Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future
Abstract
1. Introduction
2. Taxonomy and Geographical Distribution
3. Phytochemistry
CMG Composition
4. Inflammatory Bowel Disease
Effects of Pistacia lentiscus on IBD
| Study (Authors, Year) | Design | Effect |
|---|---|---|
| Animal Models | ||
| [80] Giovxari et al., 2011 | -Colitic rat model induced with TNBS assigned to seven groups: A, control; B, colitic; C–F, colitic rats treated daily with PL powder at 50, 100, 200, and 300 mg/kg/day, respectively; and G, colitic rats treated daily with cortisone (25 μg/kg) | -TNF-α, ICAM-1, IL-6, IL-8, and IL-10 ↓ -Damage ↓ -Malonaldehyde ↓ |
| [83] Papalois et al., 2012 | -Colitic rat model induced with TNBS -Treated with Chios mastic (CM) powder at 100 mg/kg/day or the respective CM components: inulin (40 mg/kg/day); acidic fraction (AF- 24 or 48 mg/kg/day); neutral fractions (NF- 24 or 48 mg/kg/day); and oleanolic acid (OA-14 mg/kg/day) for 5 days | -TNF-α, ICAM-1, IL-6, and IL-8, ↓ -CM: histologic improvement and regulation of inflammation -AF and NF: no histologic improvement, inflammatory markers reduction |
| [84] Naouar et al., 2016 | -Colitic rat model induced with TNBS -Treated with PL mastic oil at a 30 mg of oil/100 g of feed/rat/day for 2 months before colitis induction | -Weight loss, rectal bleeding, diarrhea, ulceration, hyperplasia, and cryptitis ↓ |
| [85] Ostovan et al., 2020 | -Control, colitis without treatment, and colitis induced with 3% acetic acid rat models were treated with (i) PL mastic oil, 400 mg/kg/daily, administered orally or intra-rectally; (ii) prednisolone 5 mg/kg/day; or (iii) sesame oil 2 mL/kg/day for 7 days | -TNF-α ↓ (as prednisolone) -= IL6 (as sesame oil) |
| [86] Boutemine et al., 2021 | -Control, colitis without treatment, and colitic induced with 3% dextran sulfate sodium rat models were treated with aqueous extract of PL leaves 50, 100 or 200 mg/Kg/day, respectively, for 7 days | -Activation and recruitment of immune cells ↓ (cellular level) -Blockade of pro-inflammatory cytokine receptors (membrane level) -NO, IL-6, and TNF-α ↓ -Pro-inflammatory cytokines (intracellular level) |
| [88] Cui et al., 2023 | -Acute colitis mouse model, induced with dextran sulphate sodium -Colitic rat group were treated with Masticadienonic acid (MDA), one of the most abundant constituents isolated from Chios mastic gum, solubilized with 30% PEG-400 at (i) low-dose MDA, 10 mg/kg/day, or (ii) high-dose MDA, 100 mg/kg/day, for 14 days. | -Body weight, colon length, disease activity index, and histologic score ↓ -TNFα, IL-1β and IL-6 ↓ -Intestinal barrier function by Nrf2 ↑ _ restoring ZO-1 and occluding tight junction proteins -Modulation of the composition of the intestinal microbiota |
| Cellular model | ||
| [81] Papalois et al., 2012 | -Inflammation model in co-cultured human colon epithelial HT29 cells and monocytes/macrophages | -IL-8 and NF-jB p65 ↓ -LDH↓ |
| Clinical Trials | ||
| [89] Kaliora et al., 2017 | -10 patients with active CD and 8 healthy controls -Treated with mastic caps 6 caps/day, 0.37 g/cap for 4 weeks | -CD activity index ↓ -IL-6 and CRP ↓ -TNF-α and MCP-1 ↓ not significant -Total antioxidant potential ↑ |
| [90] Kaliora et al., 2017 | -10 patients with active CD and 8 healthy controls -Treated with mastic caps, 6 caps/d, 0.37 g/cap for 4 weeks | -TNF-α secretion in PBMC ↓ -MIF ↓ PBMC -No significant changes in IL-6, MCP-1 |
| [94] Papada et al., 2018 [94] Papada et al., 2019 | -60 patients with IBD -Treated with 2.8 g of mastic daily for 3 months or placebo randomized | -Improvement in IBDQ - oxLDL ↓ -Plasma cysteine and fecal lysozyme ↓ -No impact on serum IL-6, fecal calprotectin and fecal lactoferrin |
| [98] Amerikanou et al., 2021 | -129 patients with IBD—67 randomized to mastic group and 62 to placebo -Treated with 2.8 g daily for 6 months for patients in remission and for 3 months for patients in relapse | IL-17A ↑ |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DerMarderosian, A.; Beutler, J.A. (Eds.) The Review of Natural Products: The Most Complete Source of Natural Product Information, 8th ed.; Lippincott Williams & Wilkins: Saint Louis, MO, USA, 2014. [Google Scholar]
- Buckley, S.A.; Evershed, R.P. Organic chemistry of embalming agents in Pharaonic and Graeco-Roman mummies. Nature 2001, 413, 837–841. [Google Scholar] [CrossRef]
- Avicenna. The Canon; Shrafkandi, A., Translator; Soroush: Tehran, Iran, 2008. [Google Scholar]
- Ardakani, S.M.R.; Farjadmand, F.; Rahimi, R. Makhzan al Adviyeh and Pointing to the Scientific Names of Medicinal Plants for the First Time in a Persian Book. Tradit. Integr. Med. 2018, 3, 186–195. [Google Scholar]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi, S.; Mohammad, H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [PubMed]
- Tous, J.; Ferguson, L. Mediterranean fruits. Prog. New Crops 1996, 416, 416–430. [Google Scholar]
- Durmaz, G.; Gökmen, V. Changes in oxidative stability, antioxidant capacity and phytochemical composition of Pistacia terebinthus oil with roasting. Food Chem. 2011, 128, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Britannica, T. Editors of Encyclopaedia. Pistacia. Encyclopedia Britannica. 21 November 2018. Available online: https://www.britannica.com/plant/Pistacia (accessed on 15 January 2023).
- Bai, Q.; Ma, Z.; Zhang, Y.; Su, S.; Leng, P. The sex expression and sex determining mechanism in Pistacia species. Breed. Sci. 2019, 69, 205–214. [Google Scholar] [CrossRef]
- Al-Saghir, M.G.; Porter, D.M. Taxonomic Revision of the Genus Pistacia L. (Anacardiaceae). Am. J. Plant Sci. 2012, 3, 12–32. [Google Scholar] [CrossRef]
- Raven, P.H.; Eichhorn, S.E.; Evert, R.F. Biology of Plants, 8th ed.; Freeman: New York, NY, USA, 2013. [Google Scholar]
- Christodoulakis, N.S.; Georgoudi, M.; Fasseas, C. Leaf Structure of Cistus creticus L. (Rock Rose), a Medicinal Plant Widely Used in Folk Remedies Since Ancient Times. J. Herbs Spices Med. Plants 2014, 20, 103–114. [Google Scholar] [CrossRef]
- Glampedaki, P.; Dutschk, V. Stability studies of cosmetic emulsions prepared from natural products such as wine, grape seed oil and mastic resin. Colloid Surf. A-Physicochem. Eng. Asp. 2014, 460, 306–311. [Google Scholar] [CrossRef]
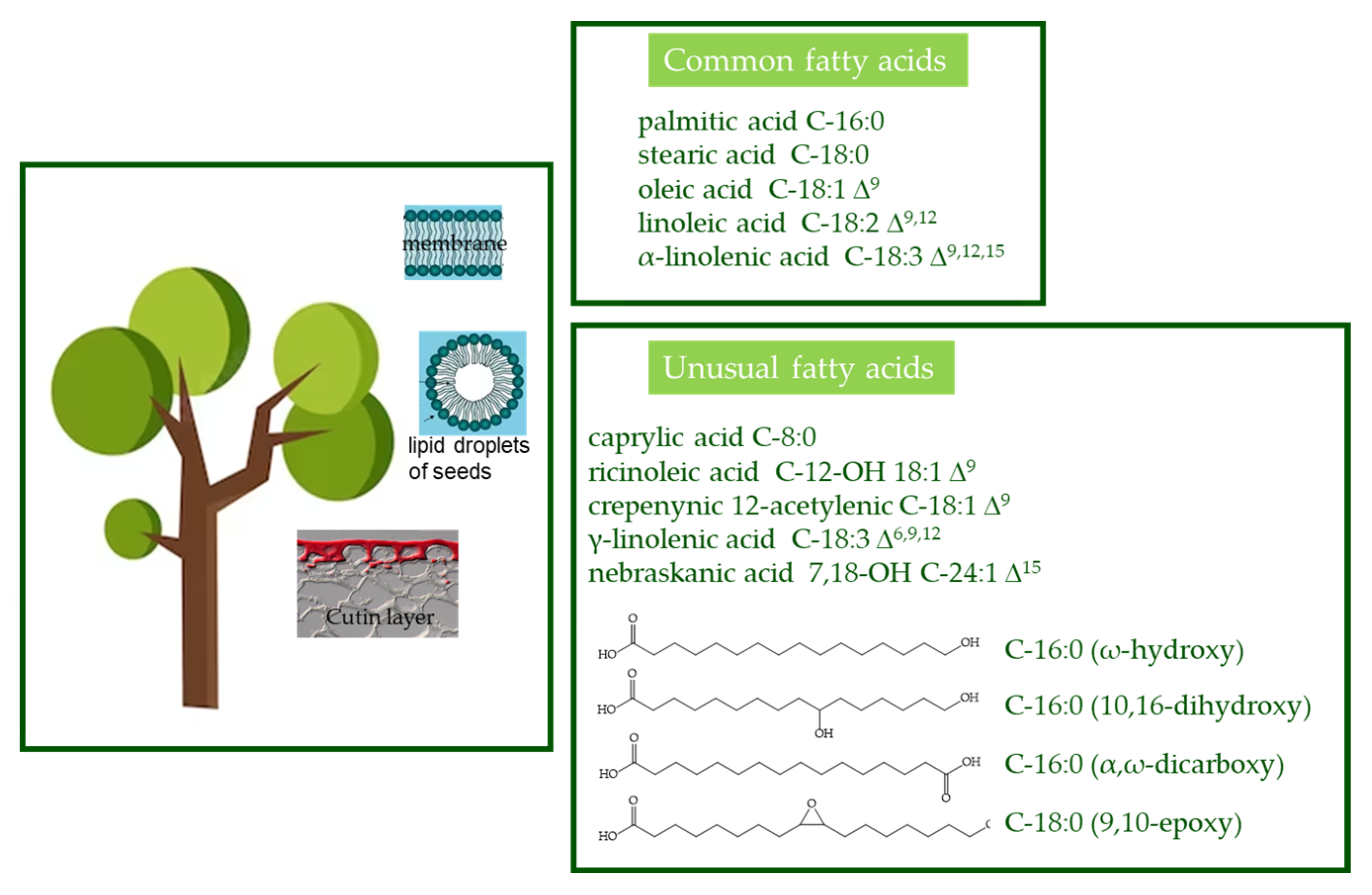
- Horn, P.J.; Benning, C. The plant lipidome in human and environmental health. Science 2016, 353, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2013, 2013, e0161. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Thrower, N.; Mhaske, V.; Stymne, S.; Baxter, M.; Yang, W.; Liu, J.; Shaw, K.; Shorrosh, B.; Zhang, M.; et al. PlantFAdb: A resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J. 2018, 96, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Satil, F.; Azcan, N.; Baser, K.H.C. Fatty Acid Composition of Pistachio Nuts in Turkey. Chem. Nat. Compd. 2003, 39, 322–324. [Google Scholar] [CrossRef]
- Farhoosh, R.; Tavakoli, J.; Khodaparast, M.H.H. Chemical Composition and Oxidative Stability of Kernel Oils from Two Current Subspecies of Pistacia atlantica in Iran. J. Am. Oil Chem. Soc. 2008, 85, 723–729. [Google Scholar] [CrossRef]
- Kizil, S.; Turk, M. Microelement contents and fatty acid compositions of Rhus coriaria L. and Pistacia terebinthus L. fruits spread commonly in the south eastern Anatolia region of Turkey. Nat. Prod. Res. 2010, 24, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, H.; Cherif, O.A.; Sakouhi, F.; Villeneuve, P.; Renaud, J.; Barouh, N.; Boukhchina, S.; Mayer, P. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chem. 2012, 131, 434–440. [Google Scholar] [CrossRef]
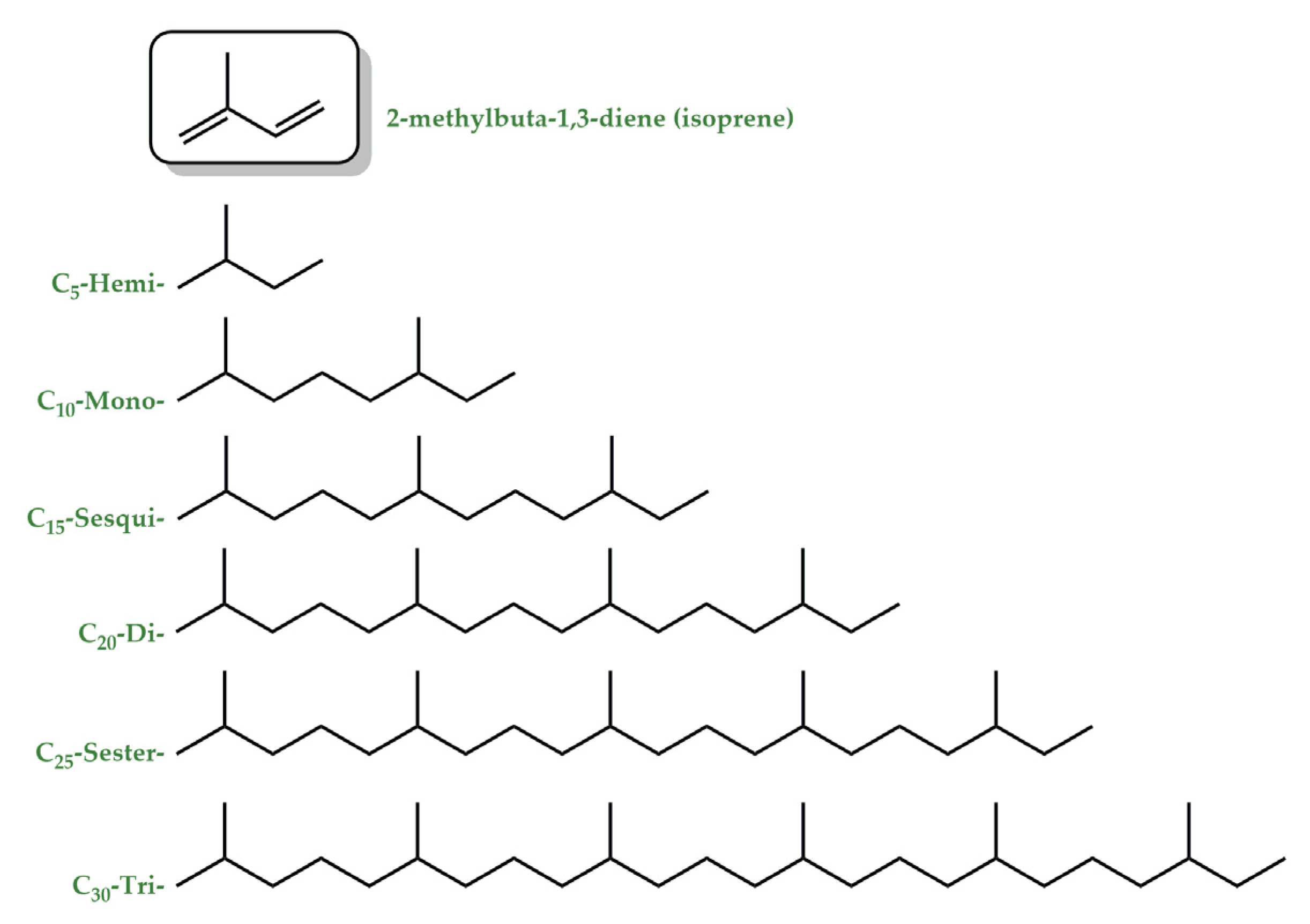
- Guimarães, A.G.; Serafini, M.R.; Quintans-Júnior, L.J. Terpenes and derivatives as a new perspective for pain treatment: A patent review. Expert Opin. Ther. Pat. 2014, 24, 243–265. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Theis, N.; Lerdau, M. The Evolution of Function in Plant Secondary Metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Vincent, N.; Lin, Z.; Jianpei, Y.; Zhenchao, F.; Tengfeng, Y.; Hongmei, Z. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Qiu, C.; Smuts, J.; Schug, K.A. Analysis of terpenes and turpentines using gas chromatography with vacuum ultraviolet detection. J. Sep. Sci. 2017, 40, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, F.; Ruiz, F. An optimal control approach to steam distillation of essential oils from aromatic plants. Comput. Chem. Eng. 2018, 117, 25–31. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Fotsing, Y.S.F.; Kezetas, J.J.B. Terpenoids as Important Bioactive Constituents of Essential Oils. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; Khan, M.S., Ed.; IntechOpen Limited: London, UK, 2020. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2016, 7, 2161. [Google Scholar] [CrossRef]
- Voon, H.C.; Bhat, R.; Rusul, G. Flower Extracts and Their Essential Oils as Potential Antimicrobial Agents for Food Uses and Pharmaceutical Applications. Compr. Rev. Food. Sci. Food Saf. 2012, 11, 34–55. [Google Scholar] [CrossRef]
- Souilah, N.; Amina, B.; Hamdi, B.; Miara, M.D.; Daoud, N.; Mustafa, A.M.; Yilmaz, M.A.; Öztürk, M.; Caprioli, G.; Maggi, F. Ethnobotanical investigation of Pistacia lentiscus L. grown in El Kala (Algeria), and phytochemical study and antioxidant activity of its essential oil and extracts. Nat. Prod. Res. 2023, 37, 1583–1588. [Google Scholar] [CrossRef]
- Sehaki, C.; Jullian, N.; Choque, E.; Dauwe, R.; Fontaine, J.X.; Molinie, R.; Ayati, F.; Fernane, F.; Gontier, E. Profiling of Essential Oils from the Leaves of Pistacia lentiscus Collected in the Algerian Region of Tizi-Ouzou: Evidence of Chemical Variations Associated with Climatic Contrasts between Littoral and Mountain Samples. Molecules 2022, 27, 4148. [Google Scholar] [CrossRef] [PubMed]
- Ailli, A.; Handaq, N.; Touijer, H.; Gourich, A.A.; Drioiche, A.; Zibouh, K.; Eddamsyry, B.; El Makhoukhi, F.; Mouradi, A.; Bin Jardan, Y.A.; et al. Phytochemistry and Biological Activities of Essential Oils from Six Aromatic Medicinal Plants with Cosmetic Properties. Antibiotics 2023, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Allaw, M.; Manconi, M.; Caboni, P.; Bacchetta, G.; Escribano-Ferrer, E.; Peris, J.E.; Nacher, A.; Diez-Sales, O.; Manca, M.L. Formulation of liposomes loading lentisk oil to ameliorate topical delivery, attenuate oxidative stress damage and improve cell migration in scratch assay. Biomed. Pharmacother. 2021, 144, 112351. [Google Scholar] [CrossRef]
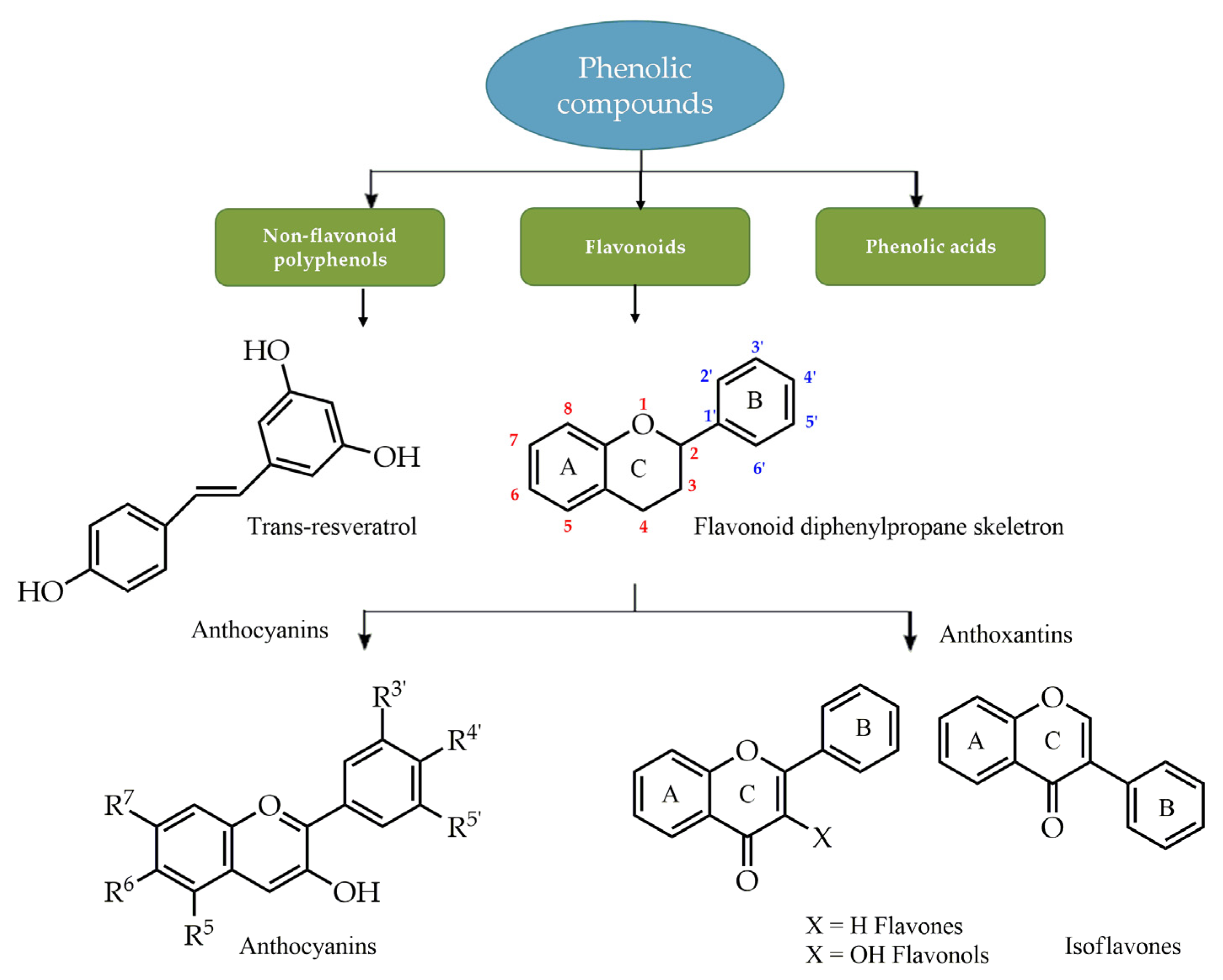
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem.-Int. Edit. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Ghani, U. Polyphenols. In Alpha-Glucosidase Inhibitors, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–100. [Google Scholar]
- Missoun, F.; Bouabedelli, F.; Benhamimed, E.; Baghdad, A.; Djebli, N. Phytochemical study and antibacterial activity of different extracts of Pistacia lentiscus L collected from Dahra Region West of Algeria. Malays. J. Fundam. Appl. Sci. 2017, 9, 669. [Google Scholar] [CrossRef][Green Version]
- Yosr, Z.; Imen, B.H.Y.; Rym, J.; Chokri, M.; Mohamed, B. Sex-related differences in essential oil composition, phenol contents and antioxidant activity of aerial parts in Pistacia lentiscus L. during seasons. Ind. Crop. Prod. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Barbouchi, M.; Elamrani, K.; El Idrissi, M.; Choukrad, M. A comparative study on phytochemical screening, quantification of phenolic contents and antioxidant properties of different solvent extracts from various parts of Pistacia lentiscus L. J. King Saud Univ.-Sci. 2020, 32, 302–306. [Google Scholar] [CrossRef]
- Alhadad, A.O.; Salem, G.S.; Elmhdwi, M.F.; Hussein, S.M.; Elshareef, S.M. Assessments of Antibacterial and Antioxidant Properties in the Methanolic and Aqueous Leaf Extracts of Pistacia lentiscus against Different Antibiotic Resistance Pathogenic Bacteria. Adv. Biosci. Biotechnol. 2022, 13, 113–133. [Google Scholar] [CrossRef]
- Dellai, A.; Souissi, H.; Borgi, W.; Bouraoui, A.; Chouchane, N. Antiinflammatory and antiulcerogenic activities of Pistacia lentiscus L. leaves extracts. Ind. Crop. Prod. 2013, 49, 879–882. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.-L. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef]
- Vuorinen, A.; Seibert, J.; Papageorgiou, V.P.; Rollinger, J.M.; Odermatt, A.; Schuster, D.; Assimopoulou, A.N. Pistacia lentiscus Oleoresin: Virtual Screening and Identification of Masticadienonic and Isomasticadienonic Acids as Inhibitors of 11β-Hydroxysteroid Dehydrogenase 1. Planta Med. 2015, 81, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Bakola-Christianopoulou, M.N.; Apazidou, K.K.; Psarros, E.E. Gas chromatographic–mass spectroscopic analysis of the acidic triterpenic fraction of mastic gum. J. Chromatogr. A 1997, 769, 263–273. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part I. Pistacia lentiscus var. Chia. Biomed. Chromatogr. 2005, 19, 285–311. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part II. Pistacia terebinthus var. Chia. Biomed. Chromatogr. 2005, 19, 586–605. [Google Scholar] [CrossRef] [PubMed]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef]
- Daferera, D.; Pappas, C.; Tarantilis, P.A.; Polissiou, M. Quantitative analysis of α-pinene and β-myrcene in mastic gum oil using FT-Raman spectroscopy. Food Chem. 2002, 77, 511–515. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Papanicolaou, D.; Melanitou, M.; Katsaboxakis, K. Changes in chemical composition of the essential oil of Chios “mastic resin” from Pistacia lentiscus var. Chia tree during solidification and storage. In Food Flavors: Generation, Analysis and Process Influence, Proceedings of the 8th International Flavor Conference, Cos, Greece, 6–8 July 1994; Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 303–310. [Google Scholar]
- Xynos, N.; Termentzi, A.; Fokialakis, N.; Skaltsounis, L.A.; Aligiannis, N. Supercritical CO2 extraction of mastic gum and chemical characterization of bioactive fractions using LC-HRMS/MS and GC–MS. J. Supercrit. Fluids 2018, 133, 349–356. [Google Scholar] [CrossRef]
- van den Berg, K.J.; van der Horst, J.; Boon, J.J.; Sudeiijer, O.O. Cis-1,4-poly-β-myrcene; the structure of the polymeric fraction of mastic resin (Pistacia lentiscus L.) elucidated. Tetrahedron Lett. 1998, 17, 2645–2648. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Mylona, A.; Chiou, A.; Petsios, D.G.; Andrikopoulos, N.K. Detection and Identification of Simple Phenolics in Pistacia lentiscus Resin. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 289–300. [Google Scholar] [CrossRef]
- Kivçak, B.; Akay, S. Quantitative determination of alpha-tocopherol in Pistacia lentiscus, Pistacia lentiscus var. chia, and Pistacia terebinthus by TLC-densitometry and colorimetry. Fitoterapia 2005, 76, 62–66. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Shelling, A.N.; Browning, B.L.; Huebner, C.; Petermann, I. Genes, diet and inflammatory bowel disease. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2007, 622, 70–83. [Google Scholar] [CrossRef]
- Damman, C.J.; Miller, S.I.; Surawicz, C.M.; Zisman, T.L. The microbiome and inflammatory bowel disease: Is there a therapeutic role for fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1452–1459. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Kim, D.H.; Cheon, J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef]
- Rogler, G.; Biedermann, L.; Scharl, M. New insights into the pathophysiology of inflammatory bowel disease: Microbiota, epigenetics and common signalling pathways. Swiss Med. Wkly. 2018, 148, w14599. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef]
- Lin, S.C.; Cheifetz, A.S. The Use of Complementary and Alternative Medicine in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2018, 14, 415–425. [Google Scholar]
- Seminerio, J. Complementary and Alternative Medicine in Crohn’s Disease. Gastroenterol. Clin. N. Am. 2022, 51, 337–351. [Google Scholar] [CrossRef]
- Torres, J.; Ellul, P.; Langhorst, J.; Mikocka-Walus, A.; Acosta, M.B.-D.; Basnayake, C.; Ding, N.J.S.; Gilardi, D.; Katsanos, K.; Moser, G.; et al. European Crohn’s and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 673–685e. [Google Scholar] [CrossRef]
- Czigle, S.; Bittner, F.S.; Tóth, J.; Mučaji, P.; Nagy, M.; On, B.O. The Oemonom Treatment of Gastrointestinal Disorders-Plants and Potential Mechanisms of Action of Their Constituents. Molecules 2022, 27, 2881. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Phytotherapies in inflammatory bowel disease. J. Res. Med. Sci. 2019, 24, 42. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Goulas, V.; Gekas, V. Health-Promoting Effects of Pistacia Resins: Recent Advances, Challenges, and Potential Applications in the Food Industry. Food Rev. Int. 2015, 31, 1–12. [Google Scholar] [CrossRef]
- Mahjoub, F.; Akhavan, R.K.; Yousefi, M.; Mohebbi, M.; Salari, R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J. Med. Life 2018, 11, 180–186. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.K. Effects of Chios mastic gum on cardiometabolic risk factors. World J. Diabetes 2022, 13, 921–925. [Google Scholar] [CrossRef]
- Rohwer, N.; Jelleschitz, J.; Höhn, A.; Weber, D.; Kühl, A.A.; Wang, C.; Ohno, R.-I.; Kampschulte, N.; Pietzner, A.; Schebb, N.H.; et al. Prevention of colitis-induced liver oxidative stress and inflammation in a transgenic mouse model with increased omega-3 polyunsaturated fatty acids. Redox Biol. 2023, 64, 102803. [Google Scholar] [CrossRef]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega3 fatty acids correlate with gut microbiome diversity and production of Ncarbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef]
- Deol, P.; Ruegger, P.; Logan, G.D.; Shawki, A.; Li, J.; Mitchell, J.D.; Yu, J.; Piamthai, V.; Radi, S.H.; Hasnain, S.; et al. Diet high in linoleic acid dysregulates the intestinal endocannabinoid system and increases susceptibility to colitis in Mice. Gut Microbes 2023, 15, 2229945. [Google Scholar] [CrossRef]
- Rodríguez, R.R.; Johnson, J.J. Terpenes: Modulating anti-inflammatory signaling in inflammatory bowel disease. Pharmacol. Ther. 2023, 248, 108456. [Google Scholar] [CrossRef]
- Jamieson, P.E.; Carbonero, F.; Stevens, J.F. Dietary (poly)phenols mitigate inflammatory bowel disease: Therapeutic targets, mechanisms of action, and clinical observations. Cur. Res. Food Sci. 2023, 6, 100521. [Google Scholar] [CrossRef]
- Gioxari, A.; Kaliora, A.C.; Papalois, A.; Agrogiannis, G.; Triantafillidis, J.K.; Andrikopoulos, N.K. Pistacia lentiscus resin regulates intestinal damage and inflammation in trinitrobenzene sulfonic acid-induced colitis. J. Med. Food 2011, 14, 1403–1411. [Google Scholar] [CrossRef]
- Neurath, M.F.; Fuss, I.; Pasparakis, M.; Alexopoulou, L.; Haralambous, S.; Meyer zum Buschenfelde, K.-H.; Strobe, W.; Kollias, G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur. J. Immunol. 1997, 27, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar] [CrossRef]
- Papalois, A.; Gioxari, A.; Kaliora, A.C.; Lymperopoulou, A.; Agrogiannis, G.; Papada, E.; Andrikopoulos, N.K. Chios mastic fractions in experimental colitis: Implication of the nuclear factor κB pathway in cultured HT29 cells. J. Med. Food 2012, 15, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Naouar, M.S.; Mekki, L.Z.; Charfi, L.; Boubaker, J.; Filali, A. Preventive and curative effect of Pistacia lentiscus oil in experimental colitis. Biomed. Pharmacother. 2016, 83, 577–583. [Google Scholar] [CrossRef]
- Ostovan, M.; Fazljou, S.M.B.; Khazraei, H.; Araj, K.M.; Torbati, M. The Anti-Inflammatory Effect of Pistacia lentiscus in a Rat Model of Colitis. J. Inflamm. Res. 2020, 13, 369–376. [Google Scholar] [CrossRef]
- Boutemine, I.-M.; Amri, M.; Dorgham, K.; Amir, Z.-C.; Benazzouz, S.; Ameur, F.; Layaida, K.; Yssel, H.; Touil-Boukoffa, C. Beneficial role of Pistacia lentiscus aqueous extract in experimental colitis: Anti-inflammatory and potential therapeutic effects. Inflammopharmacology 2021, 29, 1225–1239. [Google Scholar] [CrossRef]
- Pardis, G.; Saideh, M.; Zahra, R.; Mahban, R.; Maryam, B.; Alireza, A.; Mohammad, A. Protective Effect of a Formulation Containing Pistacia atlantica Oleo-Gum-Resin and Honey on Experimental Model of Acetic Acid-Induced Colitis in Rats. Res. J. Pharmacogn. 2021, 8, 37–49. [Google Scholar] [CrossRef]
- Cui, H.; Li, X.; An, X.-R.; Liu, W.; Yuan, T. Masticadienonic acid from Chios mastic gum mitigates colitis in mice via modulating inflammatory response, gut barrier integrity and microbiota. Phytomedicine 2023, 108, 154518. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.Z.; Andrikopoulos, N.K. Chios mastic treatment of patients with active Crohn’s disease. World J. Gastroenterol. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.Z.; Andrikopoulos, N.K. Alterations in the function of circulating mononuclear cells derived from patients with Crohn’s disease treated with mastic. World J. Gastroenterol. 2007, 13, 6031–6036. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Gioxari, A.; Amerikanou, C.; Forbes, A.; Tzavara, C.; Smyrnioudis, I.; Kaliora, A.C. (Regulation of faecal biomarkers in inflammatory bowel disease patients treated with oral mastiha (Pistacia lentiscus) supplement: A double-blind and placebo-controlled randomised trial. Phytother. Res. 2019, 33, 360–369. [Google Scholar] [CrossRef]
- Alrubaiy, L.; Rikaby, I.; Dodds, P.; Hutchings, H.A.; Williams, J.G. Systematic review of health-related quality of life measures for inflammatory bowel disease. J. Crohn’s Colitis 2015, 9, 284–292. [Google Scholar] [CrossRef]
- van der Sluys Veer, A.; Brouwer, J.; Biemond, I.; Bohbouth, G.E.; Verspaget, H.W.; Lamers, C.B. Fecal lysozyme in assessment of disease activity in inflammatory bowel disease. Dig. Dis. Sci. 1998, 43, 590–595. [Google Scholar] [CrossRef]
- Papada, E.; Forbes, A.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Triantafillidis, J.K.; Kaliora, A.C. Antioxidative Efficacy of a Pistacia lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1779. [Google Scholar] [CrossRef]
- Papada, E.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Forbes, A.; Kaliora, A.C. Plasma free amino acid profile in quiescent Inflammatory Bowel Disease patients orally administered with Mastiha (Pistacia lentiscus); a randomised clinical trial. Phytomedicine 2019, 56, 40–47. [Google Scholar] [CrossRef]
- Nakaya, M.; Xiao, Y.; Zhou, X.; Chang, J.-H.; Chang, M.; Cheng, X.; Blonska, M.; Lin, X.; Sun, S.-C. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 2014, 40, 692–705. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, C.-A.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef]
- Amerikanou, C.; Dimitropoulou, E.; Gioxari, A.; Papada, E.; Tanaini, A.; Fotakis, C.; Zoumpoulakis, P.; Kaliora, A.C. Linking the IL-17A immune response with NMR-based faecal metabolic profile in IBD patients treated with Mastiha. Biomed. Pharmacother. 2021, 138, 111535. [Google Scholar] [CrossRef]

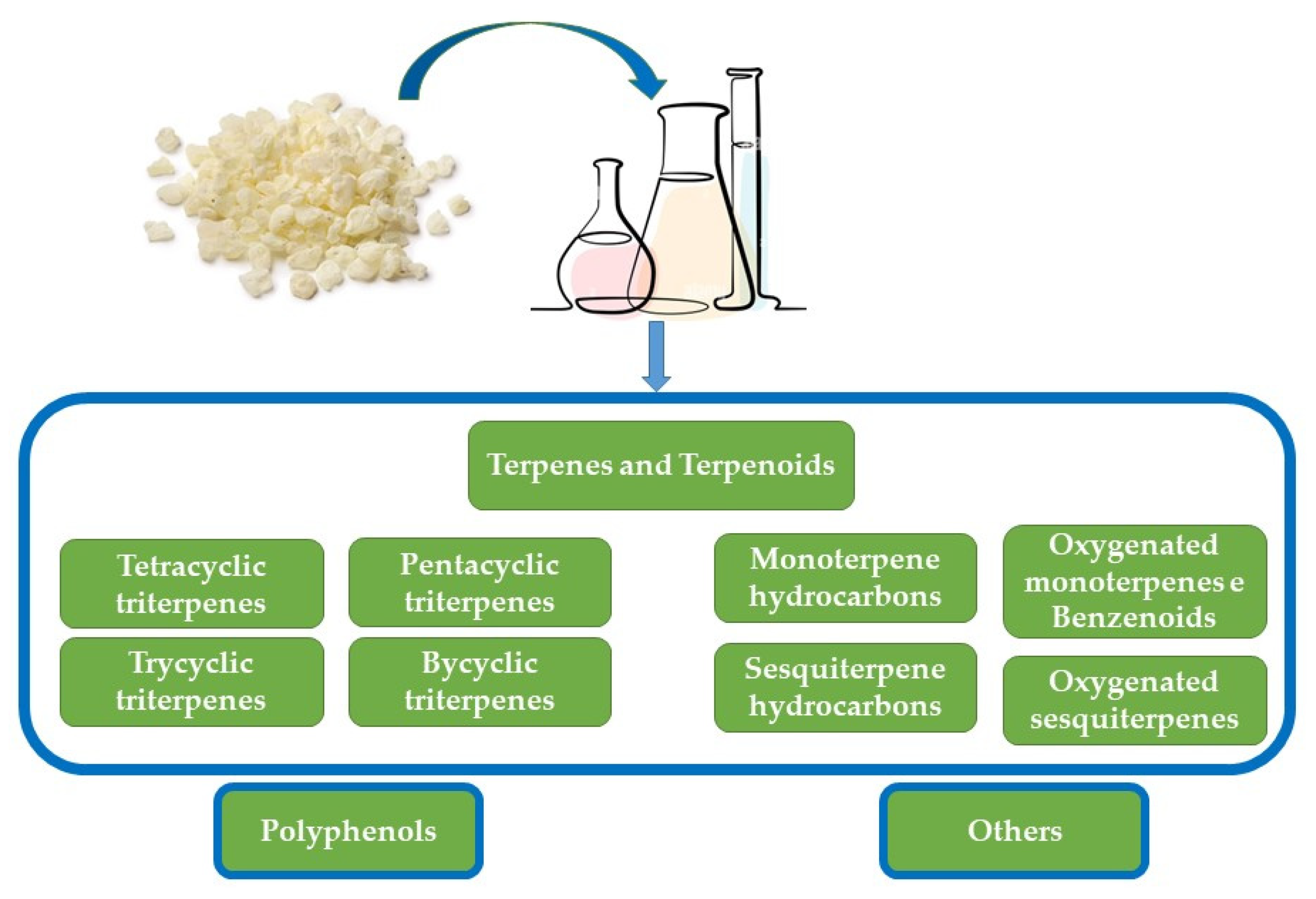



| Essential Oil |
|---|
| Monoterpene hydrocarbons |
| α-Pinene, β-pinene, β-myrcene, tricyclene, camphene, verbenene, 2-methylanisole, p-cymene, limonene, trans-linalool oxide, α-campholene aldehyde, trans-pinocarveol, trans-verbenol, pinocamphone, pinocarvone, p-mentha-1,5-dien-8-ol, myrtenal, myrtenol, verbenone, β-caryophyllene, α-caryophyllene, caryophyllene oxide, 28-nor-12,17-oleanadien-3-ol, lupenone, tirucallone, tirucallol, dammaradienol, 3-methoxy-28-norolean-12-ene, β-amyrone, 28-norolean-17-en-3-ol, 28-norolean-17-en-3-one, 6-methyl-28-norolean-17-en-3-one, olean-18-en-3-one, β-amyrin, 28-nor-12,17-oleanadien-3-one, oleanenone derivative, dammarane derivative, hydroxydammarenone, oleanonic aldehyde, moronic aldehyde, 28-nor-12,18-oleanadien-3-ol, and isomasticadienolic aldehyde |
| Oxygenated monoterpenes e Benzenoids |
| Perillene, α-linalool, camphenol, α-campholenal, pinocarveol, cis-verbenol, verbenol, verbenone, bornyl acetate, campholene, camphor, 3,6,6-trimethyl norpinan-2-one, pinocarvone, cis-3-pinanone, cis-carveol, 1-ethenyl-2,4-dimethylbenzene (or 1-Methyl-4-(2-propenyl)-benzene), o-methyl-anisole, o-cymene, m-cymene, p-cymene, β-methyl-cinnamaldehyde, myrtenal, p-cymen-8-ol, carvone, and trimethyl-hydroquinone |
| Sesquiterpene hydrocarbons |
| β-Caryophyllene, α-humulene, α-longipinene, α-ylangene, α-copaene, β-bourbonene, β-elemene, isocaryophyllene, α-muurolene, and D-germacrene |
| Oxygenated sesquiterpenes |
| Caryophyllene oxide, α-humulene epoxide, and 3,8,8-trimethyl-1,2,3,4,5,6,7,8-octahydro-2-naphthalenyl methyl acetate |
| Triterpenes |
| Pentacyclic triterpenes |
| Oleanonic acid, oleanolic acid, moronic acid, oleanonic aldehyde, oleanolic aldehyde, 28-nor-oleanone, 28-nor-oleanole, β-amyrine, β-amyrone, 28-hydroxy-β-amyrone, germanicol, lupeol, betulonal, lup-20(29)-ene-3-one, 3-oxo-28-norlup-20(29)-ene |
| Tetracyclic triterpenes |
| 24Z-Masticadienonic acid, 24Z-isomasticadienonic acid, 24Z-masticadienolic acid, 24Z-isomasticadienolic acid, mastichadienolal, isomastichadienolal, tirucallol, dammaradienone, mastichinoic acid, butyrospermol, dipterocarpol, and 20S-3β-acetoxy-20-hydroxydammar-24-ene |
| Trycyclic triterpenes and bycyclic triterpenes |
| 3β-Hydroxymalabarica-14(26),17E,21-triene, 3-oxomalabarica-14(26),17E,21-triene, (8R)-3β,8-dihydroxy-polypoda-13E,17E,21-triene, and (8R)-3-oxo-8-hydroxy-polypoda-13E,17E,21-triene. |
| Polyphenols |
| Tyrosol, p-hydroxy-benzoic, p-hydroxy-phenylacetic, vanillic acid, gallic acid, and E-cinnamic acid. |
| Others |
| 3-Ethylidene-1-methylcyclopentene, methyl-o-cresol, 1-dodecanol, 2,5-dimethoxytoluene, 3,5-dimethoxytoluene, (E)-anethole, 2-undecanone, octyl formate, 2-methyl-3-buten-2-ol, pinanediol, trans-linalool oxide, cis-linalool oxide, 6,7-dihydro-7-hydroxylinalool, 5,5-dimethyl-2(5H)-furanone, α-irone, o-methylanisol, methyleugenol, methylisoeugenol, α-fenchyl acetate, 4-acetyl-1-methylcyclohexene, and 2-undecanone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottria, R.; Xynomilakis, O.; Casati, S.; Abbiati, E.; Maconi, G.; Ciuffreda, P. Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future. Int. J. Mol. Sci. 2023, 24, 12038. https://doi.org/10.3390/ijms241512038
Ottria R, Xynomilakis O, Casati S, Abbiati E, Maconi G, Ciuffreda P. Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future. International Journal of Molecular Sciences. 2023; 24(15):12038. https://doi.org/10.3390/ijms241512038
Chicago/Turabian StyleOttria, Roberta, Ornella Xynomilakis, Silvana Casati, Ezio Abbiati, Giovanni Maconi, and Pierangela Ciuffreda. 2023. "Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future" International Journal of Molecular Sciences 24, no. 15: 12038. https://doi.org/10.3390/ijms241512038
APA StyleOttria, R., Xynomilakis, O., Casati, S., Abbiati, E., Maconi, G., & Ciuffreda, P. (2023). Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future. International Journal of Molecular Sciences, 24(15), 12038. https://doi.org/10.3390/ijms241512038







