, J.; Kory, P. A Scoping Review of the Pathophysiology of COVID-19. Int. J. Immunopathol. Pharmacol. 2021, 35, 1–16. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Wojdasiewicz, P.; Watroba, M.; Szewczyk, G. Mast Cell Activation Syndrome in COVID-19 and Female Reproductive Function: Theoretical Background vs. Accumulating Clinical Evidence. J. Immunol. Res. 2022, 2022, 9534163. [Google Scholar] [CrossRef]
- Shi, W.; Lv, J.; Lin, L. Coagulopathy in COVID-19: Focus on Vascular Thrombotic Events. J. Mol. Cell Cardiol. 2020, 146, 32–40. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.Y.; Huang, Y.; Lui, K.O. Endothelial Contribution to COVID-19: An Update on Mechanisms and Therapeutic Implications. J. Mol. Cell Cardiol. 2022, 164, 69–82. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Weng, J. ping Endothelial Dysfunction in COVID-19: An Overview of Evidence, Biomarkers, Mechanisms and Potential Therapies. Acta Pharmacol. Sin. 2022, 44, 695–709. [Google Scholar] [CrossRef]
- Juni, R.; Phelp, P.; Bink, D.; Espinoza-Gonzales, P.; van Bergen, A.; Kuster, D.; van der Velden, J.; Krijnen, P.; Niessen, H.; Boon, R. Dysregulation of Cardiac Endothelial LncRNA H19 in COVID19 Patients Induces Endothelial Dysfunction, Which Impairs Cardiomyocyte Function. J. Mol. Cell Cardiol. 2022, 173, 121. [Google Scholar] [CrossRef]
- Lowenstein, C.J.; Solomon, S.D. Severe COVID-19 Is a Microvascular Disease. Circulation 2020, 142, 1609–1611. [Google Scholar] [CrossRef]
- Viveiros, A.; Gheblawi, M.; Aujla, P.K.; Sosnowski, D.K.; Seubert, J.M.; Kassiri, Z.; Oudit, G.Y. Sex- and Age-Specific Regulation of ACE2: Insights into Severe COVID-19 Susceptibility. J. Mol. Cell Cardiol. 2022, 164, 13–16. [Google Scholar] [CrossRef]
- Gasecka, A.; Pruc, M.; Kukula, K.; Gilis-Malinowska, N.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. Post-COVID-19 Heart Syndrome. Cardiol. J. 2021, 28, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zajac, B.; Jablonska, P.; Serocki, M.; Bulinska, A.; Mierzejewska, P.; Friebe, D.; Alter, C.; Jasztal, A.; Lango, R.; Rogowski, J.; et al. Nucleotide Ecto-Enzyme Metabolic Pattern and Spatial Distribution in Calcific Aortic Valve Disease; Its Relation to Pathological Changes and Clinical Presentation. Clin. Res. Cardiol. 2020, 109, 137–160. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Kawecka, A.; Caratis, F.; Urbanowicz, K.; Braczko, A.; Furihata, T.; Karaszewski, B.; Smolenski, R.T.; Rutkowska, A. The Impaired Distribution of Adenosine Deaminase Isoenzymes in Multiple Sclerosis Plasma and Cerebrospinal Fluid. Front. Mol. Neurosci. 2022, 15, 998023. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Koszalka, P.; Mierzejewska, P.; Bulinska, A.; Zabielska, M.A.; Brodzik, K.; Skrzypkowska, A.; Zelazek, L.; Pelikant-Malecka, I.; Slominska, E.M.; et al. Adenosine Deaminase Inhibition Suppresses Progression of 4T1 Murine Breast Cancer by Adenosine Receptor-Dependent Mechanisms. J. Cell Mol. Med. 2018, 22, 5939–5954. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zajac, B.; Mierzejewska, P.; Slominska, E.M.; Smolenski, R.T.; Agrofoglio, L.A.; Roy, V.; Nicolas, C. Therapeutic Perspectives of Adenosine Deaminase Inhibition in Cardiovascular Diseases. Molecules 2020, 25, 4652. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E.; Batiha, G.E.S. Dipyridamole and Adenosinergic Pathway in COVID-19: A Juice or Holy Grail. Egypt. J. Med. Human Genet. 2022, 23, 140. [Google Scholar] [CrossRef]
- Di Nicolatonio, J.J.; Barroso-Aranda, J. Harnessing adenosine A2A receptors as a strategy for suppressing the lung inflammation and thrombotic complications of COVID-19: Potential of pentoxifylline and dipyridamole. Med. Hypotheses. 2020, 143, 110051. [Google Scholar] [CrossRef] [PubMed]
- Zhulai, G.; Oleinik, E.; Shibaev, M.; Ignatev, K. Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules 2022, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.Y. Built to Last: Gene Therapy for ADA SCID. Blood 2021, 138, 1287. [Google Scholar] [CrossRef]
- Smolenski, R.T.; Kalsi, K.K.; Zych, M.; Kochan, Z.; Yacoub, M.H. Adenine/Ribose Supply Increases Adenosine Production and Protects ATP Pool in Adenosine Kinase-Inhibited Cardiac Cells. J. Mol. Cell Cardiol. 1998, 30, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Casadó, V.; Ciruela, F.; Saura, C.; Mallol, J.; Canela, E.I.; Lluis, C. Cell Surface Adenosine Deaminase: Much More than an Ectoenzyme. Prog. Neurobiol. 1997, 52, 283–294. [Google Scholar] [CrossRef]
- Grenz, A.; Homann, D.; Eltzschig, H.K. Extracellular Adenosine: A Safety Signal That Dampens Hypoxia-Induced Inflammation During Ischemia. Antioxid. Redox Signal. 2011, 15, 2221. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Cordero, O.J.; Martini, C.; Proost, P.; Casadó, V.; Moreno, E.; Canet, J.; Gracia, E.; Lluís, C.; Mallol, J.; et al. Molecular Evidence of Adenosine Deaminase Linking Adenosine A 2A Receptor and CD26 Proteins. Front. Pharmacol. 2018, 9, 106. [Google Scholar] [CrossRef]
- Gracia, E.; Pérez-Capote, K.; Moreno, E.; Bakešová, J.; Mallol, J.; Lluis, C.; Franco, R.; Cortes, A.; Casado, V.; Canela, E.I. A2A Adenosine Receptor Ligand Binding and Signaling Is Allosterically Modulated by Adenosine Deaminase. Biochem. J. 2011, 435, 701–709. [Google Scholar] [CrossRef]
- Cameron, K.; Rozano, L.; Falasca, M.; Mancera, R.L. Does the SARS-CoV-2 Spike Protein Receptor Binding Domain Interact Effectively with the DPP4 (CD26) Receptor? A Molecular Docking Study. Int. J. Mol. Sci. 2021, 22, 7001. [Google Scholar] [CrossRef]
- Zavialov, A.V.; Gracia, E.; Glaichenhaus, N.; Franco, R.; Zavialov, A.V.; Lauvau, G. Human Adenosine Deaminase 2 Induces Differentiation of Monocytes into Macrophages and Stimulates Proliferation of T Helper Cells and Macrophages. J. Leukoc. Biol. 2010, 88, 279–290. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, S.; Watanabe, N.; Batchu, S.; Kajigaya, S.; Diamond, C.; Alemu, L.; Raffo, D.Q.; Feng, X.; Hoffmann, P.; et al. Single-Cell Profiling of T Lymphocytes in Deficiency of Adenosine Deaminase 2. J. Leukoc. Biol. 2022, 111, 301–312. [Google Scholar] [CrossRef]
- Lee, P.Y.; Schulert, G.S.; Canna, S.W.; Huang, Y.; Sundel, J.; Li, Y.; Hoyt, K.J.; Blaustein, R.B.; Wactor, A.; Do, T.; et al. Adenosine Deaminase 2 as a Biomarker of Macrophage Activation Syndrome in Systemic Juvenile Idiopathic Arthritis. Ann. Rheum. Dis. 2020, 79, 225. [Google Scholar] [CrossRef]
- Pinto, B.; Deo, P.; Sharma, S.; Syal, A.; Sharma, A. Expanding Spectrum of DADA2: A Review of Phenotypes, Genetics, Pathogenesis and Treatment. Clin. Rheumatol. 2021, 40, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zajac, B.; Mateuszuk, L.; Zukowska, P.; Jasztal, A.; Zabielska, M.A.; Toczek, M.; Jablonska, P.; Zakrzewska, A.; Sitek, B.; Rogowski, J.; et al. Increased Activity of Vascular Adenosine Deaminase in Atherosclerosis and Therapeutic Potential of Its Inhibition. Cardiovasc. Res. 2016, 112, 590–605. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Mierzejewska, P.; Sucajtys-Szulc, E.; Bulinska, A.; Zabielska, M.A.; Jablonska, P.; Serocki, M.; Koszalka, P.; Milczarek, R.; Jasztal, A.; et al. Inhibition of LPS-Stimulated Ecto-Adenosine Deaminase Attenuates Endothelial Cell Activation. J. Mol. Cell Cardiol. 2019, 128, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.H.; Estrada, A.; Ayala-Marin, Y.M.; Alvidrez-Camacho, A.Y.; Rodriguez, G.; Robles-Escajeda, E.; Cadena-Medina, D.A.; Rodriguez, A.C.; Kirken, R.A. The Many Faces of JAKs and STATs Within the COVID-19 Storm. Front. Immunol. 2021, 12, 690477. [Google Scholar] [CrossRef]
- Caorsi, R.; Penco, F.; Schena, F.; Gattorno, M. Monogenic Polyarteritis: The Lesson of ADA2 Deficiency. Pediatr. Rheumatol. 2016, 14, 51. [Google Scholar] [CrossRef][Green Version]
- Kutryb-Zajac, B.; Harasim, G.; Jedrzejewska, A.; Krol, O.; Braczko, A.; Jablonska, P.; Mierzejewska, P.; Zielinski, J.; Slominska, E.M.; Smolenski, R.T. Macrophage-Derived Adenosine Deaminase 2 Correlates with M2 Macrophage Phenotype in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 3764. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Banerjee, M. Immune Thrombocytopenia Secondary to COVID-19: A Systematic Review. SN Compr. Clin. Med. 2020, 2, 2048–2058. [Google Scholar] [CrossRef]
- Boehm, B.A.; Packer, C.D. Persistent Relapsing Immune Thrombocytopenia Following COVID-19 Infection. Cureus 2022, 14, e27133. [Google Scholar] [CrossRef] [PubMed]
- González-López, T.J.; Bárez, A.; Bernardo-Gutiérrez, A.; Bernat, S.; Canaro-Hirnyk, M.; Entrena-Ureña, L.; Fernández-Fuertes, F.; Guinea de Castro, J.M.; Jiménez-Bárcenas, R.; Pascual-Izquierdo, C.; et al. Recommendations on the Management of Patients with Immune Thrombocytopenia (ITP) in the Context of SARS-CoV-2 Infection and Vaccination: Consensus Guidelines from a Spanish ITP Expert Group. Infect. Dis. Ther. 2023, 12, 303–315. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of Thrombocytopenia in COVID-19 Patients. Ann. Hematol. 2020, 99, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, M.; Okan, V.; Sever, T.; Balci Oguzkan, S.; Yilmaz, M.; Babacan, T.; Pehlivan, S. Investigation of TNF-Alpha, TGF-Beta 1, IL-10, IL-6, IFN-Gamma, MBL, GPIA, and IL1A Gene Polymorphisms in Patients with Idiopathic Thrombocytopenic Purpura. Platelets 2011, 22, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Farazi, A.; Moharamkhani, A.; Sofian, M. Validity of Serum Adenosine Deaminase in Diagnosis of Tuberculosis. Pan Afr. Med. J. 2013, 15, 133. [Google Scholar] [CrossRef]
- Russo, M.; Giancane, R.; Apice, G.; Galanti, B. Adenosine Deaminase and Purine Nucleoside Phosphorylase Activities in Peripheral Lymphocytes from Patients with Solid Tumours. Br. J. Cancer 1981, 43, 196. [Google Scholar] [CrossRef]
- Al-Shammary, F.J. Adenosine Deaminase Activity in Serum and Pleural Effusions of Tuberculous and Non-Tuberculous Patients. Biochem. Mol. Biol. Int. 1997, 43, 763–779. [Google Scholar] [CrossRef]
- Shi, T.; Li, J.; Miao, Y.; Huang, L.; Tian, J. Adenosine Deaminase as a Marker for the Severity of Infectious Mononucleosis Secondary to EBV in Children. BMC Infect. Dis. 2022, 22, 164. [Google Scholar] [CrossRef]
- Khosla, S.N.; Kumar, D.; Singh, V. Lymphocytic Adenosine Deaminase Activity in Typhoid Fevers. Postgrad. Med. J. 1992, 68, 268–271. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.K.; Mohan, A.; Sharma, A. Miliary Tuberculosis: A New Look at an Old Foe. J. Clin. Tuberc. Other Mycobact. Dis. 2016, 3, 13–27. [Google Scholar] [CrossRef]
- Chen, Z.; John Wherry, E. T Cell Responses in Patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Park, S.H. An Impaired Inflammatory and Innate Immune Response in COVID-19. Mol. Cells 2021, 44, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Tinello, C.; Vatrella, A.; De Sarro, G.; Pelaia, G. Lung under Attack by COVID-19-Induced Cytokine Storm: Pathogenic Mechanisms and Therapeutic Implications. Ther. Adv. Respir. Dis. 2020, 14. [Google Scholar] [CrossRef]
- Hasan, A.; Al-Ozairi, E.; Al-Baqsumi, Z.; Ahmad, R.; Al-Mulla, F. Cellular and Humoral Immune Responses in Covid-19 and Immunotherapeutic Approaches. Immunotargets Ther. 2021, 10, 63–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, Y.; Wang, T.; Xie, F. Innate and Adaptive Immune Response in SARS-CoV-2 Infection-Current Perspectives. Front. Immunol. 2022, 13, 1053437. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep Immune Profiling of COVID-19 Patients Reveals Distinct Immunotypes with Therapeutic Implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef] [PubMed]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive Mapping of Immune Perturbations Associated with Severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef] [PubMed]
- HIV Nursing. The Role of Adenosine Deaminase Levels in The Diagnosis of COVID-19 Infection. 2022. Available online: http://hivnursing.net (accessed on 23 August 2023).
- Allison, T.R.; Hunsaker, J.J.H.; La’ulu, S.L.; Genzen, J.R. Evaluation of an Adenosine Deaminase (ADA) Assay in Serum, Pleural, Pericardial, Peritoneal, and Cerebrospinal Fluids on the Roche Cobas C501 Analyzer. Clin. Biochem. 2022, 109–110, 57–63. [Google Scholar] [CrossRef]
- Franco-Martínez, L.; Tecles, F.; Torres-Cantero, A.; Bernal, E.; San Lázaro, I.; Alcaraz, M.J.; Vicente-Romero, M.R.; Lamy, E.; Sánchez-Resalt, C.; Rubio, C.P.; et al. Analytical Validation of an Automated Assay for the Measurement of Adenosine Deaminase (ADA) and Its Isoenzymes in Saliva and a Pilot Evaluation of Their Changes in Patients with SARS-CoV-2 Infection. Clin. Chem. Lab. Med. 2021, 59, 1592–1599. [Google Scholar] [CrossRef]
- Chiu, K.H.Y.; Yip, C.C.Y.; Poon, R.W.S.; Leung, K.H.; Li, X.; Hung, I.F.N.; To, K.K.W.; Cheng, V.C.C.; Yuen, K.Y. Correlations of Myeloperoxidase (MPO), Adenosine Deaminase (ADA), C-C Motif Chemokine 22 (CCL22), Tumour Necrosis Factor Alpha (TNFα) and Interleukin-6 (IL-6) MRNA Expression in the Nasopharyngeal Specimens with the Diagnosis and Severity of SARS-CoV-2 Infections. Emerg. Microbes Infect. 2023, 12, 2157338. [Google Scholar] [CrossRef]
- Geiger, J.D.; Khan, N.; Murugan, M.; Boison, D. Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities. Front. Pharmacol. 2020, 11, 1901. [Google Scholar] [CrossRef]
- Tardif, V.; Muir, R.; Cubas, R.; Chakhtoura, M.; Wilkinson, P.; Metcalf, T.; Herro, R.; Haddad, E.K. Adenosine Deaminase-1 Delineates Human Follicular Helper T Cell Function and Is Altered with HIV. Nat. Commun. 2019, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Schlicht, K.; Rohmann, N.; Geisler, C.; Hollstein, T.; Knappe, C.; Hartmann, K.; Schwarz, J.; Tran, F.; Schunk, D.; Junker, R.; et al. Circulating Levels of Soluble Dipeptidylpeptidase-4 Are Reduced in Human Subjects Hospitalized for Severe COVID-19 Infections. Int. J. Obes. 2020, 44, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fu, J.; Zhang, W.; Zhang, L.; Chen, H.; Cheng, J.; He, T.; Fu, J. Effect of DPP4/CD26 Expression on SARS-CoV-2 Susceptibility, Immune Response, Adenosine (Derivatives M62A and CD) Regulations on Patients with Cancer and Healthy Individuals. Int. J. Oncol. 2023, 62, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Dang, X.; Sprague, R.S.; Mustafa, S.J.; Zhou, Z. Alteration of Purinergic Signaling in Diabetes: Focus on Vascular Function. J. Mol. Cell Cardiol. 2020, 140, 1–9. [Google Scholar] [CrossRef]
- Raj, V.S.; Smits, S.L.; Provacia, L.B.; van den Brand, J.M.A.; Wiersma, L.; Ouwendijk, W.J.D.; Bestebroer, T.M.; Spronken, M.I.; van Amerongen, G.; Rottier, P.J.M.; et al. Adenosine Deaminase Acts as a Natural Antagonist for Dipeptidyl Peptidase 4-Mediated Entry of the Middle East Respiratory Syndrome Coronavirus. J. Virol. 2014, 88, 1834–1838. [Google Scholar] [CrossRef]
- Strollo, R.; Pozzilli, P. DPP4 Inhibition: Preventing SARS-CoV-2 Infection and/or Progression of COVID-19? Diabetes Metab. Res. Rev. 2020, 36, e3330. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Hu, Y.; Wang, Q.; Qi, J.; Gao, F.; Li, Y.; Zhang, Y.; Zhang, W.; Yuan, Y.; Bao, J.; et al. Molecular Basis of Binding between Novel Human Coronavirus MERS-CoV and Its Receptor CD26. Nature 2013, 500, 227–231. [Google Scholar] [CrossRef]
- Hellmann, M.; Tarnawska, M.; Dudziak, M.; Dorniak, K.; Roustit, M.; Cracowski, J.L. Reproducibility of Flow Mediated Skin Fluorescence to Assess Microvascular Function. Microvasc. Res. 2017, 113, 60–64. [Google Scholar] [CrossRef]
- Katarzynska, J.; Lipinski, Z.; Cholewinski, T.; Piotrowski, L.; Dworzynski, W.; Urbaniak, M.; Borkowska, A.; Cypryk, K.; Purgal, R.; Marcinek, A.; et al. Non-Invasive Evaluation of Microcirculation and Metabolic Regulation Using Flow Mediated Skin Fluorescence (FMSF): Technical Aspects and Methodology. Rev. Sci. Instrum. 2019, 90, 104104. [Google Scholar] [CrossRef]
- Pajkowski, M.; Dudziak, M.; Chlebus, K.; Hellmann, M. Assessment of Microvascular Function and Pharmacological Regulation in Genetically Confirmed Familial Hypercholesterolemia. Microvasc. Res. 2021, 138, 104216. [Google Scholar] [CrossRef]
- Olkowicz, M.; Debski, J.; Jablonska, P.; Dadlez, M.; Smolenski, R.T. Application of a New Procedure for Liquid Chromatography/Mass Spectrometry Profiling of Plasma Amino Acid-Related Metabolites and Untargeted Shotgun Proteomics to Identify Mechanisms and Biomarkers of Calcific Aortic Stenosis. J. Chromatogr. A 2017, 1517, 66–78. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Controls (n = 25) | postCOVID (n = 40) |
|---|---|---|
| Age (years) | 40.0 ± 12.5 | 40.5 ± 8.7 |
| Female | n = 18 (72%) | n = 27 (67.5%) |
| BMI (kg/m2) | 23.4 ± 3.8 | 25.5 ± 4.9 |
| SBP/DBP (mmHg) | 123/78 ± 9.0/5.2 | 125/85 ± 15.6/9.2 |
| HR (beats/min) | 76 ± 9.0 | 77 ± 11.4 |
| Hospitalization | na. | n = 1 (2.5%) |
| Symptoms | ||
| Fatigue | na. | n = 26 (65%) |
| Tachycardia | na. | n = 14 (35%) |
| Chest pains | na. | n = 14 (35%) |
| Breathing difficulties | na. | n = 5 (12.5%) |
| Pharmacotherapy | ||
| Beta-adrenolitics | na. | n = 13 (32.5%) |
| ACE-inhibitors | na. | n = 5 (12.5%) |
| Blood morphology | ||
| Hb [g/dL] | 13.5 ± 1.5 | 14.2 ± 1.3 |
| HCT [%] | 40.6 ± 3.2 | 41.2 ± 3.4 |
| RBC [1012/L] | 4.62 ± 0.5 | 4.70 ± 0.4 |
| WBC [109/L] | 6.10 ± 2.4 | 6.49 ± 2.2 |
| Neutrophils [109/L] | 3.77 ± 1.5 | 3.72 ± 1.6 |
| % Neutrophils | 58.1 ± 12 | 56.0 ± 9.1 |
| Lymphocytes [109/L] | 2.46 ± 0.9 | 2.07 ± 0.6 |
| % Lymphocytes | 37.9 ± 10 | 33.1 ± 8.5 |
| Monocytes [109/L] | 0.48 ± 0.2 | 0.50 ± 0.2 |
| % Monocytes | 7.40 ± 2.5 | 7.98 ± 2.1 |
| Eosinophils [109/L] | 0.12 ± 0.05 | 0.16 ± 0.12 |
| % Eosynophils | 1.85 ± 0.8 | 2.40 ± 1.2 |
| Basophils [109/L] | 0.04 ± 0.02 | 0.04 ± 0.02 |
| % Basophils | 0.62 ± 0.2 | 0.57 ± 0.2 |
| PLT [109/L] | 276 ± 90 | 230 ± 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedrzejewska, A.; Kawecka, A.; Braczko, A.; Romanowska-Kocejko, M.; Stawarska, K.; Deptuła, M.; Zawrzykraj, M.; Franczak, M.; Krol, O.; Harasim, G.; et al. Changes in Adenosine Deaminase Activity and Endothelial Dysfunction after Mild Coronavirus Disease-2019. Int. J. Mol. Sci. 2023, 24, 13140. https://doi.org/10.3390/ijms241713140
Jedrzejewska A, Kawecka A, Braczko A, Romanowska-Kocejko M, Stawarska K, Deptuła M, Zawrzykraj M, Franczak M, Krol O, Harasim G, et al. Changes in Adenosine Deaminase Activity and Endothelial Dysfunction after Mild Coronavirus Disease-2019. International Journal of Molecular Sciences. 2023; 24(17):13140. https://doi.org/10.3390/ijms241713140
Chicago/Turabian StyleJedrzejewska, Agata, Ada Kawecka, Alicja Braczko, Marzena Romanowska-Kocejko, Klaudia Stawarska, Milena Deptuła, Małgorzata Zawrzykraj, Marika Franczak, Oliwia Krol, Gabriela Harasim, and et al. 2023. "Changes in Adenosine Deaminase Activity and Endothelial Dysfunction after Mild Coronavirus Disease-2019" International Journal of Molecular Sciences 24, no. 17: 13140. https://doi.org/10.3390/ijms241713140
APA StyleJedrzejewska, A., Kawecka, A., Braczko, A., Romanowska-Kocejko, M., Stawarska, K., Deptuła, M., Zawrzykraj, M., Franczak, M., Krol, O., Harasim, G., Walczak, I., Pikuła, M., Hellmann, M., & Kutryb-Zając, B. (2023). Changes in Adenosine Deaminase Activity and Endothelial Dysfunction after Mild Coronavirus Disease-2019. International Journal of Molecular Sciences, 24(17), 13140. https://doi.org/10.3390/ijms241713140







