The Effect of Cerebrolysin in an Animal Model of Forebrain Ischemic-Reperfusion Injury: New Insights into the Activation of the Keap1/Nrf2/Antioxidant Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. CBL Improved Motor Recovery in the Forebrain IR Mice Model
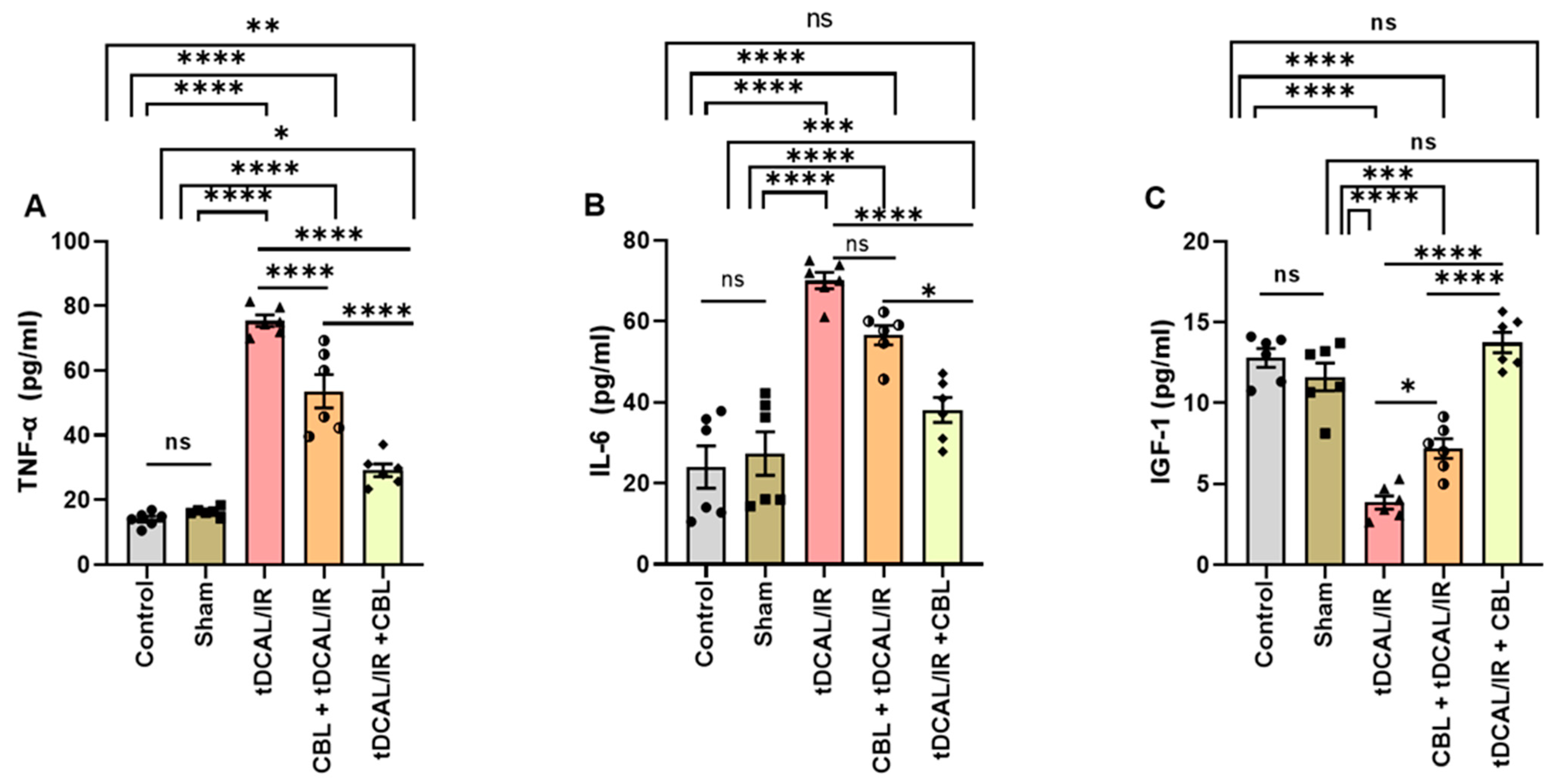
2.2. CBL Attenuated Neuroinflammation and Enhanced Tissue Regeneration and Remodeling in the Forebrain IR Mice Model
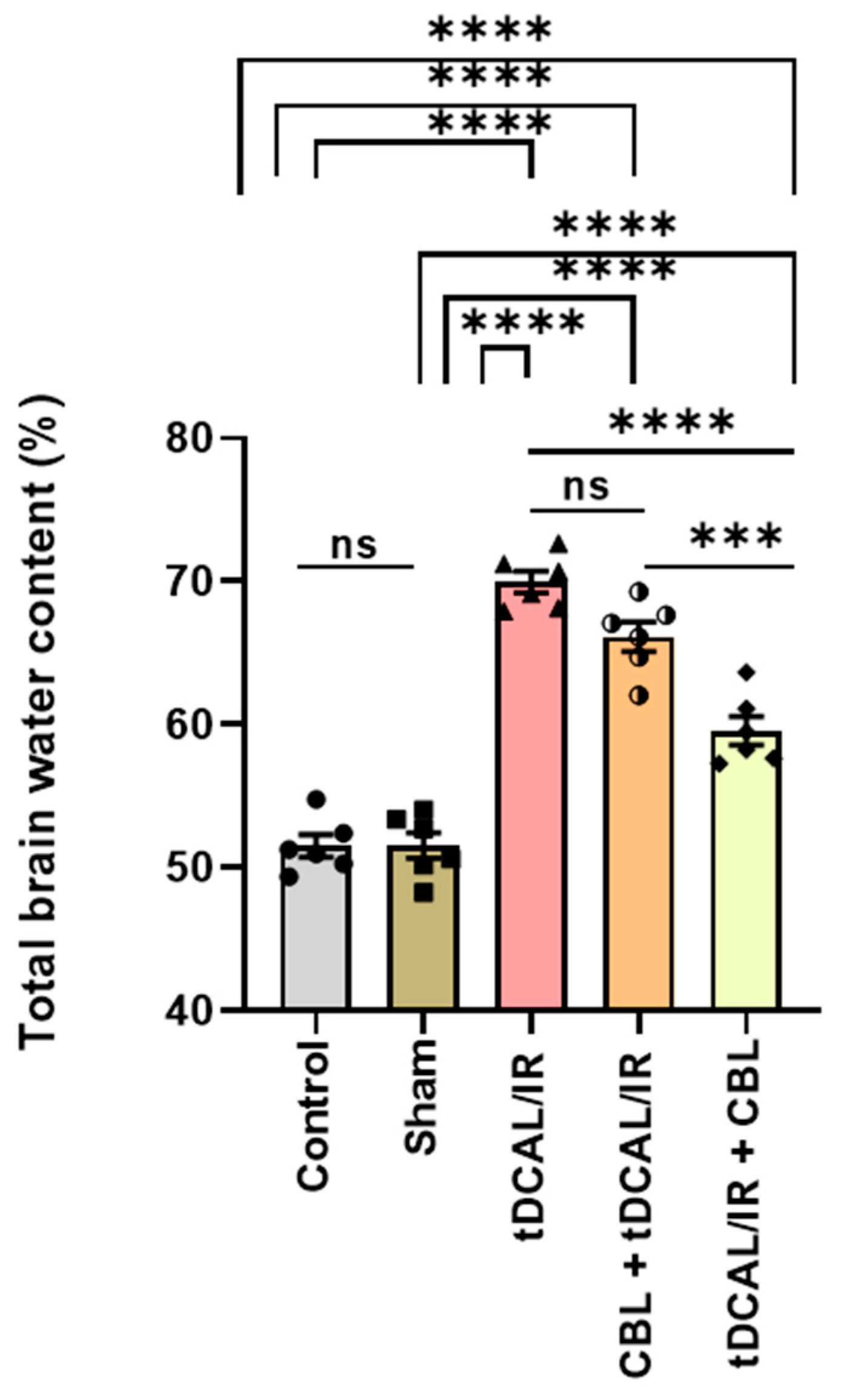
2.3. CBL Reduced the Total Brain Water Content in the Forebrain IR Mice Model
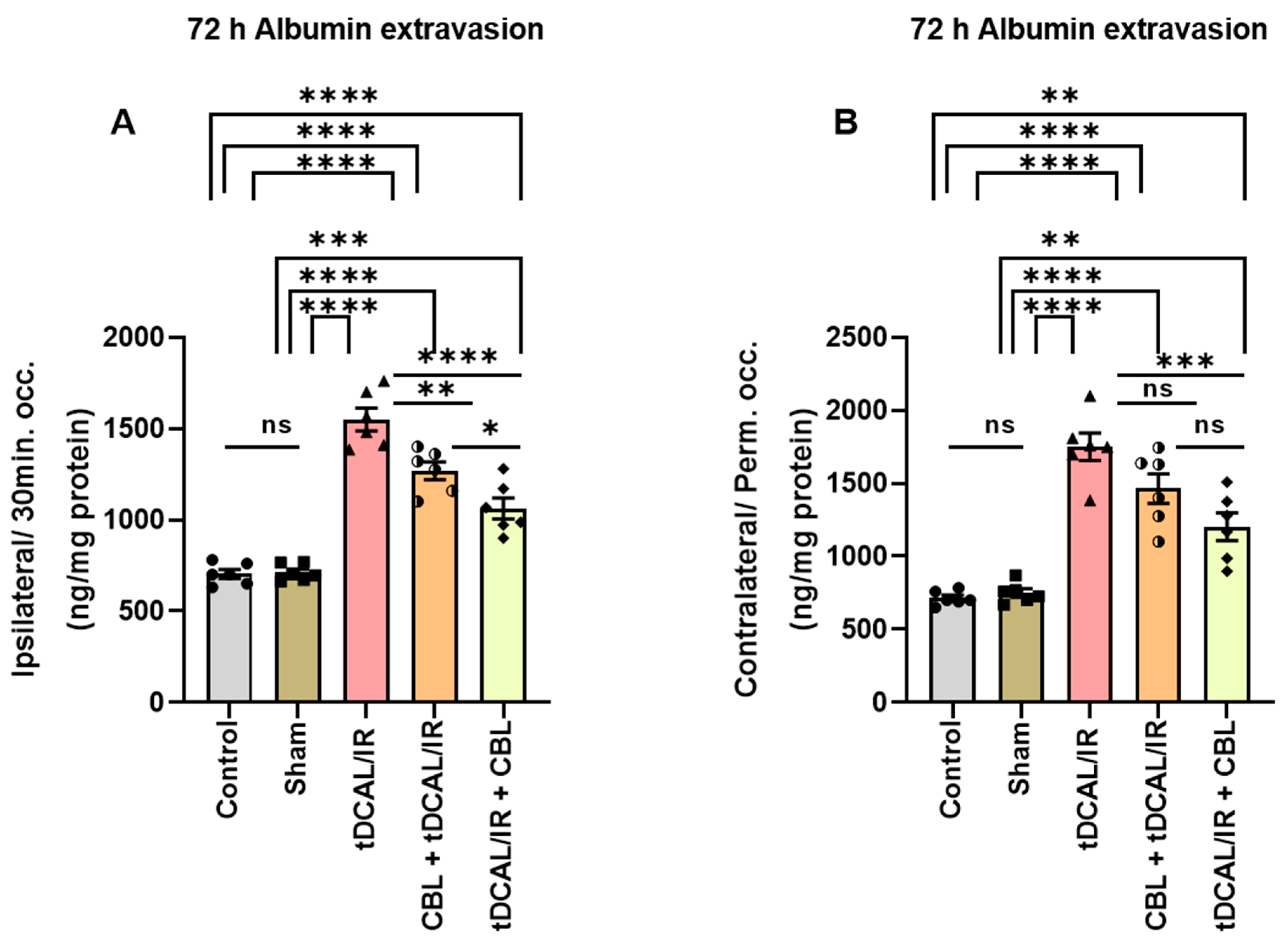
2.4. CBL Repaired the Blood–Brain Barrier Damage in the Forebrain IR Mice Model
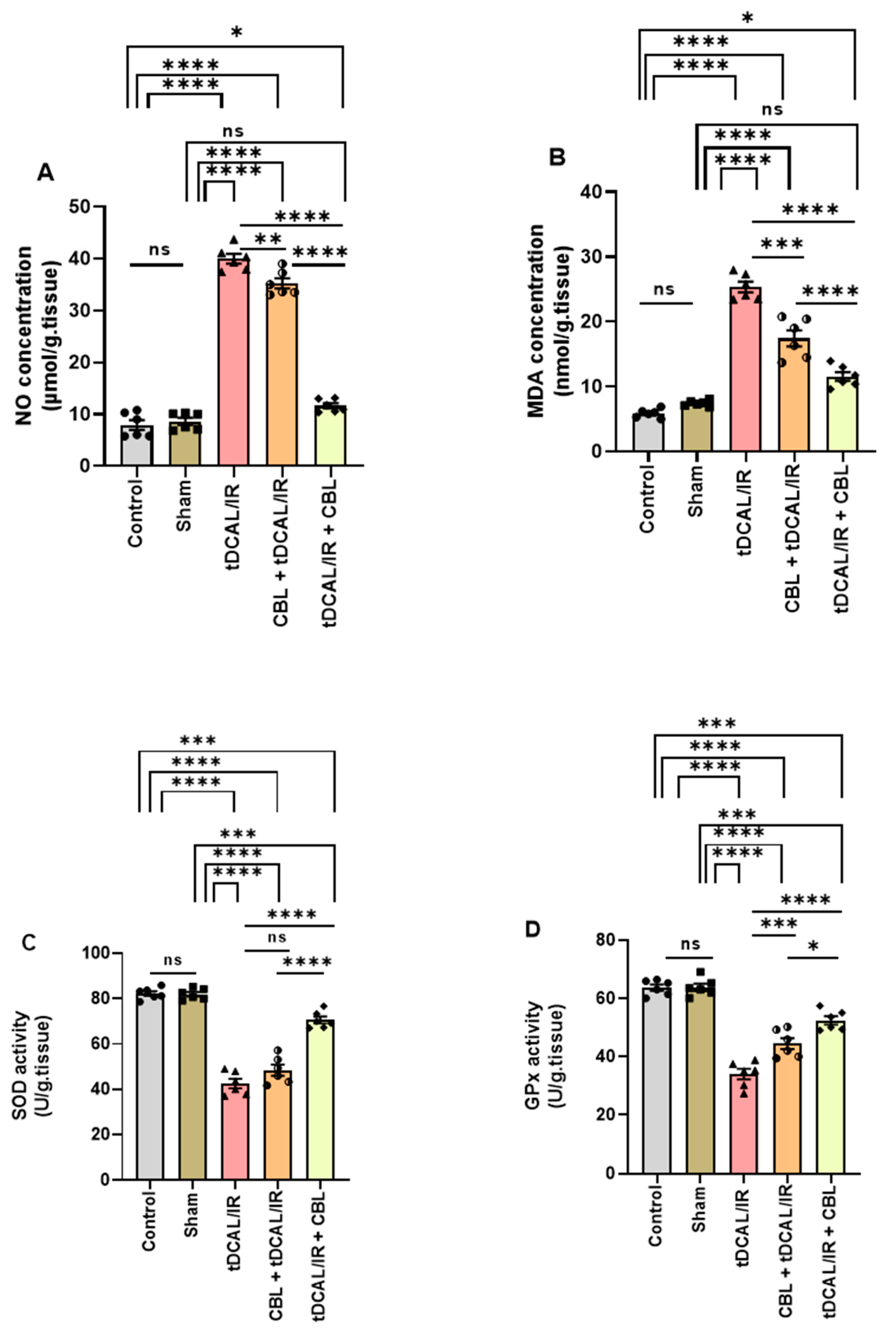
2.5. CBL Mitigated Oxidative Stress in the Forebrain IR Mice Model
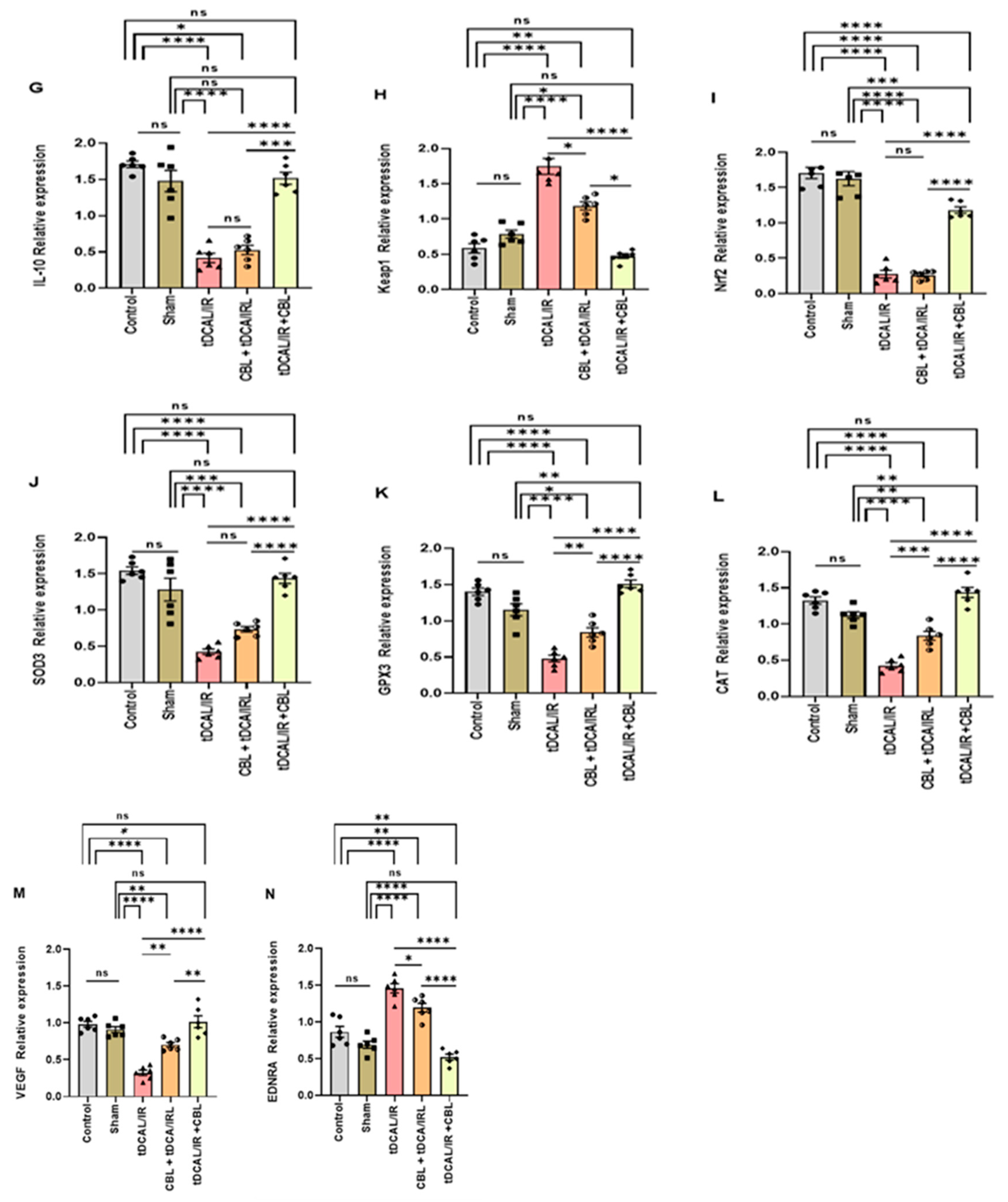
2.6. CBL, a Signaling Multi-Target Neuropeptide, Regulated the mRNA Relative Expression of the Target Genes in the Forebrain IR Mice Model
2.6.1. CBL Regulated Neuroinflammation via Inhibiting the TLRs/NF-kB/Cytokines Signaling Pathway
2.6.2. CBL Regulated Oxidative Stress via Activating the Keap1/Nrf2/Antioxidant Signaling Pathway
2.6.3. CBL Improved Postischemic Neurovascular Remodeling and BBB Integrity via Upregulating VEGF and Downregulating EDNRA Expression
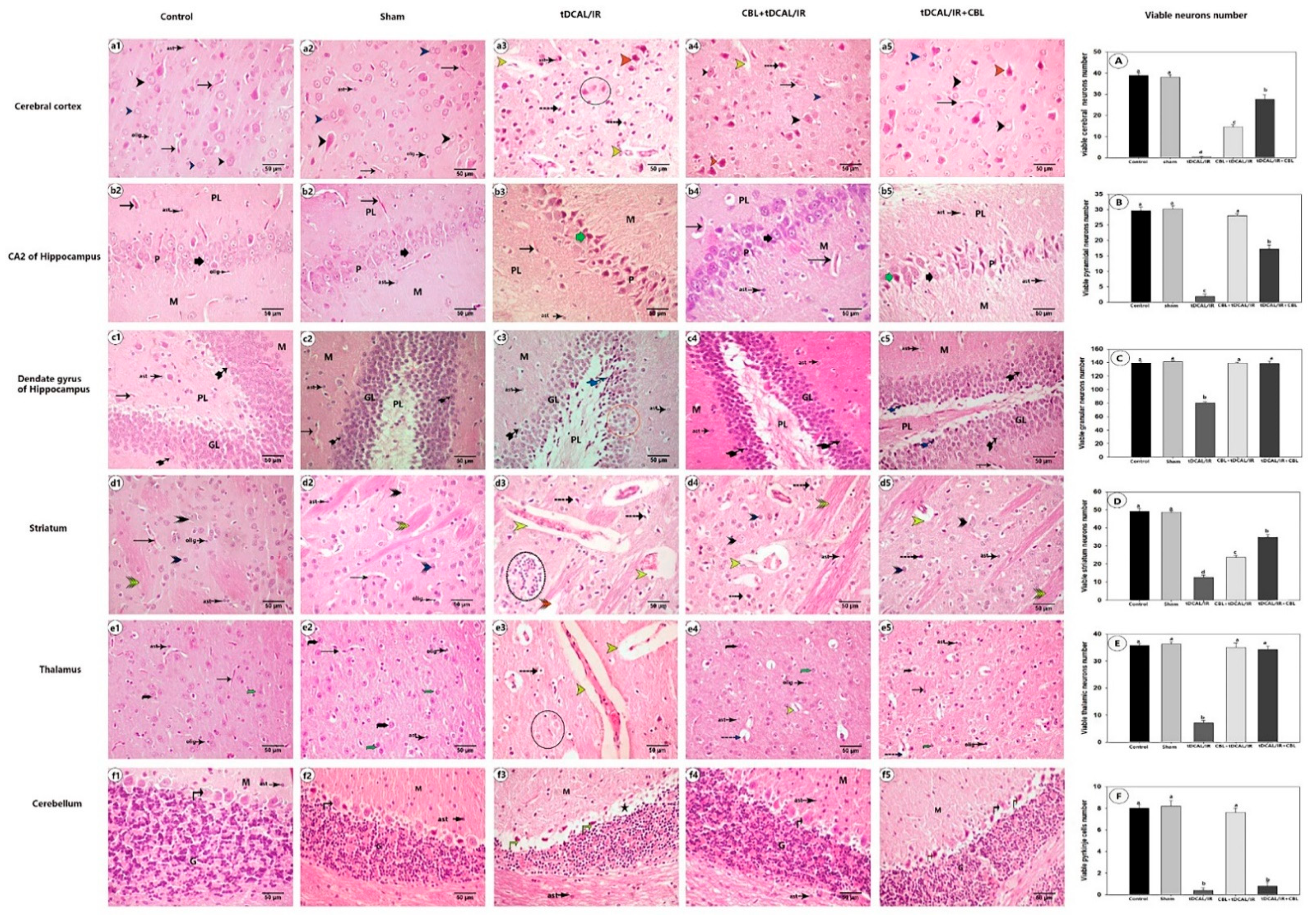
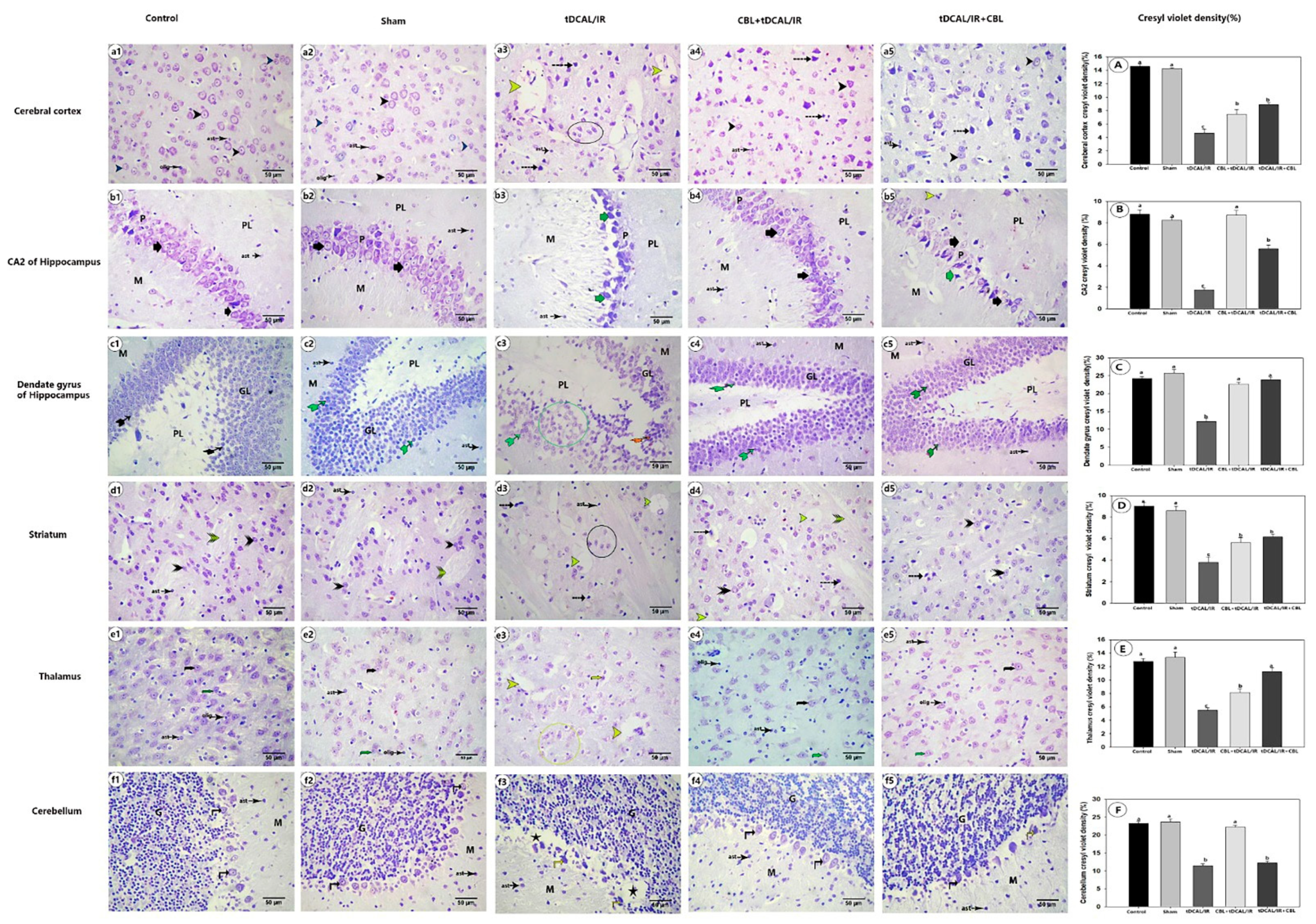
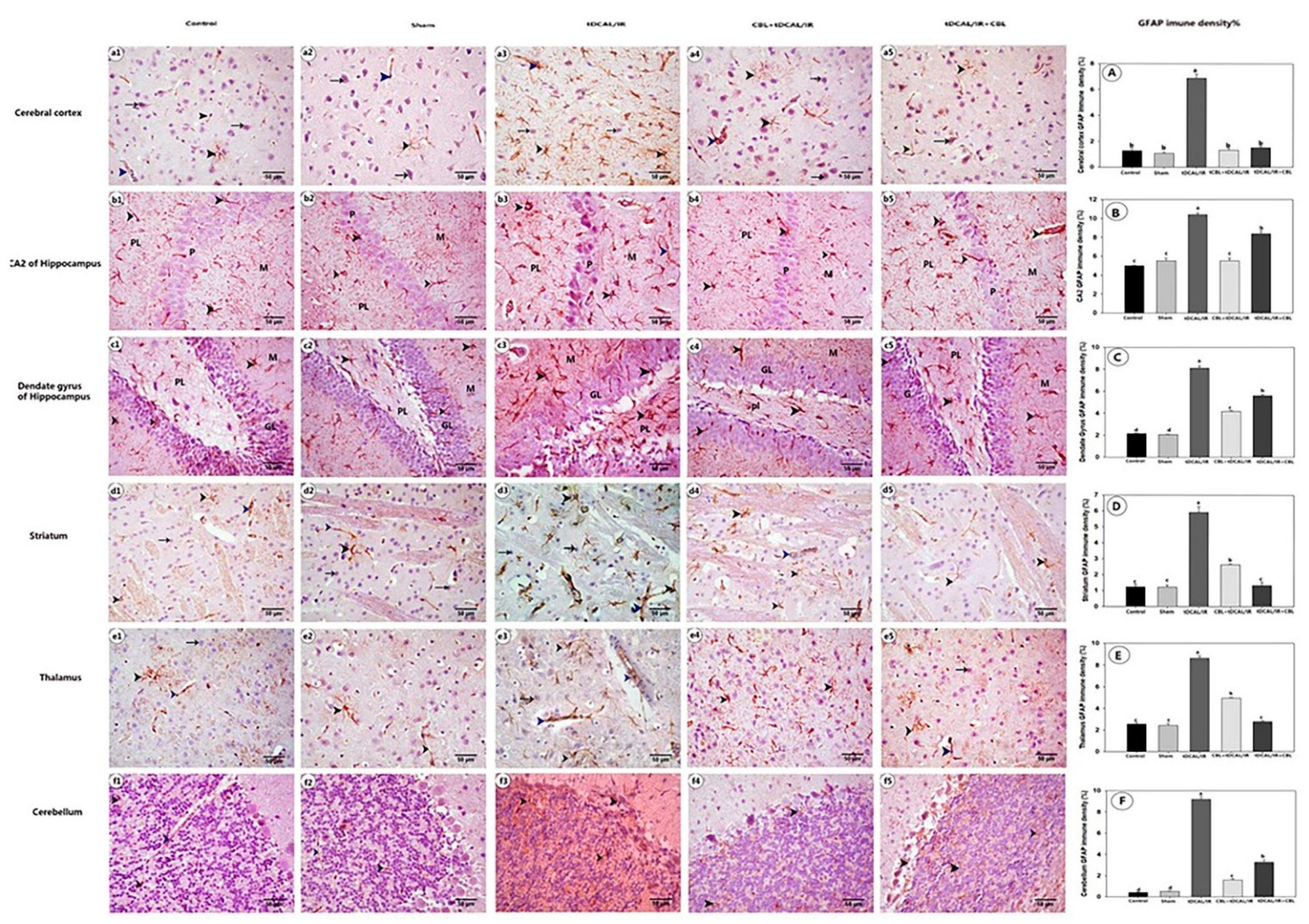
2.7. Histological Findings
2.7.1. CBL Improved Neuronal Survival and Preserved Brain Histoarchitecture in the Forebrain IR Mice Model
2.7.2. CBL Retained the Normal Distribution of Nissl Granules within the Brain Neurons in the Forebrain IR Mice Model
2.7.3. CBL Attenuated GFAP Immune Expression in the Forebrain IR Mice Model
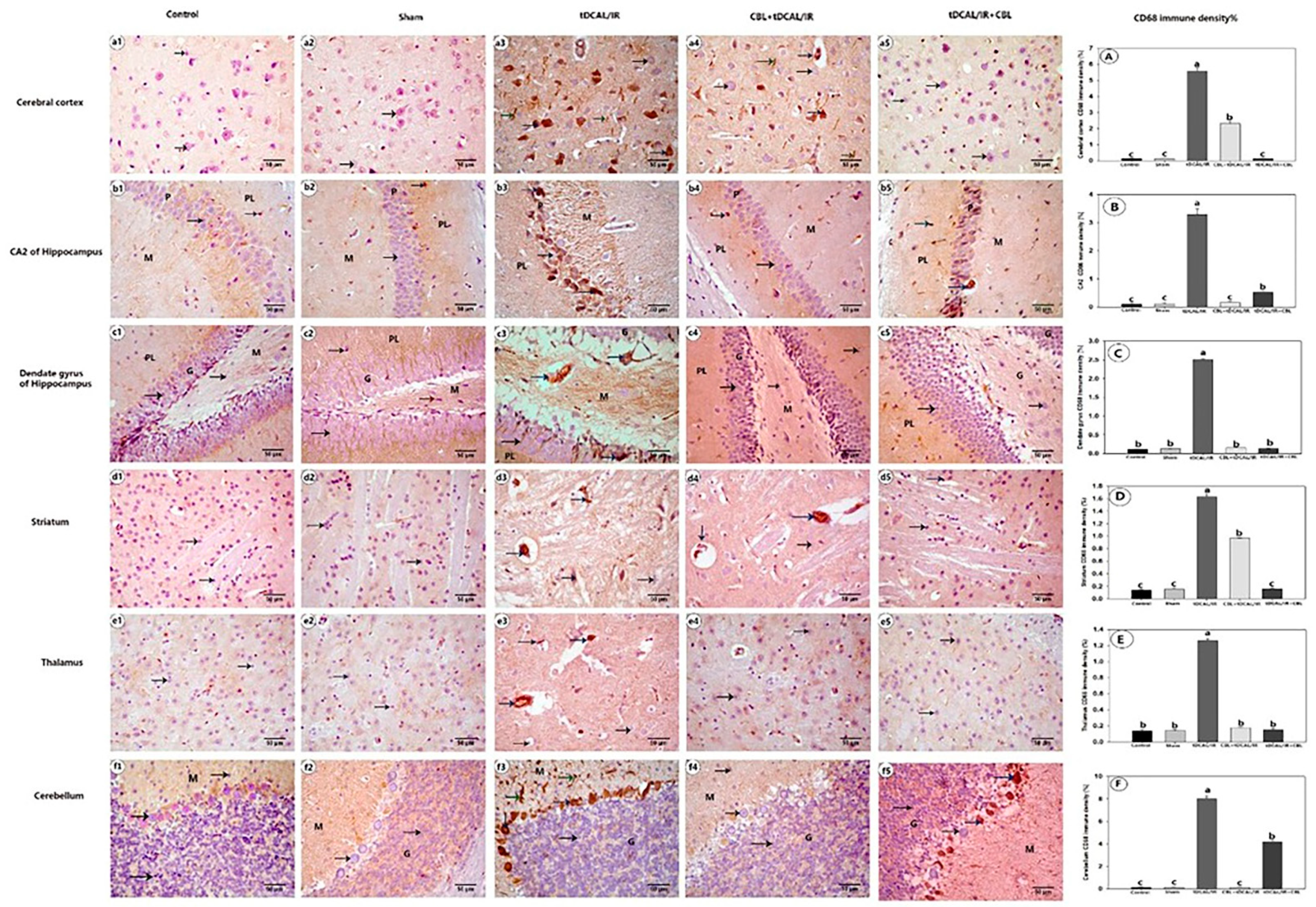
2.7.4. CBL Reduced CD68 Immune Expression in the Forebrain IR Mice Model
3. Discussion
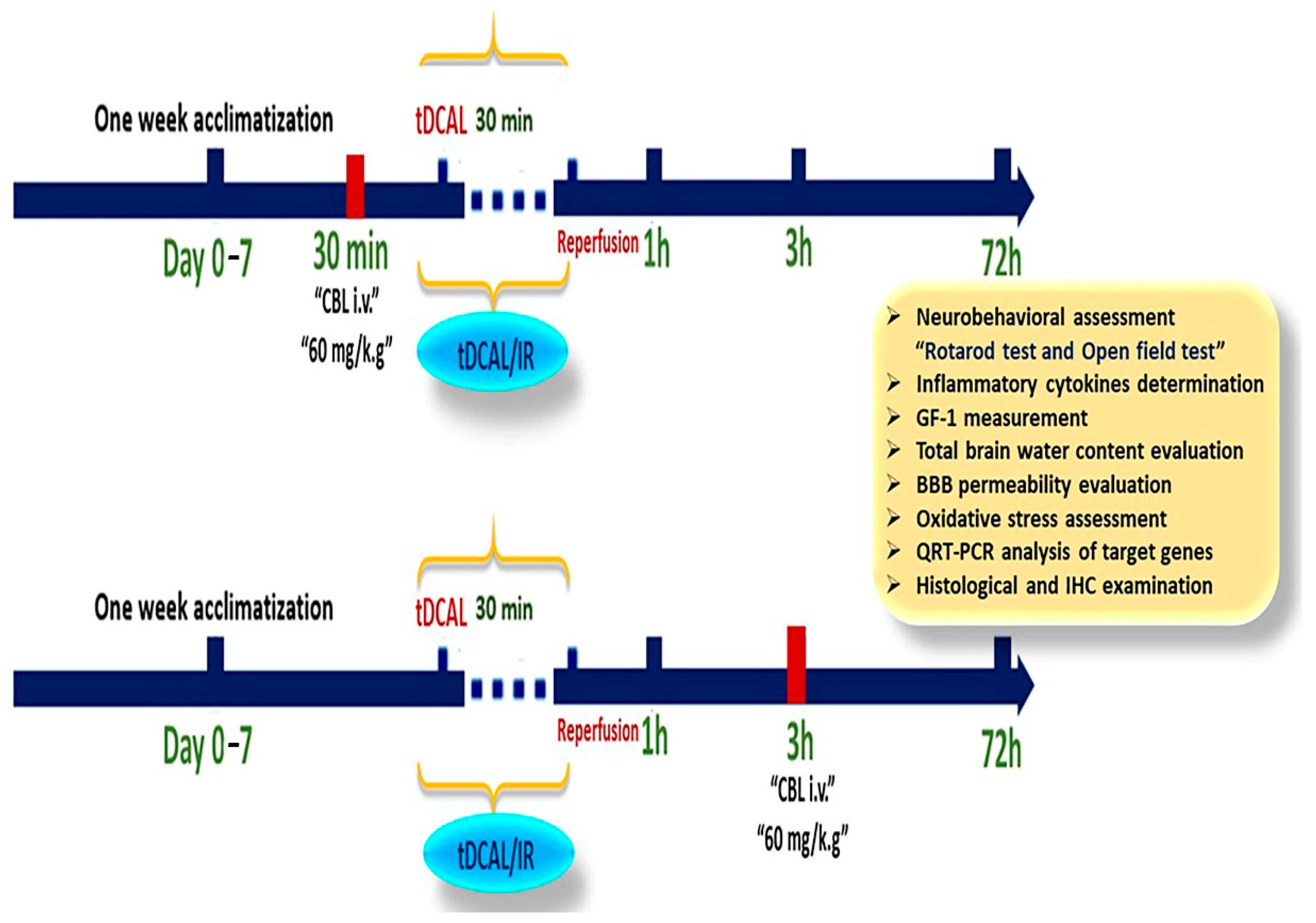
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Mice
4.3. Establishment of a Forebrain IR Mouse Model
4.4. CBL Treatment
4.5. Assessment of Neurological–Functional Recovery
4.5.1. Rotarod Test
4.5.2. Open Field Test (OFT)
4.6. Euthanasia, Blood, and Tissue Harvesting
4.7. Quantifying the Amount of Water in the Brain
4.8. Measurement of Serum TNF-α, IL-6, and IGF-1
4.9. Assessment of Blood–Brain Barrier (BBB) Permeability
4.10. Quantifying Oxidative Stress and Antioxidant Capacity
4.11. RT-qPCR
4.12. Histological Examination
4.13. Immunohistochemistry of GFAP and CD86
4.14. Morphometric Analysis
4.15. Data Analysis
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeSai, C.; Hays Shapshak, A. Cerebral Ischemia. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Li, W.; Huang, R.; Shetty, R.A.; Thangthaeng, N.; Liu, R.; Chen, Z.; Sumien, N.; Rutledge, M.; Dillon, G.H.; Yuan, F.; et al. Transient focal cerebral ischemia induces long-term cognitive function deficit in an experimental ischemic stroke model. Neurobiol. Dis. 2013, 59, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.T.; Selan, C.; Chia, J.S.J.; Sturgeon, S.A.; Wright, D.K.; Zamani, A.; Pereira, M.; Nandurkar, H.H.; Sashindranath, M. Characterization of a novel model of global forebrain ischaemia–reperfusion injury in mice and comparison with focal ischaemic and haemorrhagic stroke. Sci. Rep. 2020, 10, 18170. [Google Scholar] [CrossRef] [PubMed]
- Abumelha, H.M.; Alkhatib, F.; Alzahrani, S.; Abualnaja, M.; Alsaigh, S.; Alfaifi, M.Y.; Althagafi, I.; El-Metwaly, N. Synthesis and characterization for pharmaceutical models from Co (II), Ni (II) and Cu (II)-thiophene complexes; apoptosis, various theoretical studies and pharmacophore modeling. J. Mol. Liq. 2021, 328, 115483. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, E.; Yuan, H. Cerebral carbon dioxide embolism after kidney cancer laparoscopic surgery with full neurological recovery: A case report. Medicine 2020, 99, e20986. [Google Scholar] [CrossRef] [PubMed]
- Donnino, M.W.; Andersen, L.W.; Berg, K.M.; Reynolds, J.C.; Nolan, J.P.; Morley, P.T.; Lang, E.; Cocchi, M.N.; Xanthos, T.; Callaway, C.W. Temperature management after cardiac arrest: An advisory statement by the advanced life support task force of the international liaison committee on resuscitation and the American Heart Association emergency cardiovascular care committee and the council on cardiopulmonary, critical care, Perioperative and Resuscitation. Circulation 2015, 132, 2448–2456. [Google Scholar] [PubMed] [Green Version]
- Girotra, S.; Chan, P.S.; Bradley, S.M. Post-resuscitation care following out-of-hospital and in-hospital cardiac arrest. Heart 2015, 101, 1943–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Splichal, Z.; Jurajda, M.; Duris, K. The Role of Inflammatory Response in Stroke Associated Programmed Cell Death. Curr. Neuropharmacol. 2018, 16, 1365–1374. [Google Scholar] [CrossRef]
- Kang, H.B.; Kim, G.; Kim, H.; Han, S.R.; Chae, D.J.; Song, H.-J.; Kim, D.W. Cerebrolysin Attenuates Astrocyte Activation Following Repetitive Mild Traumatic Brain Injury: Implications for Chronic Traumatic Encephalopathy. J. Life Sci. 2013, 23, 1096–1103. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.K.; Park, Y.S.; Woo, J.S.; Kim, J.H.; Beom, J.H.; Chung, S.P.; You, J.S.; Suh, S.W. Transient Global Ischemia-Induced Brain Inflammatory Cascades Attenuated by Targeted Temperature Management. Int. J. Mol. Sci. 2021, 22, 5114. [Google Scholar] [CrossRef]
- Naumann, T.; Schnell, O.; Zhi, Q.; Kirsch, M.; Schubert, K.O.; Sendtner, M.; Hofmann, H.D. Endogenous Ciliary Neurotrophic Factor Protects GABAergic, But Not Cholinergic, Septohippocam pal Neurons Following Fimbria-fornix Transection. Brain Pathol. 2003, 13, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Wronski, R.; Kronawetter, S.; Hutter-Paier, B.; Crailsheim, K.; Windisch, M. A brain derived peptide preparation reduces the translation dependent loss of a cytoskeletal protein in primary cultured chicken neurons. In Advances in Dementia Research; Springer: Vienna, Austria, 2000; pp. 263–272. [Google Scholar] [CrossRef]
- Bornstein, N.M.; Guekht, A.; Vester, J.; Heiss, W.-D.; Gusev, E.; Hömberg, V.; Rahlfs, V.W.; Bajenaru, O.; Popescu, B.O.; Muresanu, D. Safety and efficacy of Cerebrolysin in early post-stroke recovery: A meta-analysis of nine randomized clinical trials. Neurol. Sci. 2018, 39, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Sadigh-Eteghad, S.; Geranmayeh, M.H.; Majdi, A.; Salehpour, F.; Mahmoudi, J.; Farhoudi, M. Intranasal cerebrolysin improves cognitive function and structural synaptic plasticity in photothrombotic mouse model of medial prefrontal cortex ischemia. Neuropeptides 2018, 71, 61–69. [Google Scholar] [CrossRef]
- Huemer, J.; Plattner, B.; Planer, N.; Steiner, H.; Feucht, M. Psychopathology in adolescents with TLE and FLE. Eur. J. Paediatr. Neurol. 2016, 20, 880–887. [Google Scholar] [CrossRef]
- Yang, G.; Kitagawa, K.; Matsushita, K.; Mabuchi, T.; Yagita, Y.; Yanagihara, T.; Matsumoto, M. C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: Selective neuronal death in the murine transient forebrain ischemia. Brain Res. 1997, 752, 209–218. [Google Scholar] [CrossRef]
- Ziganshina, L.E.; Abakumova, T.; Hoyle, C.H. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst. Rev. 2020, 7, CD007026. [Google Scholar] [PubMed]
- Milot, M.R.; Plamondon, H. Time-dependent effects of global cerebral ischemia on anxiety, locomotion, and habituation in rats. Behav. Brain Res. 2009, 200, 173–180. [Google Scholar] [CrossRef]
- Wilson, M.; Staniforth, A.; Till, R.; das Nair, R.; Vesey, P. The psychosocial outcomes of anoxic brain injury following cardiac arrest. Resuscitation 2014, 85, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Neigh, G.N.; Karelina, K.; Glasper, E.R.; Bowers, S.L.; Zhang, N.; Popovich, P.G.; DeVries, A.C. Anxiety after cardiac arrest/cardiopulmonary resuscitation: Exacerbated by stress and prevented by minocycline. Stroke 2009, 40, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Wang, Y.; Kai, G.; Zhao, S.; Huang, T.; Li, Y.; Xu, Y.; Zhang, L.; Pang, T. Cerebrolysin Ameliorates Focal Cerebral Ischemia Injury Through Neuroinflammatory Inhibition via CREB/PGC-1α Pathway. Front. Pharmacol. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Godinho, J.; de Oliveira, R.M.W.; de Sa-Nakanishi, A.B.; Bacarin, C.C.; Huzita, C.H.; Longhini, R.; Mello, J.C.P.; Nakamura, C.V.; Previdelli, I.S.; Ribeiro, M.H.D.M.; et al. Ethyl-acetate fraction of Trichilia catigua restores long-term retrograde memory and reduces oxidative stress and inflammation after global cerebral ischemia in rats. Behav. Brain Res. 2018, 337, 173–182. [Google Scholar] [CrossRef]
- Edel Hennessy, E.; Griffin, W.; Cunningham, C. Cajal course on Neuroinflammation and how to study it Course directors. Glia 2018, 61, 71–90. [Google Scholar]
- Jiang, M.; Liu, X.; Zhang, D.; Wang, Y.; Hu, X.; Xu, F.; Jin, M.; Cao, F.; Xu, L. Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization. J. Neuroinflammation 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhong, Q.; Chen, X.; Wu, X.; Sha, R.; Song, G.; Zhang, C.; Chen, X. Neuroprotective effects of celastrol on transient global cerebral ischemia rats via regulating HMGB1/NF-κB signaling pathway. Front. Neurosci. 2020, 14, 847. [Google Scholar] [CrossRef]
- Wine, R.N.; McPherson, C.A.; Harry, G.J. IGF-1 and pAKT Signaling Promote Hippocampal CA1 Neuronal Survival Following Injury to Dentate Granule Cells. Neurotox. Res. 2009, 16, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.; Berger, S.; Kristian, T. Simple model of forebrain ischemia in mouse. J. Neurosci. Methods 2012, 204, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Åberg, N.D.; Åberg, D.; Jood, K.; Nilsson, M.; Blomstrand, C.; Kuhn, H.G.; Svensson, J.; Jern, C.; Isgaard, J. Altered levels of circulating insulin-like growth factor I (IGF-I) following ischemic stroke are associated with outcome—A prospective observational study. BMC Neurol. 2018, 18, 108. [Google Scholar] [CrossRef] [Green Version]
- Obaid, R. Synthesis and biological evaluation of some new imidazo [1, 2-c] pyrimido [5, 4-e] pyrimidin-5-amine derivatives. J. Umm Al-Qura Univ. Appl. Sci. 2021, 7, 16–22. [Google Scholar]
- El-Beeh, M.E.; El-Badawi, A.A.; Amin, A.H.; Qari, S.H.; Ramadan, M.F.; Filfilan, W.M.; El-Sayyad, H.I. Anti-aging trait of whey protein against brain damage of senile rats. J. Umm Al-Qura Univ. Appl. Sci. 2022, 8, 8–20. [Google Scholar] [CrossRef]
- Sage, J.; Van Uitert, R.L.; Duffy, T. Early changes in blood brain barrier permeability to small molecules after transient cerebral ischemia. Stroke 1984, 15, 46–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamanna, J.C.; Kaiserman-Abramof, I.; Xu, K.; Daugherty, S.; Chávez, J.C.; Pichiule, P. Acute and Delayed Effects of Transient Global Cerebral Ischemia on Rat Brain Capillary Endothelial Cells In Vivo. In Ischemic Blood Flow in the Brain; Springer: Tokyo, Japan, 2001; pp. 319–325. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Mo, Y.; Li, R.; Huang, S.; Zhang, A.; Ni, X.; Dai, Q.; Wang, J. DCA Protects against Oxidation Injury Attributed to Cerebral Ischemia-Reperfusion by Regulating Glycolysis through PDK2-PDH-Nrf2 Axis. Oxidative Med. Cell. Longev. 2021, 2021, 5173035. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H.; et al. Oxidative Stress in Ischemic Brain Damage: Mechanisms of Cell Death and Potential Molecular Targets for Neuroprotection. Antioxid. Redox Signal. 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.S.; Muresanu, D.F.; Ozkizilcik, A.; Sahib, S.; Tian, Z.R.; Lafuente, J.V.; Nozari, A.; Feng, L.; Buzoianu, A.D.; Menon, P.K.; et al. Superior antioxidant and anti-ischemic neuroprotective effects of cerebrolysin in heat stroke following intoxication of engineered metal Ag and Cu nanoparticles: A comparative biochemical and physiological study with other stroke therapies. Prog. Brain Res. 2021, 266, 301–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, L.; Zhao, Y. Modulation of immune responses through direct activation of Toll-like receptors to T cells. Clin. Exp. Immunol. 2010, 160, 168–175. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Ahmad-Nejad, P.; da Costa, C.; Miethke, T.; Kirschning, C.J.; Häcker, H.; Wagner, H. Endocytosed HSP60s Use Toll-like Receptor 2 (TLR2) and TLR4 to Activate the Toll/Interleukin-1 Receptor Signaling Pathway in Innate Immune Cells. J. Biol. Chem. 2001, 276, 31332–31339. [Google Scholar] [CrossRef] [Green Version]
- Shan, R.; Zhou, H.; Liu, X.; Su, G.; Liu, G.; Zhang, X.; Sun, C.; Yu, Z.; Zhan, L.; Huang, Z. Neuroprotective effects of four different fluids on cerebral ischaemia/reperfusion injury in rats through stabilization of the blood–brain barrier. Eur. J. Neurosci. 2021, 54, 5586–5600. [Google Scholar] [CrossRef]
- Cores, Á.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. Biomolecules 2020, 10, 904. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.; Sandercock, P.; Dennis, M.; Starr, J. Is Breakdown of the Blood-Brain Barrier Responsible for Lacunar Stroke, Leukoaraiosis, and Dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Pinard, E.; Nallet, H.; MacKenzie, E.T.; Seylaz, J.; Roussel, S. Penumbral microcirculatory changes associated with peri-infarct depolarizations in the rat. Stroke 2002, 33, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, D.M.; Zechariah, A. Implications of Vascular Endothelial Growth Factor for Postischemic Neurovascular Remodeling. J. Cereb. Blood Flow Metab. 2009, 29, 1620–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marghani, B.H.; Fehaid, A.; Ateya, A.I.; Ezz, M.A.; Saleh, R.M. Photothermal therapeutic potency of plasmonic silver nanoparticles for apoptosis and anti-angiogenesis in testosterone induced benign prostate hyperplasia in rats. Life Sci. 2022, 291, 120240. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, D.; Mccarron, R.; Bertrand, N.; Spatz, M. Endothelins Release 51Cr from Cultured Human Cerebromicrovascular Endothelium. Biochem. Biophys. Res. Commun. 1993, 191, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Zimmermann-Meinzingen, S.; Johanson, C.E. Cerebrolysin reduces blood-cerebrospinal fluid barrier permeability change, brain pathology, and functional deficits following traumatic brain injury in the rat. Ann. N. Y. Acad. Sci. 2010, 1199, 125–137. [Google Scholar] [CrossRef]
- Irmak, M.K.; Fadillioglu, E.; Sogut, S.; Erdogan, H.; Gulec, M.; Ozer, M.; Yagmurca, M.; Gozukara, M.E. Effects of caffeic acid phenethyl ester and alpha-tocopherol on reperfusion injury in rat brain. Cell Biochem. Funct. 2003, 21, 283–289. [Google Scholar] [CrossRef]
- Chan, P.H. Reactive Oxygen Radicals in Signaling and Damage in the Ischemic Brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef]
- El Sayed, N.S.; Kandil, E.A.; Ghoneum, M.H. Probiotics Fermentation Technology, a Novel Kefir Product, Ameliorates Cognitive Impairment in Streptozotocin-Induced Sporadic Alzheimer’s Disease in Mice. Oxidative Med. Cell. Longev. 2021, 2021, 5525306. [Google Scholar] [CrossRef]
- Türeyen, K.; Vemuganti, R.; Sailor, K.A.; Dempsey, R.J. Infarct volume quantification in mouse focal cerebral ischemia: A comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J. Neurosci. Methods 2004, 139, 203–207. [Google Scholar] [CrossRef]
- Zhang, L.; Chopp, M.; Zhang, Z.G. Abstract WMP36: The Sonic Hedgehog Signaling Pathway Mediates Cerebrolysin-Improved Neurological Functions after Stroke. Stroke 2013, 44, AWMP36. [Google Scholar] [CrossRef] [Green Version]
- Ubhi, K.; Rockenstein, E.; Doppler, E.; Mante, M.; Adame, A.; Patrick, C.; Trejo, M.; Crews, L.; Paulino, A.; Moessler, H.; et al. Neurofibrillary and neurodegenerative pathology in APP-transgenic mice injected with AAV2-mutant TAU: Neuroprotective effects of Cerebrolysin. Acta Neuropathol. 2009, 117, 699–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, D.C.; Brizuela, N.O.; Álvarez, R.G.; García, E.H.; Mejía, G.B.; Olguín, H.J. Cerebrolysin and morphine decrease glutathione and 5-hydroxyindole acetic acid levels in fasted rat brain. Biomed. Pharmacother. 2009, 63, 517–521. [Google Scholar] [CrossRef]
- Gutmann, B.; Hutter-Paier, B.; Skofitsch, G.; Windisch, M.; Gmeinbauer, R. In vitro models of brain ischemia: The peptidergic drug cerebrolysin protects cultured chick cortical neurons from cell death. Neurotox. Res. 2002, 4, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef]
- Strużyńska, L.; Dąbrowska-Bouta, B.; Koza, K.; Sulkowski, G. Inflammation-Like Glial Response in Lead-Exposed Immature Rat Brain. Toxicol. Sci. 2007, 95, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Kingham, P.J.; Cuzner, M.L.; Pocock, J.M. Apoptotic Pathways Mobilized in Microglia and Neurones as a Consequence of Chromogranin A-Induced Microglial Activation. J. Neurochem. 1999, 73, 538–547. [Google Scholar] [CrossRef]
- Schnieder, T.P.; Trencevska, I.; Rosoklija, G.; Stankov, A.; Mann, J.J.; Smiley, J.; Dwork, A.J. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. Neurol. 2014, 73, 880–890. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.-Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Annunziato, L.; Boscia, F.; Pignataro, G. Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia. J. Cereb. Blood Flow Metab. 2013, 33, 969–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.-R.; Liu, J.-C.; Bao, J.-S.; Bai, Q.-Q.; Wang, G.-Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Barakat, W.; Safwet, N.; El-Maraghy, N.N.; Zakaria, M.N. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur. J. Pharmacol. 2014, 724, 43–50. [Google Scholar] [CrossRef]
- Kane, M.J.; Angoa-Pérez, M.; Briggs, D.I.; Viano, D.C.; Kreipke, C.W.; Kuhn, D.M. A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods 2012, 203, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Caston, J.; Jones, N.; Stelz, T. Role of Preoperative and Postoperative Sensorimotor Training on Restoration of the Equilibrium Behavior in Adult Mice Following Cerebellectomy. Neurobiol. Learn. Mem. 1995, 64, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, D.C.; Campbell, C.A.; Stretton, J.L.; Mackay, K. Correlation Between Motor Impairment and Infarct Volume After Permanent and Transient Middle Cerebral Artery Occlusion in the Rat. Stroke 1997, 28, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Archer, J. Tests for emotionality in rats and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Menard, J.; Treit, D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci. Biobehav. Rev. 1999, 23, 591–613. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Brain water content: A misunderstood measurement? Transl. Stroke Res. 2012, 3, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Marghani, B.H.; El-Adl, M.; Ateya, A.I.; Othman, B.H.; Ghamry, H.I.; Shukry, M.; Soliman, M.M.; Rizk, M.A. The Potential Protective Effect and Underlying Mechanisms of Physiological Unconjugated Hyperbilirubinemia Mediated by UGT1A1 Antisense Oligonucleotide Therapy in a Mouse Model of Cyclosporine A-Induced Chronic Kidney Disease. Metabolites 2022, 12, 999. [Google Scholar] [CrossRef]
- Pfaffl, M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Yin, W.; Badr, A.E.; Mychaskiw, G.; Zhang, J.H. Down regulation of COX-2 is involved in hyperbaric oxygen treatment in a rat transient focal cerebral ischemia model. Brain Res. 2002, 926, 165–171. [Google Scholar] [CrossRef]
- Horobin, R.W. Theory of histological staining. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; E-Book; Elsevier Health Sciences: Beijing, China, 2018; pp. 114–123. [Google Scholar]
- Jones, M.L. Histotechnology: A Self Instructional Text. J. Histotechnol. 2015, 38, 97. [Google Scholar] [CrossRef]
- Petrosyan, K.; Tamayo, R.; Joseph, D. Sensitivity of a novel biotin-free detection reagent (Powervision+™) for immunohistochemistry. J. Histotechnol. 2002, 25, 247–250. [Google Scholar] [CrossRef]
- Lehr, C.A.; Tan, C.S.; Ysseldyke, J. Alternative schools: A synthesis of state-level policy and research. Remedial Spec. Educ. 2009, 30, 19–32. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Habeby, M.; El-Sherif, N.; Al-Gholam, M. Influence of global cerebral ischemia/reperfusion injury on rat dentate gyrus and the possible protective effect of beetroot (Beta vulgaris L.) extract. Eur. J. Anat. 2021, 25, 4. [Google Scholar]
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Chandar, S.S.; Rao, K.V.B. Protease Inhibitors from Marine Actinobacteria as a Potential Source for Antimalarial Compound. PLoS ONE 2014, 9, e90972. [Google Scholar] [CrossRef] [Green Version]


| Gene | GenBank Accession Number | Oligonucleotide Sequence | Annealing Temperature (°C) | Size (bp) |
|---|---|---|---|---|
| IL-1β | NM_008361.4 | f5,-TGCCACCTTTTGACAGTGATG-3, r5,-TGATGTGCTGCTGCGAGATT-3, | 60 | 138 |
| IL-6 | NM_001314054.1 | f5,-GACAAAGCCAGAGTCCTTCAGA-3, r5,-TGTGACTCCAGCTTATCTCTTGG-3, | 59 | 76 |
| TNF-α | NM_001278601.1 | f5,-ACTGAACTTCGGGGTGATCG-3, r5,-CCACTTGGTGGTTTGTGAGTG-3, | 60 | 107 |
| IL10 | NM_010548.2 | f5,-AGTGGAGCAGGTGAAGAGTG-3, r5,-TGGAGTCCAGCAGACTCAATAC-3, | 58 | 160 |
| NF-κB | AY388959.1 | F 5,-CTGGCAAGCGTATCCCAAGA-3, R5,-TTCCGAAGTCGAACAGCCTC-3, | 60 | 127 |
| TLR2 | NM_011905.3 | F 5,-GCAGGAGATGTGTCCGCAAT-3, R 5,-AGAAGGAAACAGTCCGCACC-3, | 62 | 111 |
| TLR4 | NM_021297.3 | F 5,-GGACTCTGATCATGGCACTGT-3, R 5,-TCTTCAAGGGGTTGAAGCTC-3, | 58 | 174 |
| Keap1 | NM_001110307.1 | F 5,-GATGGGCAGGACCAGTTGAA-3, R 5,-CCGAGGACGTAGATCTTGCC-3, | 60 | 134 |
| Nrf2 | NM_010902.4 | F 5,-CCTCACCTCTGCTGCAAGTA-3, R 5,-AGCTCATAGTCCTTCTGTCGC-3, | 59 | 205 |
| CAT | NM_009804.2 | F 5,-GCGGATTCCTGAGAGAGTGG-3, R 5,-TGTGGAGAATCGAACGGCAA-3, | 59 | 145 |
| SOD3 | NM_011435.3 | F 5,-GAGAAGATAGGCGACACGCA-3, R 5,-GAGAACCAAGCCGGTGATCT-3, | 59 | 156 |
| GPX3 | NM_001329860.1 | F 5,-CATCCTGCCTTCTGTCCCTG-3, R 5,-CGATGGTGAGGGCTCCATAC-3, | 62 | 126 |
| VEGF | NM_001025257.3 | F 5,-TGAGACCCTGGTGGACATCT-3, R 5,-CACTCCAGGGCTTCATCGTT-3, | 59 | 117 |
| EDNRA | NM_010332.2 | F 5,-TTGACCTCCCCATCAACGTG-3, R 5,-AGCACAGAGGTTCAAGACGG-3, | 60 | 140 |
| β-actin | AY618569.1 | F 5,-GAGAGGGAAATCGTGCGTGA-3, R 5,-AACCGCTCGTTGCCAATAGT-3, | 60 | 152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marghani, B.H.; Rezk, S.; Ateya, A.I.; Alotaibi, B.S.; Othman, B.H.; Sayed, S.M.; Alshehri, M.A.; Shukry, M.; Mansour, M.M. The Effect of Cerebrolysin in an Animal Model of Forebrain Ischemic-Reperfusion Injury: New Insights into the Activation of the Keap1/Nrf2/Antioxidant Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12080. https://doi.org/10.3390/ijms241512080
Marghani BH, Rezk S, Ateya AI, Alotaibi BS, Othman BH, Sayed SM, Alshehri MA, Shukry M, Mansour MM. The Effect of Cerebrolysin in an Animal Model of Forebrain Ischemic-Reperfusion Injury: New Insights into the Activation of the Keap1/Nrf2/Antioxidant Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(15):12080. https://doi.org/10.3390/ijms241512080
Chicago/Turabian StyleMarghani, Basma H., Shaymaa Rezk, Ahmed I. Ateya, Badriyah S. Alotaibi, Basma H. Othman, Samy M. Sayed, Mohammed Ali Alshehri, Mustafa Shukry, and Mohamed M. Mansour. 2023. "The Effect of Cerebrolysin in an Animal Model of Forebrain Ischemic-Reperfusion Injury: New Insights into the Activation of the Keap1/Nrf2/Antioxidant Signaling Pathway" International Journal of Molecular Sciences 24, no. 15: 12080. https://doi.org/10.3390/ijms241512080
APA StyleMarghani, B. H., Rezk, S., Ateya, A. I., Alotaibi, B. S., Othman, B. H., Sayed, S. M., Alshehri, M. A., Shukry, M., & Mansour, M. M. (2023). The Effect of Cerebrolysin in an Animal Model of Forebrain Ischemic-Reperfusion Injury: New Insights into the Activation of the Keap1/Nrf2/Antioxidant Signaling Pathway. International Journal of Molecular Sciences, 24(15), 12080. https://doi.org/10.3390/ijms241512080









