Thyroid Hormone Regulates the Lipid Content of Muscle Fibers, Thus Affecting Physical Exercise Performance
Abstract
:1. Introduction
2. Results
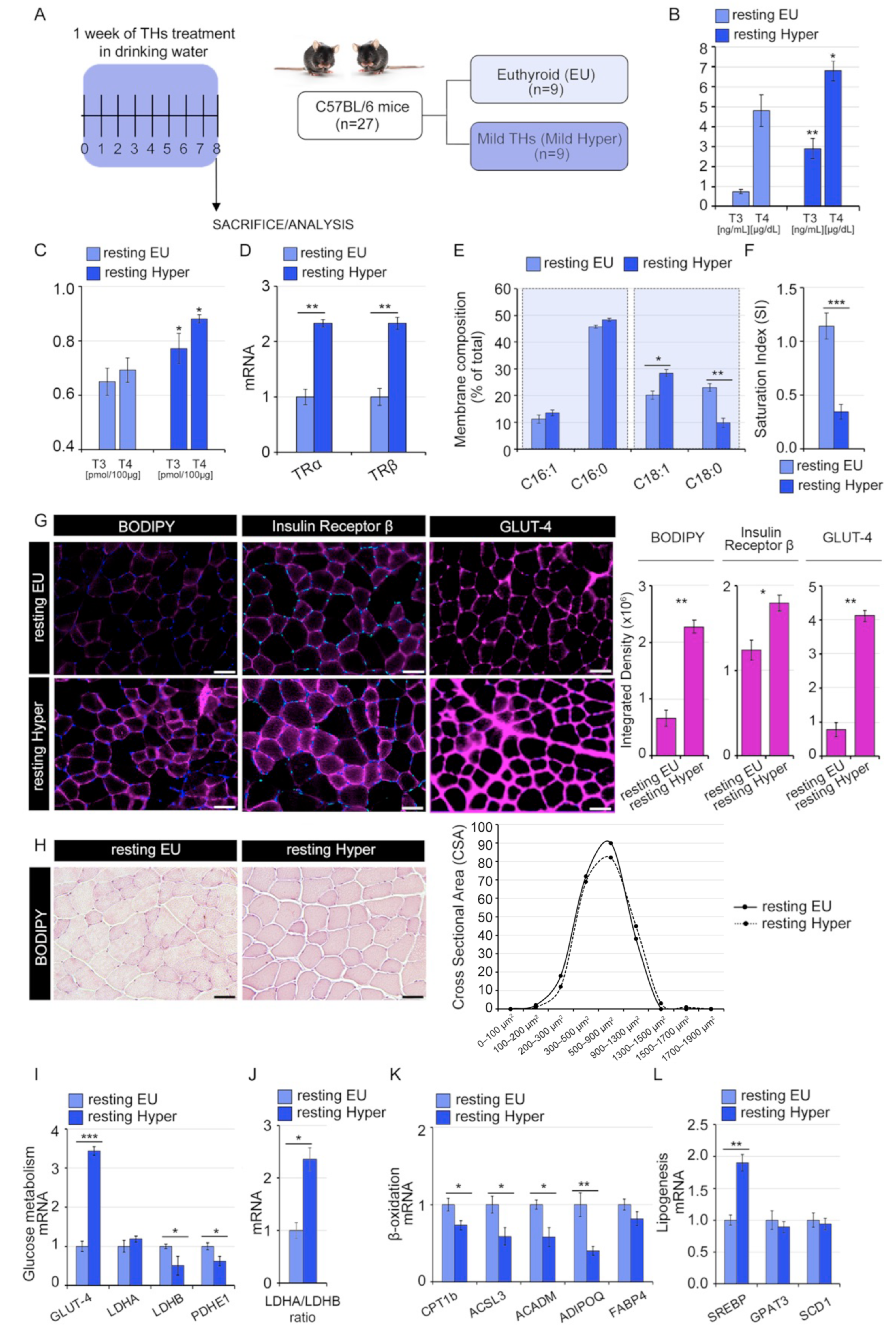
2.1. Thyroid Hormones Alter Lipids Profile and Saturated/Unsaturated FAs Ratio in Skeletal Muscle Cells
2.2. Thyroid Hormones Reduce the Saturation Index of the Skeletal Muscle Membrane
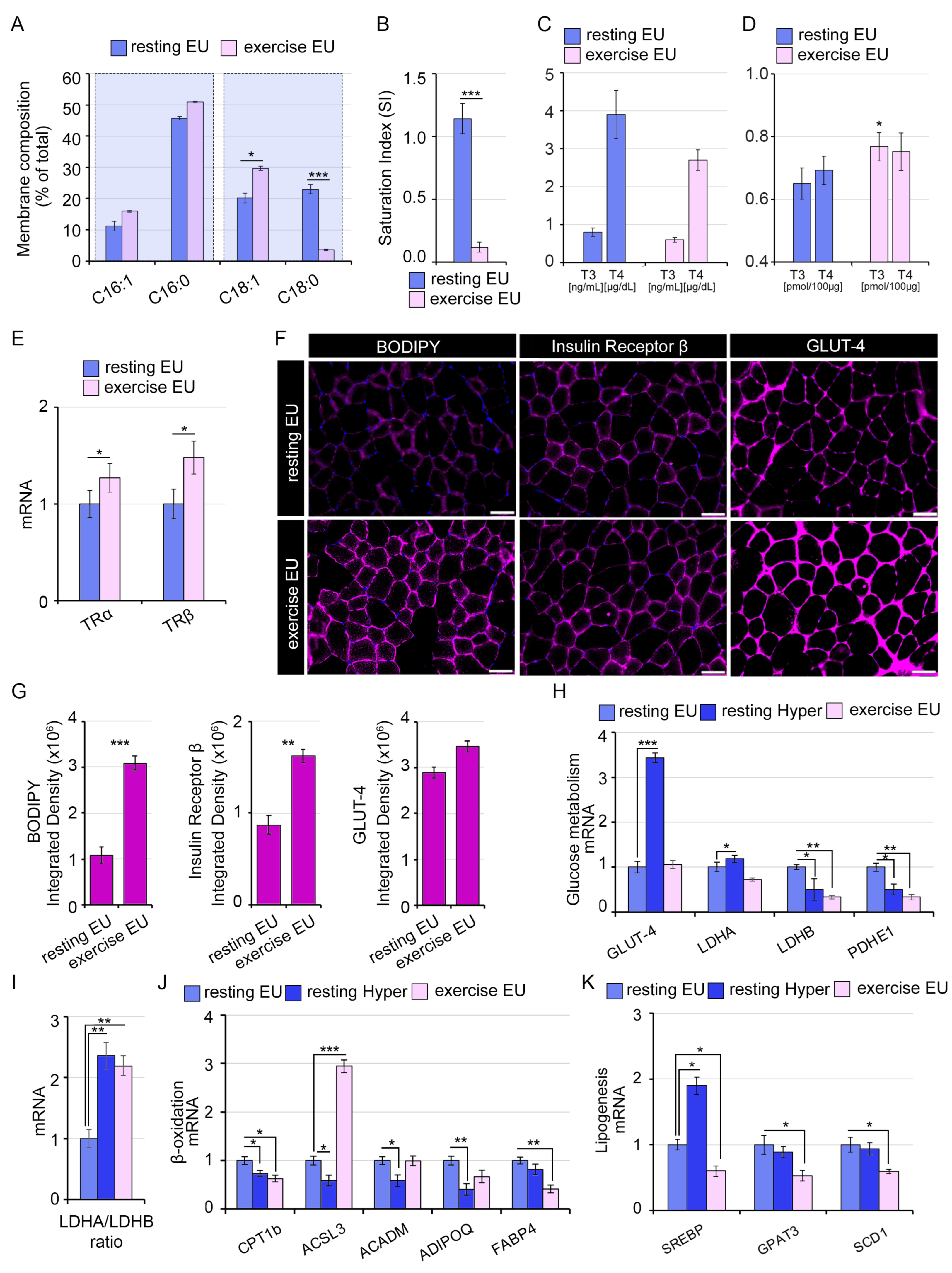
2.3. The TH-Mediated Changes in Skeletal Muscle Membrane Lipidomic Profile Partially Reflect the Exercise-Induced Modifications
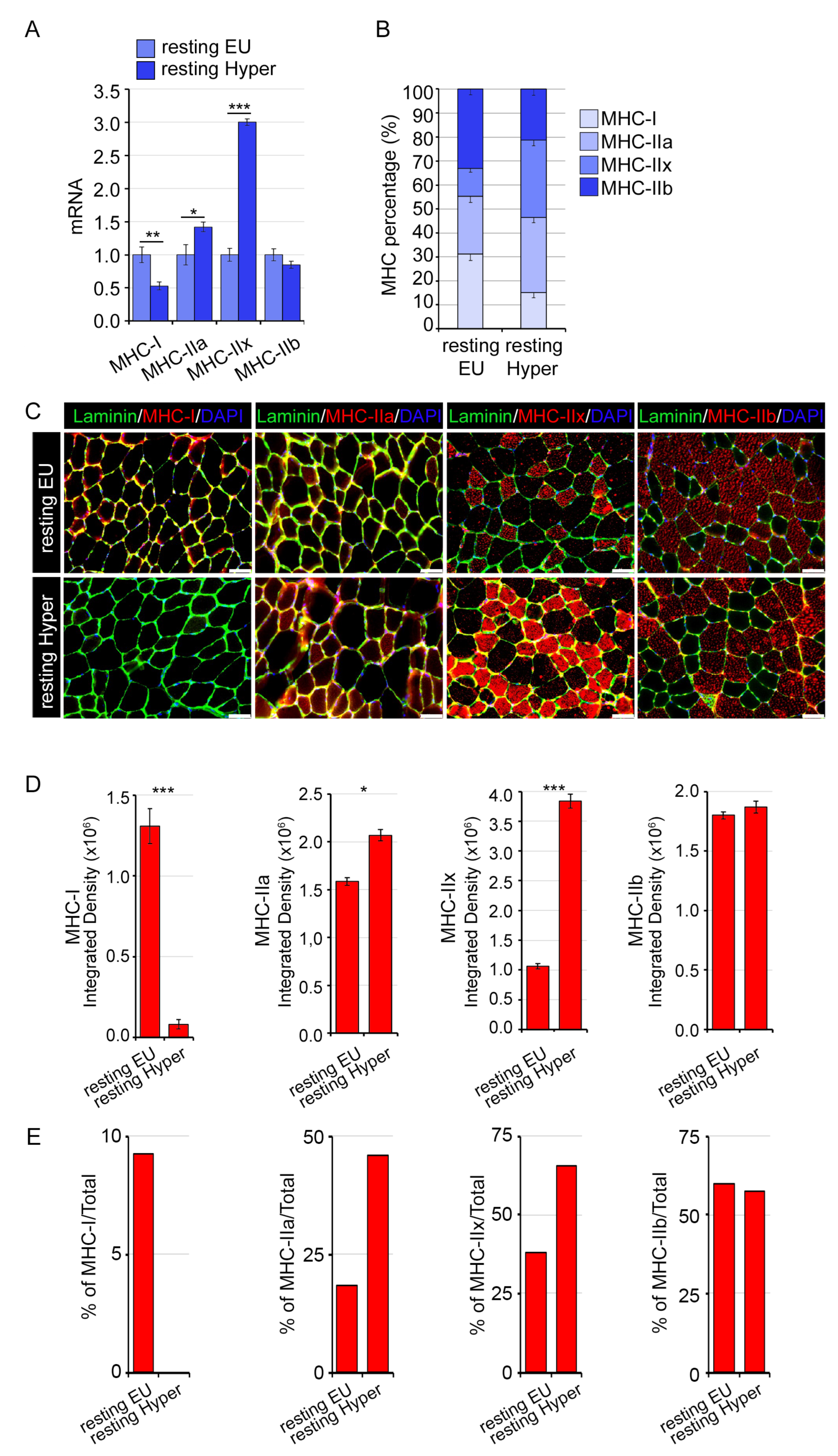
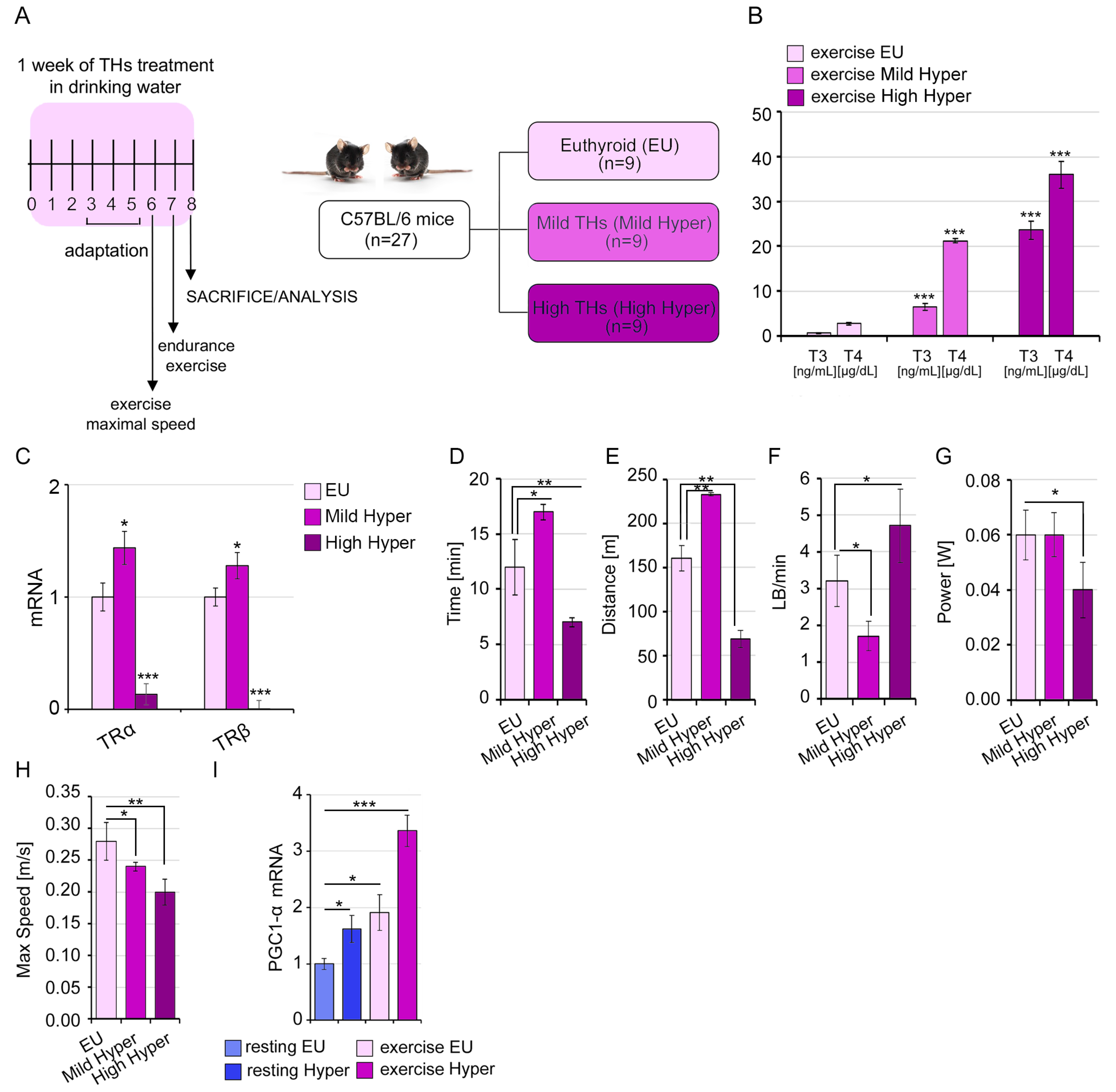
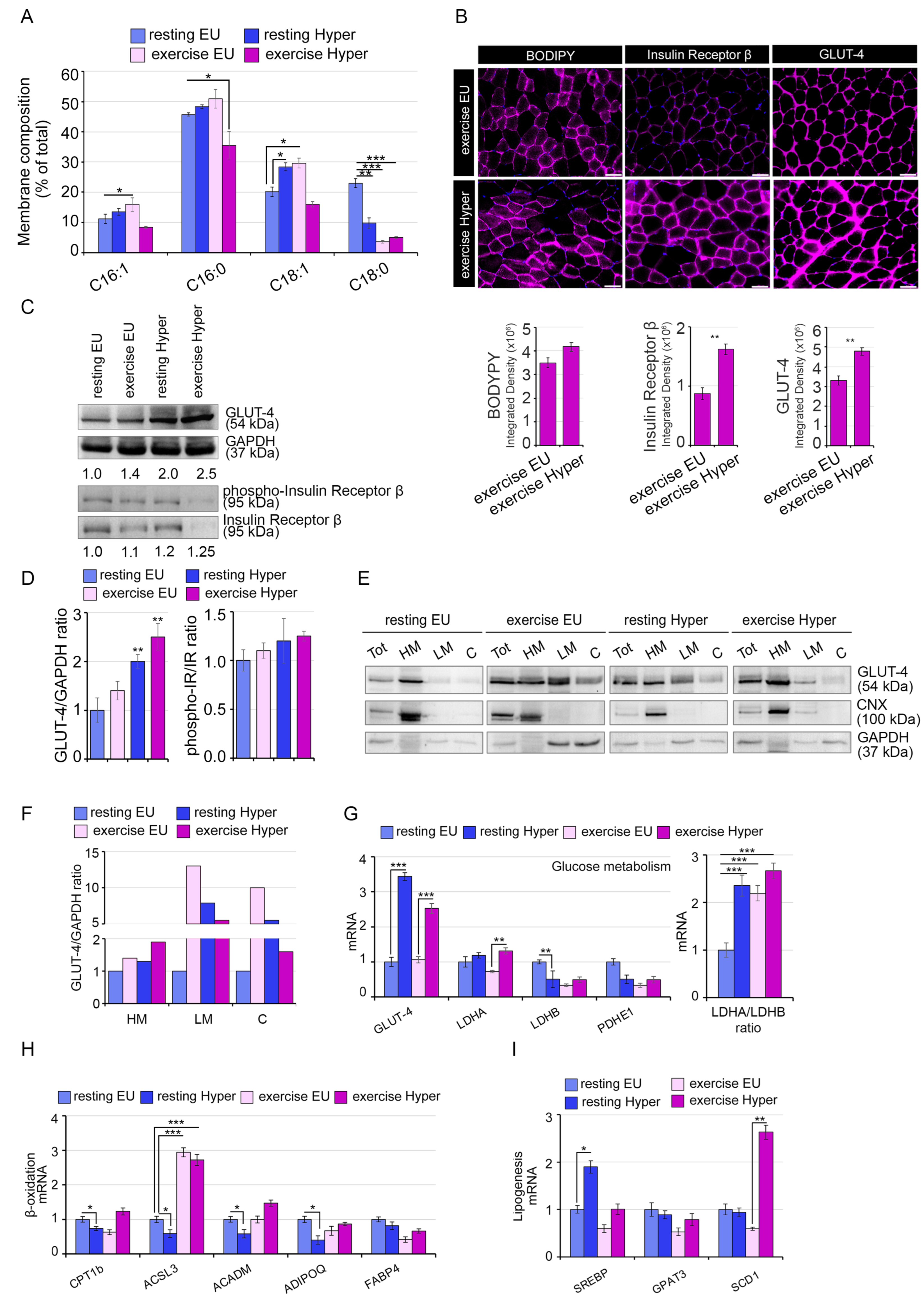
2.4. A Mild THs Stimulation Impacts the Structural and Functional Proprieties of Skeletal Muscle Influencing the Exercise Performance
2.5. Systemic Hyperthyroidism Improves Insulin Sensitivity In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Mouse Strains
4.3. Elisa Immunoassay
4.4. LC-MS/MS Analysis for Measurements of Intramuscular Thyroid Hormones
4.5. Treadmill Exercise Running
4.6. Lipidomic Analysis
4.7. Glucose Tolerance Test and Insulin Tolerance Test
4.8. Quantitative RT-PCR
4.9. Western Blot Analysis
4.10. Cell Fractionation
4.11. Histology, MHC Immunostaining, and BODIPY (493/503) Staining and Visualization
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steeves, J.A.; Bassett, D.R.; Thompson, D.L.; Fitzhugh, E.C. Relationships of occupational and non-occupational physical activity to abdominal obesity. Int. J. Obes. 2012, 36, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Hac-Wydro, K.; Wydro, P. The influence of fatty acids on model cholesterol/phospholipid membranes. Chem. Phys. Lipids 2007, 150, 66–81. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Weijers, R.N. Membrane flexibility, free fatty acids, and the onset of vascular and neurological lesions in type 2 diabetes. J. Diabetes Metab. Disord. 2015, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wei, Y.; Huang, Q.; Chen, Y.; Zeng, K.; Yang, W.; Chen, J.; Chen, J. Important hormones regulating lipid metabolism. Molecules 2022, 27, 7052. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The regulation of fat metabolism during aerobic exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef]
- Andersson, A.; Nälsén, C.; Tengblad, S.; Vessby, B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am. J. Clin. Nutr. 2002, 76, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Melanson, E.L.; MacLean, P.S.; Hill, J.O. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc. Sport Sci. Rev. 2009, 37, 93–101. [Google Scholar] [CrossRef]
- Paquin, J.; Lagacé, J.C.; Brochu, M.; Dionne, I.J. Exercising for insulin sensitivity—Is there a mechanistic relationship with quantitative changes in skeletal muscle mass? Front. Physiol. 2021, 12, 656909. [Google Scholar] [CrossRef]
- Lee, S.; Norheim, F.; Gulseth, H.L.; Langleite, T.M.; Aker, A.; Gundersen, T.E.; Holen, T.; Birkeland, K.I.; Drevon, C.A. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine respond to exercise and influence insulin sensitivity in men. Sci. Rep. 2018, 8, 6531. [Google Scholar] [CrossRef] [Green Version]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef]
- Phillips, S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014, 44, S71–S77. [Google Scholar] [CrossRef] [Green Version]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Cicatiello, A.G.; Di Girolamo, D.; Dentice, M. Metabolic effects of the intracellular regulation of thyroid hormone: Old players, new concepts. Front. Endocrinol. 2018, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Russo, S.C.; Salas-Lucia, F.; Bianco, A.C. Deiodinases and the metabolic code for thyroid hormone action. Endocrinology 2021, 162, bqab059. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef]
- Sagliocchi, S.; Cicatiello, A.G.; Di Cicco, E.; Ambrosio, R.; Miro, C.; Di Girolamo, D.; Nappi, A.; Mancino, G.; De Stefano, M.A.; Luongo, C.; et al. The thyroid hormone activating enzyme, type 2 deiodinase, induces myogenic differentiation by regulating mitochondrial metabolism and reducing oxidative stress. Redox Biol. 2019, 24, 101228. [Google Scholar] [CrossRef]
- Dentice, M.; Marsili, A.; Ambrosio, R.; Guardiola, O.; Sibilio, A.; Paik, J.H.; Minchiotti, G.; DePinho, R.A.; Fenzi, G.; Larsen, P.R.; et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J. Clin. Investig. 2010, 120, 4021–4030. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Sagliocchi, S.; Nappi, A.; Di Cicco, E.; Miro, C.; Murolo, M.; Stornaiuolo, M.; Dentice, M. Thyroid hormone regulates glutamine metabolism and anaplerotic fluxes by inducing mitochondrial glutamate aminotransferase GPT2. Cell Rep. 2022, 38, 110562. [Google Scholar] [CrossRef] [PubMed]
- Marsili, A.; Tang, D.; Harney, J.W.; Singh, P.; Zavacki, A.M.; Dentice, M.; Salvatore, D.; Larsen, P.R. Type II iodothyronine deiodinase provides intracellular 3,5,3′-triiodothyronine to normal and regenerating mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E818–E824. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Ogawa-Wong, A.; Carmody, M.C.; Ambrosio, R.; Cicatiello, A.G.; Luongo, C.; Salvatore, D.; Handy, D.E.; Larsen, P.R.; Wajner, S.M.; et al. A Type 2 deiodinase-dependent increase in Vegfa mediates myoblast-endothelial cell crosstalk during skeletal muscle regeneration. Thyroid 2021, 31, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.; Murolo, M.; Cicatiello, A.G.; Sagliocchi, S.; Di Cicco, E.; Raia, M.; Stornaiuolo, M.; Dentice, M.; Miro, C. Thyroid hormone receptor isoforms alpha and beta play convergent roles in muscle physiology and metabolic regulation. Metabolites 2022, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Rizos, C.V.; Elisaf, M.S.; Liberopoulos, E.N. effects of thyroid dysfunction on lipid profile. Open Cardiovasc. Med. J. 2011, 5, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Notarnicola, M.; Caruso, M.G.; Tutino, V.; Bonfiglio, C.; Cozzolongo, R.; Giannuzzi, V.; De Nunzio, V.; De Leonardis, G.; Abbrescia, D.I.; Franco, I.; et al. Significant decrease of saturation index in erythrocytes membrane from subjects with non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2017, 16, 160. [Google Scholar] [CrossRef] [Green Version]
- Verbrugge, S.A.J.; Gehlert, S.; Stadhouders, L.E.M.; Jacko, D.; Aussieker, T.; de Wit, G.M.J.; Vogel, I.S.P.; Offringa, C.; Schönfelder, M.; Jaspers, R.T.; et al. PKM2 Determines myofiber hypertrophy in vitro and increases in response to resistance exercise in human skeletal muscle. Int. J. Mol. Sci. 2020, 21, 7062. [Google Scholar] [CrossRef]
- Liang, X.; Liu, L.; Fu, T.; Zhou, Q.; Zhou, D.; Xiao, L.; Liu, J.; Kong, Y.; Xie, H.; Yi, F.; et al. Exercise inducible lactate dehydrogenase B regulates mitochondrial function in skeletal muscle. J. Biol. Chem. 2016, 291, 25306–25318. [Google Scholar] [CrossRef] [Green Version]
- Svensson, K.; Dent, J.R.; Tahvilian, S.; Martins, V.F.; Sathe, A.; Ochala, J.; Patel, M.S.; Schenk, S. Defining the contribution of skeletal muscle pyruvate dehydrogenase alpha1 to exercise performance and insulin action. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1034–E1045. [Google Scholar] [CrossRef]
- Leyton, J.; Drury, P.; Crawford, M. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 1987, 57, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef]
- Juel, C. Training-induced changes in membrane transport proteins of human skeletal muscle. Eur. J. Appl. Physiol. 2006, 96, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Giacco, A.; Cioffi, F.; Cuomo, A.; Simiele, R.; Senese, R.; Silvestri, E.; Amoresano, A.; Fontanarosa, C.; Petito, G.; Moreno, M.; et al. Mild endurance exercise during fasting increases gastrocnemius muscle and prefrontal cortex thyroid hormone levels through differential BHB and BCAA-mediated BDNF-mTOR signaling in rats. Nutrients 2022, 14, 1166. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Ignácio, D.L.; Padron, S.; Peçanha, R.; Marassi, M.P.; Rosenthal, D.; Werneck-De-Castro, J.P.S.; Carvalho, D.P. The effect of acute exercise session on thyroid hormone economy in rats. J. Endocrinol. 2008, 198, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Pilon, M. Revisiting the membrane-centric view of diabetes. Lipids Health Dis. 2016, 15, 167. [Google Scholar] [CrossRef] [Green Version]
- Borkman, M.; Storlien, L.H.; Pan, D.A.; Jenkins, A.B.; Chisholm, D.J.; Campbell, L.V. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N. Engl. J. Med. 1993, 328, 238–244. [Google Scholar] [CrossRef]
- Dimitriadis, G.D.; Raptis, S.A. Thyroid hormone excess and glucose intolerance. Exp. Clin. Endocrinol. Diabetes 2001, 109, 225–239. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A.; Stouth, D.W.; Manta, A.; Ljubicic, V.; Hinkley, J.M.; Zou, K.; Park, S.; et al. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Bomkamp, M.; Ozdemir, M.; Hyatt, H. Mechanisms of exercise-induced preconditioning in skeletal muscles. Redox Biol. 2020, 35, 101462. [Google Scholar] [CrossRef]
- Booth, F.W.; Thomason, D.B. Molecular and cellular adaptation of muscle in response to exercise: Perspectives of various models. Physiol. Rev. 1991, 71, 541–585. [Google Scholar] [CrossRef]
- Salvatore, D.; Simonides, W.S.; Dentice, M.; Zavacki, A.M.; Larsen, P.R. Thyroid hormones and skeletal muscle--new insights and potential implications. Nat. Rev. Endocrinol. 2014, 10, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanesi, A.; Lee, J.W.; Kim, N.H.; Liu, Y.Y.; Yang, A.; Sedrakyan, S.; Kahng, A.; Cervantes, V.; Tripuraneni, N.; Cheng, S.Y.; et al. Thyroid hormone receptor alpha plays an essential role in male skeletal muscle myoblast proliferation, differentiation, and response to injury. Endocrinology 2016, 157, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, C.; Agostini, M.; Visser, W.E.; Schoenmakers, E.; Schoenmakers, N.; Offiah, A.C.; Poole, K.; Rajanayagam, O.; Lyons, G.; Halsall, D.; et al. Resistance to thyroid hormone caused by a mutation in thyroid hormone receptor (TR)alpha1 and TRalpha2: Clinical, biochemical, and genetic analyses of three related patients. Lancet Diabetes Endocrinol. 2014, 2, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Nappi, A.; Murolo, M.; Sagliocchi, S.; Miro, C.; Cicatiello, A.G.; Di Cicco, E.; Di Paola, R.; Raia, M.; D’Esposito, L.; Stornaiuolo, M.; et al. Selective inhibition of genomic and non-genomic effects of thyroid hormone regulates muscle cell differentiation and metabolic behavior. Int. J. Mol. Sci. 2021, 22, 7175. [Google Scholar] [CrossRef]
- Hones, G.S.; Rakov, H.; Logan, J.; Liao, X.H.; Werbenko, E.; Pollard, A.S.; Præstholm, S.M.; Siersbæk, M.S.; Rijntjes, E.; Gassen, J.; et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E11323–E11332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iaccarino, G.; Franco, D.; Sorriento, D.; Strisciuglio, T.; Barbato, E.; Morisco, C. Modulation of insulin sensitivity by exercise training: Implications for cardiovascular prevention. J. Cardiovasc. Transl. Res. 2021, 14, 256–270. [Google Scholar] [CrossRef]
- Venkatasamy, V.V.; Pericherla, S.; Manthuruthil, S.; Mishra, S.; Hanno, R. Effect of physical activity on insulin resistance, inflammation and oxidative stress in diabetes mellitus. J. Clin. Diagn. Res. 2013, 7, 1764–1766. [Google Scholar]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2016, 2, e000143. [Google Scholar] [CrossRef] [Green Version]
- Miro, C.; Di Giovanni, A.; Murolo, M.; Cicatiello, A.G.; Nappi, A.; Sagliocchi, S.; Di Cicco, E.; Morra, F.; Celetti, A.; Pacifico, F.; et al. Thyroid hormone and androgen signals mutually interplay and enhance inflammation and tumorigenic activation of tumor microenvironment in prostate cancer. Cancer Lett. 2022, 532, 215581. [Google Scholar] [CrossRef]
- Nappi, A.; Miro, C.; Pezone, A.; Tramontano, A.; Di Cicco, E.; Sagliocchi, S.; Cicatiello, A.G.; Murolo, M.; Torabinejad, S.; Abbotto, E.; et al. Loss of p53 activates thyroid hormone via type 2 deiodinase and enhances DNA damage. Nat. Commun. 2023, 14, 1244. [Google Scholar] [CrossRef]
- Davis, P.J.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Editorial: Non genomic actions of thyroid hormones in cancer. Front. Endocrinol. 2019, 10, 847. [Google Scholar] [CrossRef] [Green Version]
- Cicatiello, A.G.; Ambrosio, R.; Dentice, M. Thyroid hormone promotes differentiation of colon cancer stem cells. Mol. Cell. Endocrinol. 2017, 459, 84–89. [Google Scholar] [CrossRef]
- Bocco, B.M.; Louzada, R.A.; Silvestre, D.H.; Santos, M.C.; Anne-Palmer, E.; Rangel, I.F.; Abdalla, S.; Ferreira, A.C.; Ribeiro, M.O.; Gereben, B.; et al. Thyroid hormone activation by type 2 deiodinase mediates exercise-induced peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression in skeletal muscle. J. Physiol. 2016, 594, 5255–5269. [Google Scholar] [CrossRef] [Green Version]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance OJ L 276, 20.10.2010, p. 33–79 (BG, ES, CS, DA, DE, ET, EL, EN, FR, IT, LV, LT, HU, MT, NL, PL, PT, RO, SK, SL, FI, SV) Special Edition in Croatian: Chapter 15 Volume 028, P. 82–128. In Force: This Act has Been Changed. Current consolidated version: 26/06/2019. ELI. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 1 July 2023).
- Festing, M.F.W.; Overend, P.; Gaines Das, R.; Cortina Borja, M.; Berdoy, M. The ARRIVE Guidelines—Animal Research: Reporting In Vivo Experiments; The design of animal experiments: Reducing the number of animals in research through better experimental design; Laboratory Animal Handbooks Series, 14; Royal Society of Medicine Press: London, UK, 2002. [Google Scholar]
- Sagliocchi, S.; Murolo, M.; Cicatiello, A.G.; Miro, C.; Nappi, A.; Di Cicco, E.; Torabinejad, S.; La Civita, E.; Romano, V.; Terracciano, D.; et al. Repositioning of Cefuroxime as novel selective inhibitor of the thyroid hormone activating enzyme type 2 deiodinase. Pharmacol. Res. 2023, 189, 106685. [Google Scholar] [CrossRef]
- Generoso, S.F.; Giustiniano, M.; La Regina, G.; Bottone, S.; Passacantilli, S.; Di Maro, S.; Cassese, H.; Bruno, A.; Mallardo, M.; Dentice, M.; et al. Pharmacological folding chaperones act as allosteric ligands of Frizzled4. Nat Chem Biol. 2015, 11, 280–286. [Google Scholar] [CrossRef]
- Werneck-de-Castro, J.P.; Fonseca, T.L.; Ignacio, D.L.; Fernandes, G.W.; Andrade-Feraud, C.M.; Lartey, L.J.; Ribeiro, M.B.; Ribeiro, M.O.; Gereben, B.; Bianco, A.C. Thyroid Hormone Signaling in Male Mouse Skeletal Muscle Is Largely Independent of D2 in Myocytes. Endocrinology 2015, 156, 3842–3852. [Google Scholar] [CrossRef] [Green Version]
- Sommella, E.; Badolati, N.; Riccio, G.; Salviati, E.; Bottone, S.; Dentice, M.; Campiglia, P.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. A Boost in mitochondrial activity underpins the cholesterol-lowering effect of annurca apple polyphenols on hepatic cells. Nutrients 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, G.; Sommella, E.; Badolati, N.; Salviati, E.; Bottone, S.; Campiglia, P.; Dentice, M.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. Annurca apple polyphenols protect murine hair follicles from taxane induced dystrophy and hijacks polyunsaturated fatty acid metabolism toward beta-oxidation. Nutrients 2018, 10, 1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Badolati, N.; Masselli, R.; Sommella, E.; Sagliocchi, S.; Di Minno, A.; Salviati, E.; Campiglia, P.; Dentice, M.; Tenore, G.C.; Stornaiuolo, M.; et al. The Hepatoprotective Effect of Taurisolo, a nutraceutical enriched in resveratrol and polyphenols, involves activation of mitochondrial metabolism in mice liver. Antioxidants 2020, 9, 410. [Google Scholar] [CrossRef]
- Stornaiuolo, M.; Lotti, L.V.; Borgese, N.; Torrisi, M.-R.; Mottola, G.; Martire, G.; Bonatti, S. KDEL and KKXX retrieval signals appended to the same reporter protein determine different trafficking between endoplasmic reticulum, intermediate compartment, and Golgi complex. Mol. Biol. Cell. 2003, 14, 889–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubowitz, V. Muscle biopsy--technical and diagnostic aspects. Ann. Clin. Res. 1974, 6, 69–79. [Google Scholar]
- Ardite, E.; Perdiguero, E.; Vidal, B.; Gutarra, S.; Serrano, A.L.; Muñoz-Cánoves, P. PAI-1-regulated miR-21 defines a novel age-associated fibrogenic pathway in muscular dystrophy. J. Cell Biol. 2012, 196, 163–175. [Google Scholar] [CrossRef]
- Sawano, S.; Komiya, Y.; Ichitsubo, R.; Ohkawa, Y.; Nakamura, M.; Tatsumi, R.; Ikeuchi, Y.; Mizunoya, W. One-step immunostaining method to visualize rodent muscle fiber type within a single specimen. PLoS ONE 2016, 11, e0166080. [Google Scholar] [CrossRef] [Green Version]


| Gene | Sense | Sequence |
|---|---|---|
| ACADM | Forward | AGGGTTTAGTTTTGAGTTGACGG |
| Reverse | CCCCGCTTTTGTCATATTCCG | |
| ACSL3 | Forward | AACCACGTATCTTCAACACCATC |
| Reverse | AGTCCGGTTTGGAACTGACAG | |
| ADIPOQ | Forward | GTGACGACACCAAAAGGGCTC |
| Reverse | TCCAACCTGCACAAGTTCCC | |
| CPT1b | Forward | GCACACCAGGCAGTAGCTTT |
| Reverse | CAGGAGTTGATTCCAGACAGGTA | |
| ETFA | Forward | GCCTCATTGCTCCGTTTTCAG |
| Reverse | GCTACTAAGCAGGACACTTCAC | |
| ETFB | Forward | CTGTCAAGAGGGTCATCGACT |
| Reverse | CACAGAAGGGGTTCATGGAGT | |
| FABP4 | Forward | TCTCACCTGGAAGACAGCTCC |
| Reverse | GCTGATGATCATGTTGGGCTTGG | |
| GLUT-4 | Forward | CAGAAGGTGATTGAACAGAG |
| Reverse | AATGATGCCAATGAGAAA | |
| GPAT3 | Forward | GTGTCCTAGTGCGCTATTGC |
| Reverse | TCTGGGGTCTGTACTGCTTG | |
| LDHA | Forward | GGCATGGCTTGTGCCATCAGTATC |
| Reverse | GGAGATCCATCATCTCGCCCTTGA | |
| LDHB | Forward | GGGAGCTTGTTCCTCCAGAC |
| Reverse | TGGGTTGGAAACCACGATGAT | |
| PDHE1a | Forward | GAAATGTGACCTTCATCGGCT |
| Reverse | TGATCCGCCTTTAGCTCCATC | |
| PDK3 | Forward | TCCTGGACTTCGGAAGGGATA |
| Reverse | GAAGGGCGGTTCAACAAGTTA | |
| PKM1 | Forward | TATAAGAGGCCTCCACGCTG |
| Reverse | GGGCATTGAGATTCCTGCAG | |
| PKM2 | Forward | TTGGTGAGCACGATAATGGC |
| Reverse | GGGCATTGAGATTCCTGCAG | |
| PYC | Forward | CTGAAGTTCCAAACAGTTCGAGG |
| Reverse | GCAACGAAACACTCGGATG | |
| SCD1 | Forward | CATTCTCATGGTCCTGCTGC |
| Reverse | GCCGTGCCTTGTAAGTTCTG | |
| SREBP1c | Forward | ACAGACACTGGCCGAGATG |
| Reverse | GCTCTCAGGAGAGTTGGCAC | |
| TRα | Forward | ACCACCGCAAACACAACATT |
| Reverse | CATTCCGAGAAGCTGCTGTC | |
| TRβ | Forward | CACAGGGTACCACTATCGCTGC |
| Reverse | CAGCACCAAGTCTGTTGCCATGC |
| Antibodies Used for Western Blot and Immunofluorescence Analysis | |||
|---|---|---|---|
| Antibody | Source | Identifier | Dilution |
| Insulin Receptor b (4B8) | Cell Signaling Technology (Danvers, MA, USA) | #3025 | 1:1000 IF |
| Anti-Glucose Transporter GLUT-4 (1F8) | Abcam (Cambridge, UK) | Ab35826 | 1:1000 WB/1:500 IF |
| GAPDH | Elabscience (Houston, TX, USA) | E-AB-20059 | 1:1000 WB |
| Mouse monoclonal anti-Myosin Heavy Chain Type I | DSHB (Iowa City, IA, USA) | BA-F8 | 1:500 IF |
| Mouse monoclonal anti-Myosin Heavy Chain Type IIA | DSHB | SC-71 | 1:500 IF |
| Mouse monoclonal anti-Myosin Heavy Chain Type IIX | DSHB | 6H1 | 1:500 IF |
| Mouse monoclonal anti-Myosin Heavy Chain Type IIB | DSHB | BF-F3 | 1:500 IF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miro, C.; Nappi, A.; Sagliocchi, S.; Di Cicco, E.; Murolo, M.; Torabinejad, S.; Acampora, L.; Pastore, A.; Luciano, P.; La Civita, E.; et al. Thyroid Hormone Regulates the Lipid Content of Muscle Fibers, Thus Affecting Physical Exercise Performance. Int. J. Mol. Sci. 2023, 24, 12074. https://doi.org/10.3390/ijms241512074
Miro C, Nappi A, Sagliocchi S, Di Cicco E, Murolo M, Torabinejad S, Acampora L, Pastore A, Luciano P, La Civita E, et al. Thyroid Hormone Regulates the Lipid Content of Muscle Fibers, Thus Affecting Physical Exercise Performance. International Journal of Molecular Sciences. 2023; 24(15):12074. https://doi.org/10.3390/ijms241512074
Chicago/Turabian StyleMiro, Caterina, Annarita Nappi, Serena Sagliocchi, Emery Di Cicco, Melania Murolo, Sepehr Torabinejad, Lucia Acampora, Arianna Pastore, Paolo Luciano, Evelina La Civita, and et al. 2023. "Thyroid Hormone Regulates the Lipid Content of Muscle Fibers, Thus Affecting Physical Exercise Performance" International Journal of Molecular Sciences 24, no. 15: 12074. https://doi.org/10.3390/ijms241512074
APA StyleMiro, C., Nappi, A., Sagliocchi, S., Di Cicco, E., Murolo, M., Torabinejad, S., Acampora, L., Pastore, A., Luciano, P., La Civita, E., Terracciano, D., Stornaiuolo, M., Dentice, M., & Cicatiello, A. G. (2023). Thyroid Hormone Regulates the Lipid Content of Muscle Fibers, Thus Affecting Physical Exercise Performance. International Journal of Molecular Sciences, 24(15), 12074. https://doi.org/10.3390/ijms241512074










