Wheatgrass-and-Aronia-Mixed Extract Suppresses Immunoglobulin E-Mediated Allergic Reactions In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
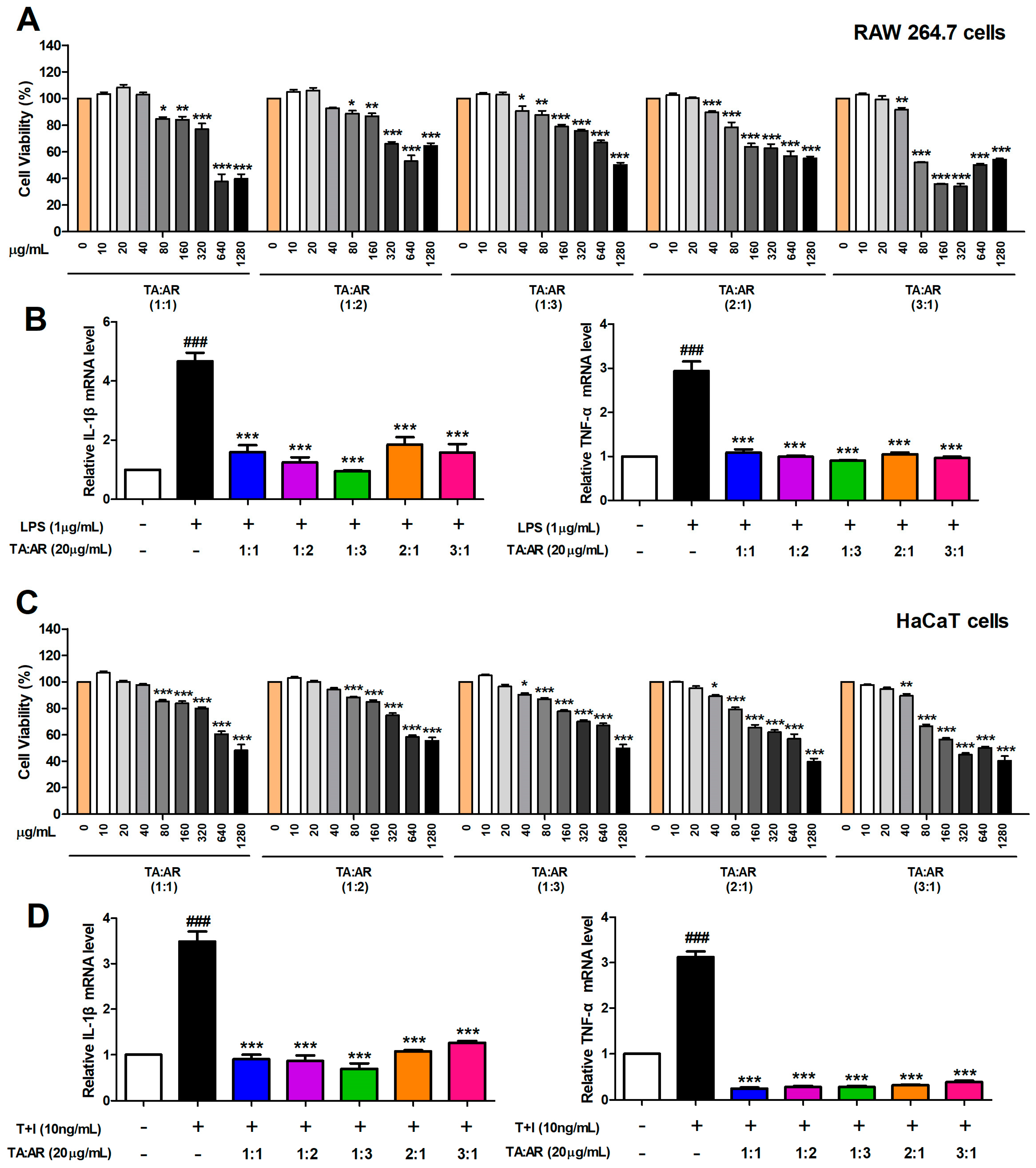
2.1. The Mixing Ratio Affects the Anti-Inflammatory Effect of Wheatgrass and Aronia Mixed Extracts
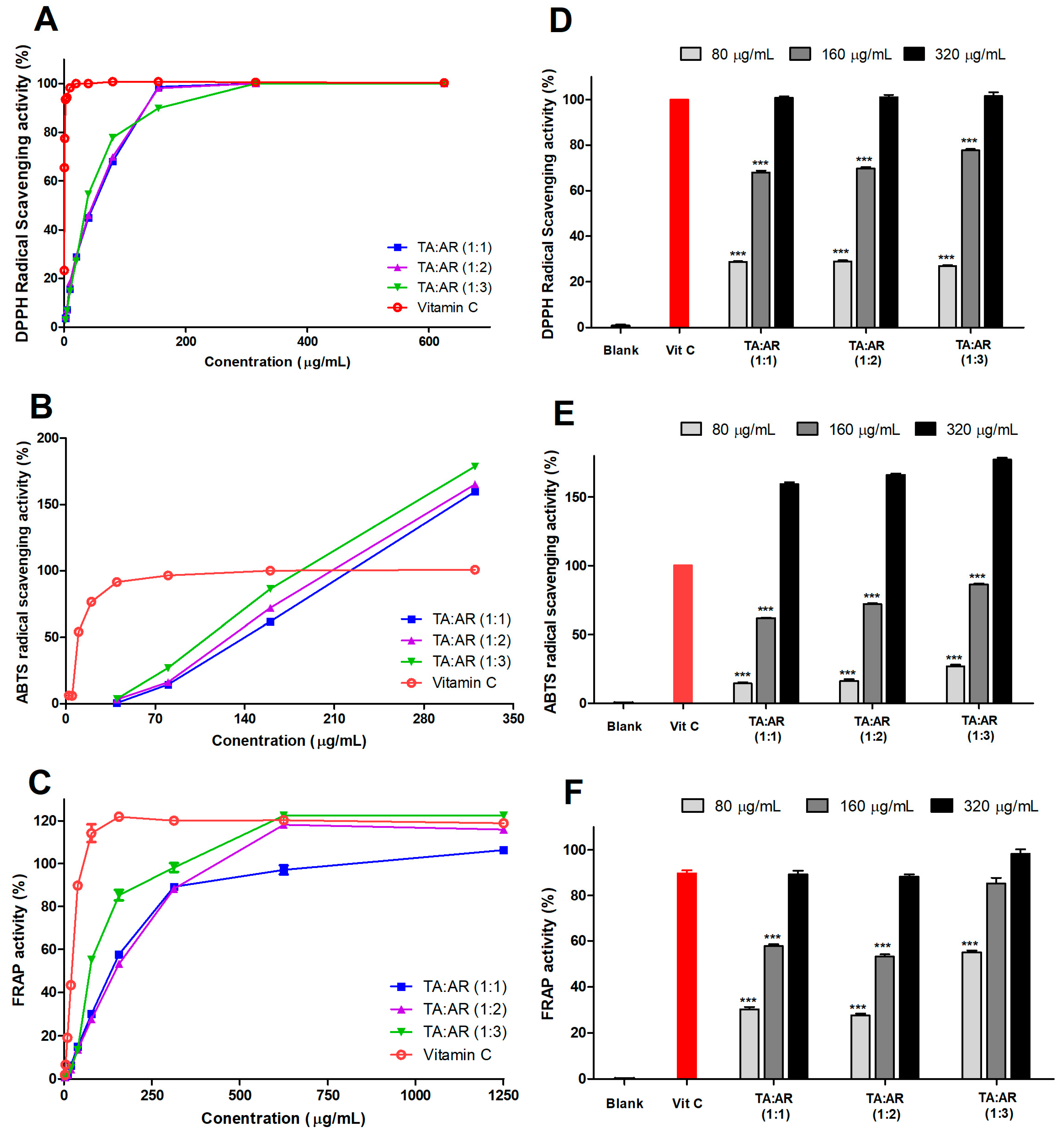
2.2. The Mixing Ratio of Wheatgrass and Aronia Extract Is Associated with an Antioxidant Effect
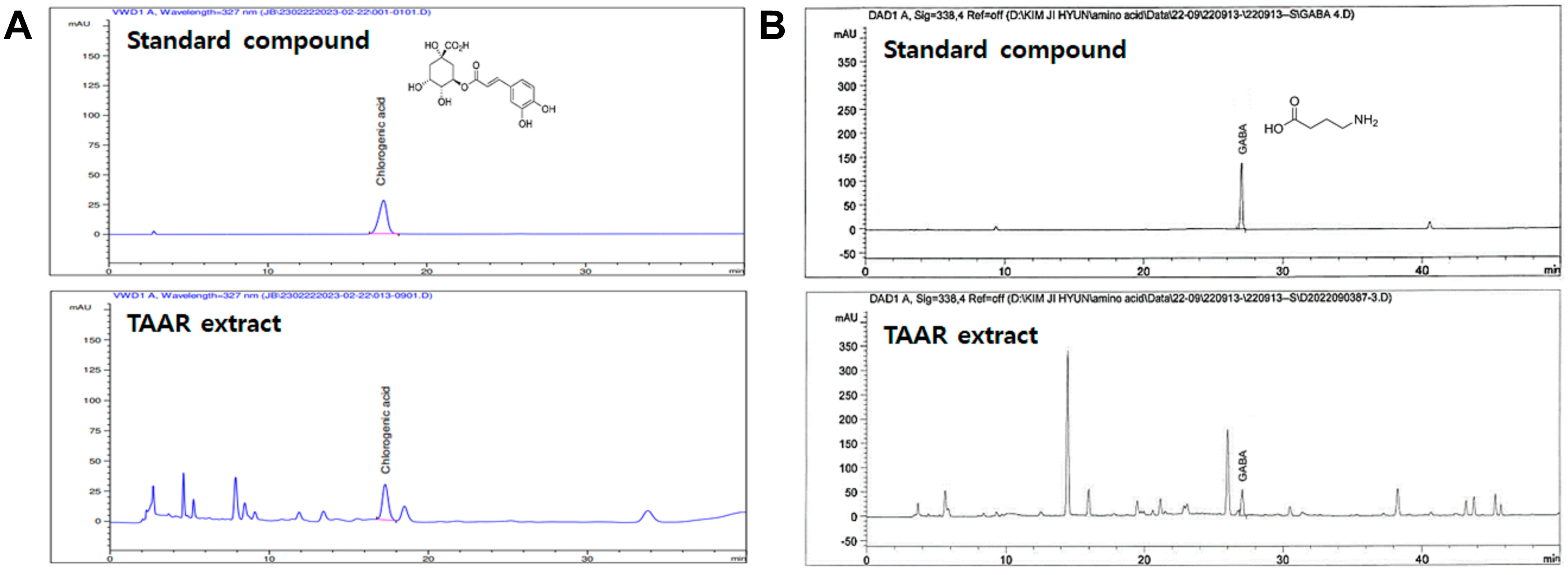
2.3. Quantification of Active Components of the TAAR Extract by HPLC
2.4. TAAR Extract Reduces Mast Cell Degranulation and Mast Cell-Mediated Pro-Inflammatory Cytokine Secretion in Anti-DNP IgE Plus DNP-BSA-Induced RBL-2H3 Cells
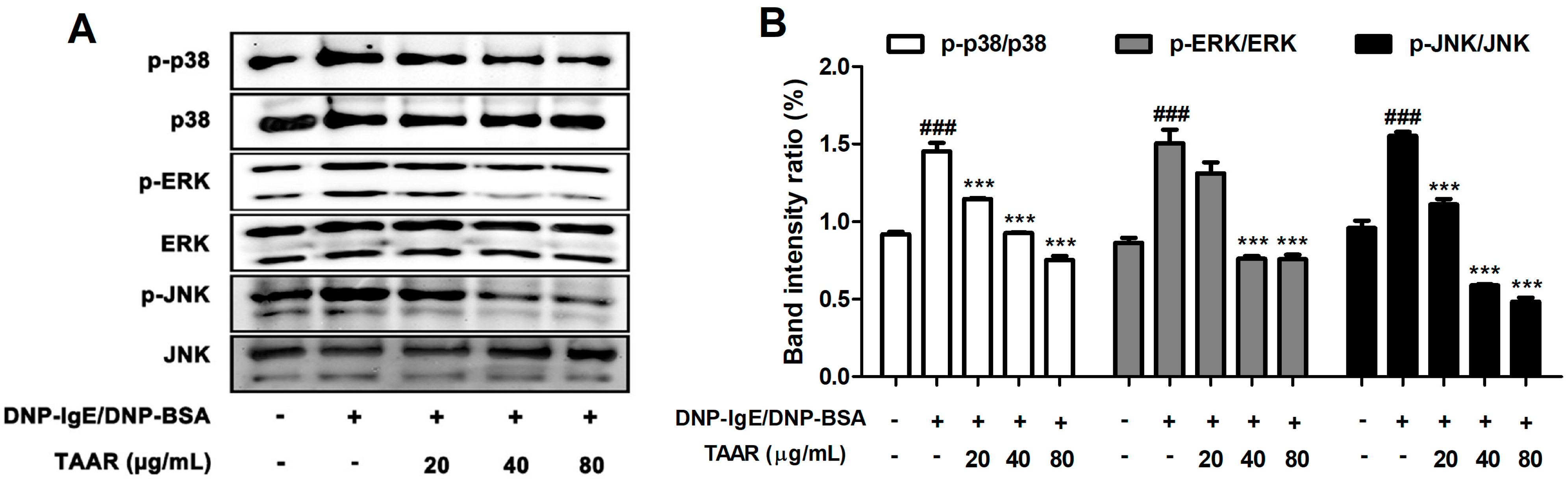
2.5. TAAR Extract Inhibits Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway Activation in Anti-DNP IgE Plus DNP-BSA-Induced RBL-2H3 Cells
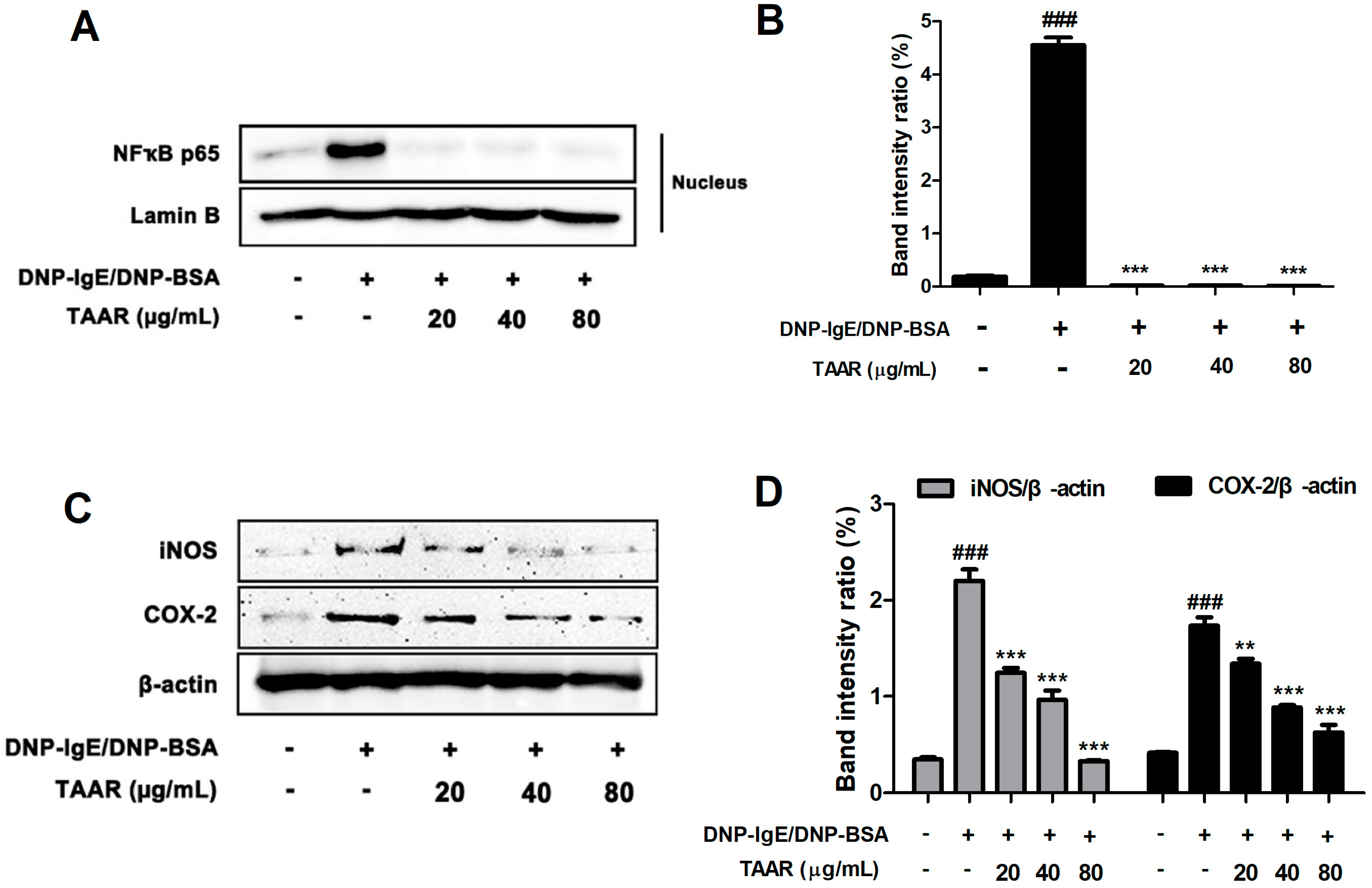
2.6. TAAR Extract Attenuates Nuclear Factor-κB (NF-κB) Nuclear Translocation and Related Inflammatory Mediators in Anti-DNP IgE Plus DNP-BSA-Induced RBL-2H3 Cells
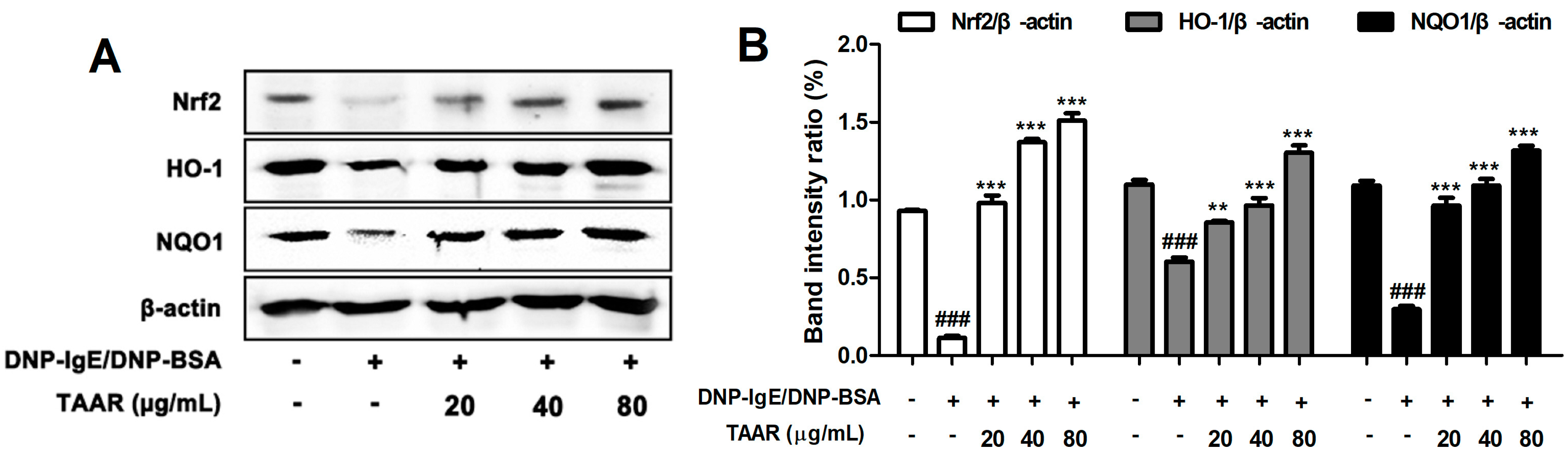
2.7. TAAR Extract Regulates the Activation of Nrf2/HO-1/NQO1 Signaling Pathway in Anti-DNP IgE Plus DNP-BSA-Induced RBL-2H3 Cells
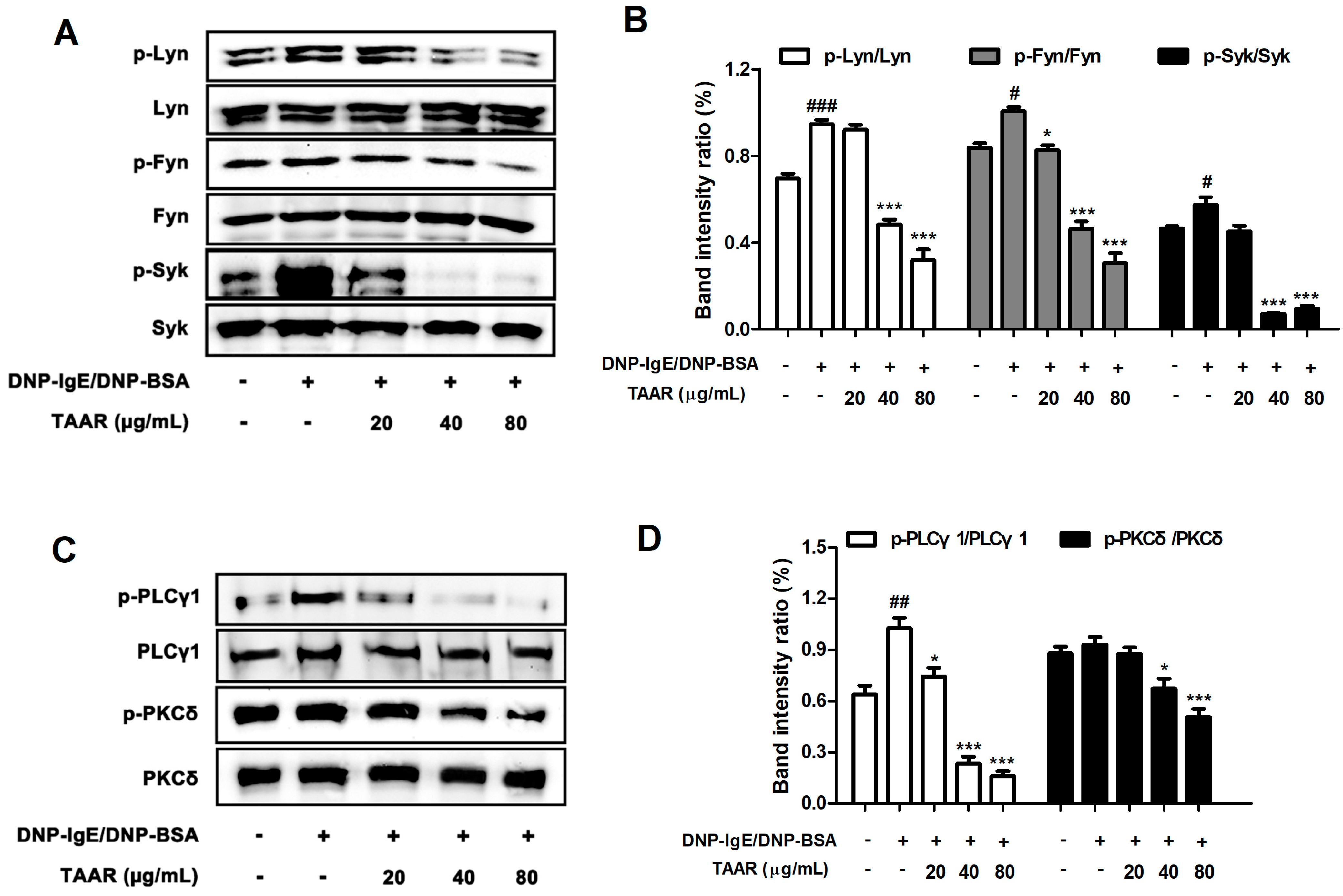
2.8. TAAR Extract Decreases the Activation of FcεRI Signaling Cascade in Anti-DNP IgE Plus DNP-BSA-Induced RBL-2H3 Cells
2.9. TAAR Extract Weakens the IgE-Mediated Cutaneous Anaphylaxis Reaction in Mice
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Wheatgrass-and-Aronia-Mixed Extract
3.3. HPLC Analysis
3.4. DPPH Radical Scavenging Activity
3.5. ABTS Radical Scavenging Assay
3.6. Fluorescent Recovery after Photobleaching (FRAP) Assay
3.7. Cell Culture
3.8. MTT Assay
3.9. Quantitative Real-Time PCR (qRT-PCR)
3.10. β-hexosaminidase Release Assay
3.11. Histamine Release
3.12. Western Blotting
3.13. Animals
3.14. Induction of IgE-Mediated Passive Cutaneous Anaphylaxis (PCA) Mouse Model
3.15. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakayama, T. Introduction to “allergic inflammation”. Immunol. Rev. 2017, 278, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Tsai, M. IgE and Mast Cells in Allergic Disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [PubMed] [Green Version]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar]
- Frieri, M. Mast cell activation syndrome. Clin. Rev. Allergy Immunol. 2015, 54, 353–365. [Google Scholar]
- Slater, J.W.; Zechnich, A.D.; Haxby, D.G. Second-generation antihistamines. Drugs 1999, 57, 31–47. [Google Scholar] [CrossRef]
- Shewry, P.P.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Tirgar, P.R.; Thumber, B.L.; Desai, T.R. Isolation, characterization and biological evaluation of iron chelator from Triticum aestivum (wheat grass). Int. J. Pharma Bio Sci. 2011, 2, 288–296. [Google Scholar]
- Banerjee, S.; Katiyar, P.; Kumar, V.; Waghmode, B.; Nathani, S.; Krishnan, V.; Sircar, D.; Roy, P. Wheatgrass inhibits the lipopolysaccharide-stimulated inflammatory effect in RAW 264.7 macrophages. Curr. Res. Toxicol. 2021, 2, 116–127. [Google Scholar]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int. J. Food Sci. Nutr. 2012, 63, 718–721. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.W.; Lee, Y.M.; Lee, H.S.; Kim, D.K. Polysaccharide isolated from Triticum aestivum stimulates insulin release from pancreatic cells via the ATP-sensitive K+ channel. Int. J. Mol. Med. 2012, 29, 913–919. [Google Scholar]
- Nepali, S.; Ki, H.H.; Lee, J.H.; Lee, H.Y.; Kim, D.K.; Lee, Y.M. Wheatgrass-derived polysaccharide has antiinflammatory, anti-oxidative and anti-apoptotic effects on lps-induced hepatic injury in mice. Phytother. Res. 2017, 31, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Nepali, S.; Ki, H.H.; Lee, J.H.; Cha, J.Y.; Lee, Y.M.; Kim, D.K. Triticum aestivum sprout-derived polysaccharide exerts hepatoprotective effects against ethanol-induced liver damage by enhancing the antioxidant system in mice. Int. J. Mol. Med. 2017, 40, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudel, B.; Nepali, S.; Xin, M.; Ki, H.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. Flavonoids from Triticum aestivum inhibit adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol. Med. Rep. 2015, 12, 3139–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Ki, H.H.; Kim, D.K.; Lee, Y.M. Triticum aestivum sprout extract attenuates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice and the expression of chemokines in human keratinocytes. Mol. Med. Rep. 2018, 18, 3461–3468. [Google Scholar] [CrossRef] [Green Version]
- Ki, H.H.; Hwang, S.W.; Lee, J.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. A dichloromethane fraction of Triticum aestivum sprouts reduces allergic immune response through inhibiting Th2 differentiation in ovalbumin immunized mice. Mol. Med. Rep. 2017, 16, 3535–3541. [Google Scholar] [CrossRef] [Green Version]
- Ben Arye, E.; Goldin, E.; Wengrower, D.; Stamper, A.; Kohn, R.; Berry, E. Wheat grass juice in the treatment of active distal ulcerative colitis: A randomized double blind placebo controlled trial. Scand. J. Gastroenterol. 2002, 37, 444–449. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal Plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Wei, Y.; Hao, J.; Lei, Y.; Zhao, W.; Xiao, Y.; Sun, A. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. microbiota and improves lipid metabolism in diet-induced obese rats. Nutr. Metab. 2020, 17, 54. [Google Scholar] [CrossRef]
- Yang, H.; Oh, K.H.; Yoo, Y.C. Anti-Inflammatory Effect of Hot Water Extract of Aronia Fruits in LPS-Stimulated RAW 264.7 Macrophages. J. Korean Soc. Food Sci. Nut. 2015, 44, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Carle, R.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) concentrate inhibits NF-κB and synergizes with selenium to inhibit the release of pro-inflammatory mediators in macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar]
- King, E.S.; Bolling, B.W. Composition, polyphenol bioavailability, and health benefits of aronia berry: A review. J. Food Bioact. 2020, 11, 13–30. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food. 2010, 13, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lim, J.Y.; Jeon, Y.D.; Yun, D.H.; Lee, Y.M.; Kim, D.K. Extract of wheatgrass and aronia mixture ameliorates atopic dermatitis-related symptoms by suppressing inflammatory response and oxidative stress in vitro and in vivo. Antioxidants 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [PubMed]
- Yoo, J.M.; Yang, J.H.; Kim, Y.S.; Cho, W.K.; Ma, J.Y. Inhibitory effect of Loranthus parasiticus on IgE-mediated allergic responses in RBL-2H3 Cells. Mediators. Inflamm. 2016, 2016, 8742562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.T.; Kim, H.W.; Kim, H.S.; Park, Y.H.; Lee, D.; Lee, M.B.; Min, K.Y.; Kim, Y.M.; Choi, W.S. Furaltadone suppresses IgE-mediated allergic response through the inhibition of Lyn/Syk pathway in mast cells. Eur. J. Pharmacol. 2018, 828, 119–125. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, J.H.; Lee, B.R.; Kim, M.A.; Lee, Y.M.; Kim, D.K.; Choi, J.K. Extract of Boehmeria nivea suppresses mast cell-mediated allergic inflammation by inhibiting mitogen-activated protein kinase and nuclear factor-κB. Molecules 2020, 25, 4178. [Google Scholar] [CrossRef]
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93–104. [Google Scholar] [CrossRef]
- Akin, C. Mast cell activation disorders. J. Allergy Clin. Immunol. Pract. 2014, 2, 252–258. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Peavy, R.D.; Gilfillan, A.M. Mechanisms of mast cell signaling in anaphylaxis. J. Allergy Clin. Immunol. 2009, 124, 639–646. [Google Scholar]
- Dong, F.; Tan, J.; Zheng, Y. Chlorogenic Acid Alleviates Allergic Inflammatory Responses through Regulating Th1/Th2 Balance in Ovalbumin-Induced Allergic Rhinitis Mice. Med. Sci. Monit. 2020, 26, e923358. [Google Scholar] [CrossRef] [PubMed]
- Azusa, K.; Takashi, H.; Toshio, J. Inhibitory Effect of γ-Aminobutyric Acid (GABA) on Histamine Release from Rat Basophilic Leukemia RBL-2H3 Cells and Rat Peritoneal Exudate Cells. J. Jpn. Soc. Food Sci. Technol.-Nippon Shokuhin Kagaku Kogaku Kaishi 2014, 61, 362–366. [Google Scholar]
- Fukuishi, N.; Murakami, S.; Ohno, A.; Yamanaka, N.; Matsui, N.; Fukutsuji, K.; Yamada, S.; Itoh, K.; Akagi, M. Does βhexosaminidase function only as a degranulation indicator in mast cells? The primary role of β-hexosaminidase in mast cell granules. J. Immunol. 2014, 193, 1886–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Kempuraj, D.; Di Gioacchino, M.; Boucher, W.; Letourneau, R.; Kandere, K.; Barbacane, R.C.; Reale, M.; Felaco, M.; Frydas, S.; et al. Interleukin-6 and mast cells. Allergy. Asthma. Proc. 2002, 23, 331–335. [Google Scholar] [PubMed]
- Azzolina, A.; Guarneri, P.; Lampiasi, N. Involvement of p38 and JNK MAPKs pathways in substance P-induced production of TNF-alpha by peritoneal mast cells. Cytokine 2002, 18, 72–80. [Google Scholar]
- Frisk, J.M.H.; Kjellén, L.; Melo, F.R.; Öhrvik, H.; Pejler, G. Mitogen-Activated Protein Kinase Signaling Regulates Proteoglycan Composition of Mast Cell Secretory Granules. Front. Immunol. 2018, 9, 2018. [Google Scholar] [CrossRef]
- Marquardt, D.L.; Walker, L.L. Dependence of mast cell IgE-mediated cytokine production on nuclear factor-kappaB activity. J. Allergy Clin. Immunol. 2000, 105, 500–505. [Google Scholar] [CrossRef]
- Zhao, W.; Gan, X.; Su, G.; Wanling, G.; Li, S.; Hei, Z.; Yang, C.; Wang, H. The Interaction between Oxidative Stress and Mast Cell Activation Plays a Role in Acute Lung Injuries Induced by Intestinal Ischemia–Reperfusion. J. Surg. Res. 2014, 187, 542–552. [Google Scholar] [CrossRef]
- Kalesnikoff, J.; Galli, S.J. New developments in mast cell biology. Nat. Immunol. 2008, 9, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Koyasu, S. The role of PI3K in immune cells. Nat. Immunol. 2003, 4, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.; Killoran, K.E.; Mitre, E. Measuring local anaphylaxis in mice. J. Vis. Exp. 2014, 92, e52005. [Google Scholar]


| Gene | Forward | Reverse |
|---|---|---|
| mTNF-α | TAGCCAGGAGGGAGAACAGA | TTTTCTGGAGGGAGATGTGG |
| mIL-1β | CTCCATGAGCTTTGTACAAGG | TGCTGATGTACCAGTTGGGG |
| hTNF-α | TTGGAGTGATCGGCCCCCAG | ACAGGCTTGTCACTCGGGGTT |
| hIL-1β | CAGCTCTCTCCTTTCAGGGCCA | GGCCGTGGTTTCTGTCAGGC |
| rTNF-α | GAAAGCATGATCCGAGATGTGG | TCATACCAGGGCTTGAGCTCA |
| rIL-1β | CCCTGCAGCTGGAGAGTGTGG | TGTGCTCTGCTTGAGTGCT |
| rIL-6 | GGAGACTTCACAGAGGATAC | CCATTAGGAGAGCATTGGAAG |
| rIL-4 | ACCCTGTTCTGCTTTCTC | GTTCTCCGTGGTGTTCCT |
| mGAPDH | CATGGCCTTCCGTGTTC | CCTGGTCCTCAGTGTAGC |
| hGAPDH | GAAGGTGAAGGTCGGAGT | GAAGATGGTGATGGGATTTC |
| rGAPDH | AACGGCACAGTCAAGGCTGA | ACGCCAGTAGACTCCACGACAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Lim, J.-Y.; Jeon, Y.-D.; Yun, D.-H.; Lee, Y.-M.; Kim, D.-K. Wheatgrass-and-Aronia-Mixed Extract Suppresses Immunoglobulin E-Mediated Allergic Reactions In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 11979. https://doi.org/10.3390/ijms241511979
Lee J-H, Lim J-Y, Jeon Y-D, Yun D-H, Lee Y-M, Kim D-K. Wheatgrass-and-Aronia-Mixed Extract Suppresses Immunoglobulin E-Mediated Allergic Reactions In Vitro and In Vivo. International Journal of Molecular Sciences. 2023; 24(15):11979. https://doi.org/10.3390/ijms241511979
Chicago/Turabian StyleLee, Ji-Hyun, Ji-Ye Lim, Yong-Deok Jeon, Dae-Ho Yun, Young-Mi Lee, and Dae-Ki Kim. 2023. "Wheatgrass-and-Aronia-Mixed Extract Suppresses Immunoglobulin E-Mediated Allergic Reactions In Vitro and In Vivo" International Journal of Molecular Sciences 24, no. 15: 11979. https://doi.org/10.3390/ijms241511979




