Local Administration of Minocycline Improves Nerve Regeneration in Two Rat Nerve Injury Models
Abstract
1. Introduction
2. Results
2.1. Minocycline Decreases RAW 264.7 Cell Activation
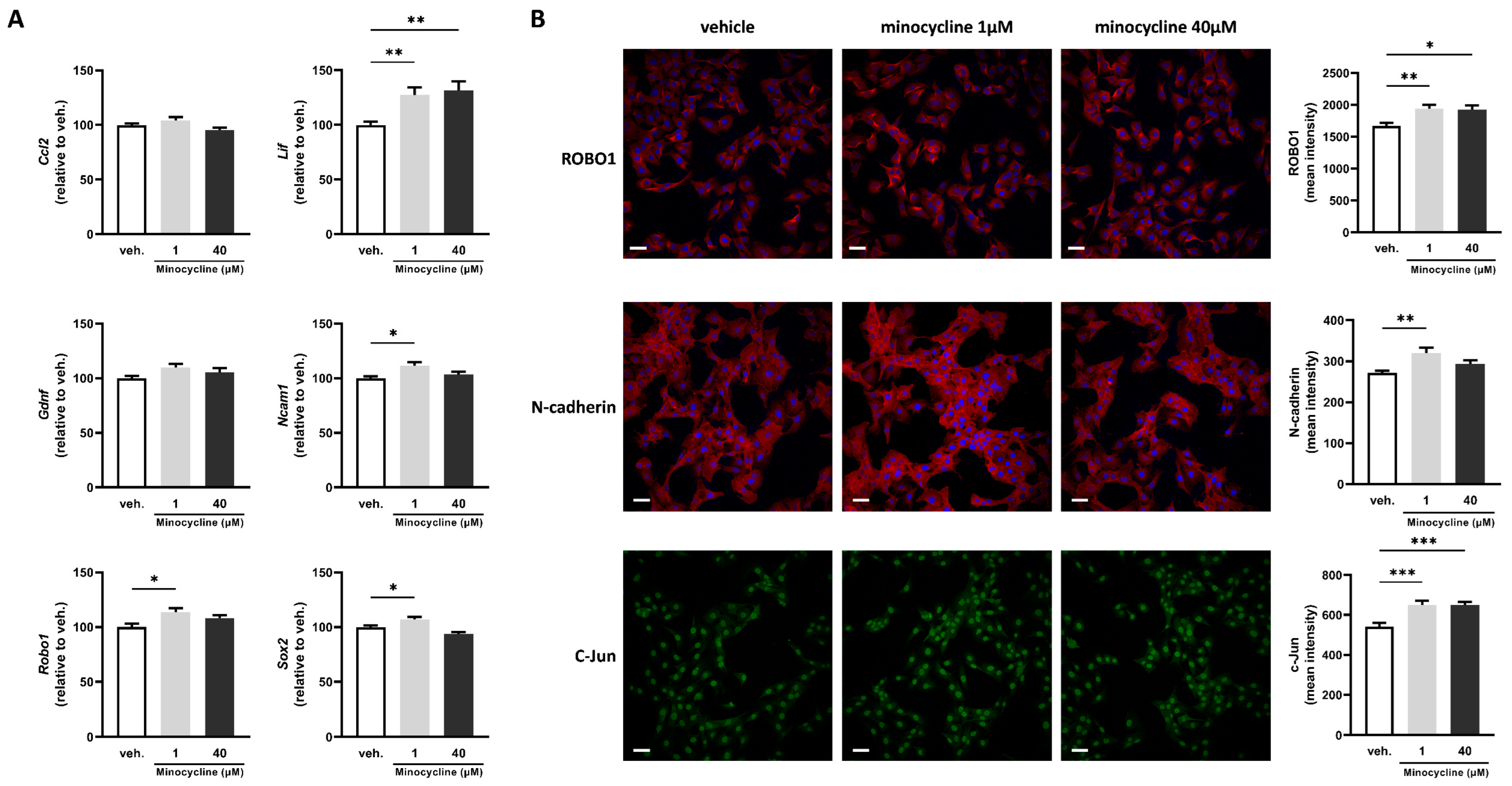
2.2. Minocycline Alters the Expression of Key Factors Associated with Schwann Cell Repair Phenotype in SCL 4.1/F7 Cells
2.3. Minocycline Alters the Expression of Key Factors Associated with Schwann Cell Repair Phenotype in Sciatic Nerve Explants
2.4. Minocycline Locally Delivered in Fibrin Gel Improves Nerve Regeneration following Sciatic Nerve Transection and Direct Repair
2.5. Minocycline Locally Delivered in Fibrin Gel Improves Nerve Regeneration in Sciatic Nerve Autograft
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Sciatic Nerve Explants
4.3. RT-qPCR
4.4. CCL2 Quantification by ELISA
4.5. Nerve Regeneration Models
4.6. Electrophysiology Recordings
4.7. Immunofluorescence
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruijs, A.C.; Jaquet, J.B.; Kalmijn, S.; Giele, H.; Hovius, S.E. Median and ulnar nerve injuries: A meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast. Reconstr. Surg. 2005, 116, 484–494; discussion 486–495. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Sameem, M.; Wood, T.J.; Bain, J.R. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast. Reconstr. Surg. 2011, 127, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.E.; Duraku, L.S.; de Jong, T.; de Vries, R.B.M.; Michiel Zuidam, J.; Hundepool, C.A. A systematic review and meta-analysis on the use of fibrin glue in peripheral nerve repair: Can we just glue it? J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1018–1033. [Google Scholar] [CrossRef] [PubMed]
- Tikka, T.M.; Koistinaho, J.E. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J. Immunol. 2001, 166, 7527–7533. [Google Scholar] [CrossRef]
- Vandooren, J.; Knoops, S.; Aldinucci Buzzo, J.L.; Boon, L.; Martens, E.; Opdenakker, G.; Kolaczkowska, E. Differential inhibition of activity, activation and gene expression of MMP-9 in THP-1 cells by azithromycin and minocycline versus bortezomib: A comparative study. PLoS ONE 2017, 12, e0174853. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. What is behind the non-antibiotic properties of minocycline? Pharmacol. Res. 2013, 67, 18–30. [Google Scholar] [CrossRef]
- Yong, V.W.; Wells, J.; Giuliani, F.; Casha, S.; Power, C.; Metz, L.M. The promise of minocycline in neurology. Lancet Neurol. 2004, 3, 744–751. [Google Scholar] [CrossRef]
- Keilhoff, G.; Langnaese, K.; Wolf, G.; Fansa, H. Inhibiting effect of minocycline on the regeneration of peripheral nerves. Dev. Neurobiol. 2007, 67, 1382–1395. [Google Scholar] [CrossRef]
- Keilhoff, G.; Schild, L.; Fansa, H. Minocycline protects Schwann cells from ischemia-like injury and promotes axonal outgrowth in bioartificial nerve grafts lacking Wallerian degeneration. Exp. Neurol. 2008, 212, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Ydens, E.; Amann, L.; Asselbergh, B.; Scott, C.L.; Martens, L.; Sichien, D.; Mossad, O.; Blank, T.; De Prijck, S.; Low, D.; et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci. 2020, 23, 676–689. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Mikos, A.G. Fibrin glue as a drug delivery system. J. Control. Release 2010, 148, 49–55. [Google Scholar] [CrossRef]
- Wood, M.D.; Gordon, T.; Kim, H.; Szynkaruk, M.; Phua, P.; Lafontaine, C.; Kemp, S.W.; Shoichet, M.S.; Borschel, G.H. Fibrin gels containing GDNF microspheres increase axonal regeneration after delayed peripheral nerve repair. Regen. Med. 2013, 8, 27–37. [Google Scholar] [CrossRef]
- Liu, F.Y.; Wu, Y.H.; Zhou, S.J.; Deng, Y.L.; Zhang, Z.Y.; Zhang, E.L.; Huang, Z.Y. Minocycline and cisplatin exert synergistic growth suppression on hepatocellular carcinoma by inducing S phase arrest and apoptosis. Oncol. Rep. 2014, 32, 835–844. [Google Scholar] [CrossRef]
- Filipovic, R.; Zecevic, N. Neuroprotective role of minocycline in co-cultures of human fetal neurons and microglia. Exp. Neurol. 2008, 211, 41–51. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.S.; Shin, K.Y.; Kim, E.M.; Kim, M.; Kim, H.S.; Park, C.H.; Jeong, Y.H.; Yoo, J.; Lee, J.P.; et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology 2007, 32, 2393–2404. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef]
- Amin, A.R.; Attur, M.G.; Thakker, G.D.; Patel, P.D.; Vyas, P.R.; Patel, R.N.; Patel, I.R.; Abramson, S.B. A novel mechanism of action of tetracyclines: Effects on nitric oxide synthases. Proc. Natl. Acad. Sci. USA 1996, 93, 14014–14019. [Google Scholar] [CrossRef]
- Kohno, H.; Chen, Y.; Kevany, B.M.; Pearlman, E.; Miyagi, M.; Maeda, T.; Palczewski, K.; Maeda, A. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J. Biol. Chem. 2013, 288, 15326–15341. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Q.; Zhang, Y.; Li, S.; Yi, S. Leukemia inhibitory factor regulates Schwann cell proliferation and migration and affects peripheral nerve regeneration. Cell Death Dis. 2021, 12, 417. [Google Scholar] [CrossRef]
- Mei, X.P.; Xu, H.; Xie, C.; Ren, J.; Zhou, Y.; Zhang, H.; Xu, L.X. Post-injury administration of minocycline: An effective treatment for nerve-injury induced neuropathic pain. Neurosci. Res. 2011, 70, 305–312. [Google Scholar] [CrossRef]
- Hua, X.Y.; Svensson, C.I.; Matsui, T.; Fitzsimmons, B.; Yaksh, T.L.; Webb, M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur. J. Neurosci. 2005, 22, 2431–2440. [Google Scholar] [CrossRef]
- Ismail, C.A.N.; Suppian, R.; Aziz, C.B.A.; Long, I. Minocycline attenuates the development of diabetic neuropathy by modulating DREAM and BDNF protein expression in rat spinal cord. J. Diabetes Metab. Disord. 2019, 18, 181–190. [Google Scholar] [CrossRef]
- Carriel, V.; Garzon, I.; Campos, A.; Cornelissen, M.; Alaminos, M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. J. Tissue Eng. Regen. Med. 2017, 11, 553–563. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hoshikawa, S.; Ueno, T.; Hirata, M.; Saito, T.; Ikeda, T.; Kawaguchi, H.; Nakamura, K.; Tanaka, S.; Ogata, T. SOX10 transactivates S100B to suppress Schwann cell proliferation and to promote myelination. PLoS ONE 2014, 9, e115400. [Google Scholar] [CrossRef]
- Hung, H.A.; Sun, G.; Keles, S.; Svaren, J. Dynamic regulation of Schwann cell enhancers after peripheral nerve injury. J. Biol. Chem. 2015, 290, 6937–6950. [Google Scholar] [CrossRef]
- Wanner, I.B.; Wood, P.M. N-cadherin mediates axon-aligned process growth and cell-cell interaction in rat Schwann cells. J. Neurosci. 2002, 22, 4066–4079. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; Kilpatrick, A.M.; Michael, K.; Wong, L.Y.N.; Ma, K.H.; Turmaine, M.; Svaren, J.; et al. Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. Elife 2021, 10, e62232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Tang, Y.; He, Z.; Ma, H.; Wang, L.; Liu, Y.; Yang, Q.; Pan, D.; Zhu, C.; Qian, S.; et al. Slit3 secreted from M2-like macrophages increases sympathetic activity and thermogenesis in adipose tissue. Nat. Metab. 2021, 3, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, D.; Tamburino, A.; Krasowska-Zoladek, A.; Sanoja, R.; Li, L.; Peterson, V.; Wang, X.; Uslaner, J. scRNA-seq generates a molecular map of emerging cell subtypes after sciatic nerve injury in rats. Commun. Biol. 2022, 5, 1105. [Google Scholar] [CrossRef]
- Cheng, K.I.; Wang, H.C.; Wu, Y.C.; Tseng, K.Y.; Chuang, Y.T.; Chou, C.W.; Chen, P.L.; Chang, L.L.; Lai, C.S. Sciatic Nerve Intrafascicular Lidocaine Injection-induced Peripheral Neuropathic Pain: Alleviation by Systemic Minocycline Administration. Clin. J. Pain 2016, 32, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.S.; Nagi, S.S.; Mahns, D.A. Minocycline reduces experimental muscle hyperalgesia induced by repeated nerve growth factor injections in humans: A placebo-controlled double-blind drug-crossover study. Eur. J. Pain 2020, 24, 1138–1150. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Li, X.; Chen, Z.; Chen, J.; Zhang, T.; Gu, X.; Yang, J. Deciphering the dynamic niches and regeneration-associated transcriptional program of motoneurons following peripheral nerve injury. iScience 2022, 25, 104917. [Google Scholar] [CrossRef]
- Chung, D.; Shum, A.; Caraveo, G. GAP-43 and BASP1 in Axon Regeneration: Implications for the Treatment of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 567537. [Google Scholar] [CrossRef]
- Yuan, Q.; Hu, B.; Su, H.; So, K.F.; Lin, Z.; Wu, W. GAP-43 expression correlates with spinal motoneuron regeneration following root avulsion. J. Brachial Plex. Peripher. Nerve Inj. 2009, 4, 18. [Google Scholar] [CrossRef]
- Bremer, M.; Frob, F.; Kichko, T.; Reeh, P.; Tamm, E.R.; Suter, U.; Wegner, M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia 2011, 59, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X. Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: A critical overview. Eur. J. Neurosci. 2016, 43, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.L.D.; Kellaway, S.C.; Kingston, I.; Guillemot-Legris, O.; Gregory, H.; Healy, J.; Phillips, J.B. Exploring the Nerve Regenerative Capacity of Compounds with Differing Affinity for PPARgamma In Vitro and In Vivo. Cells 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.L.; Dun, X.P.; Doddrell, R.D.S.; Mindos, T.; Drake, L.K.; Onaitis, M.W.; Florio, F.; Quattrini, A.; Lloyd, A.C.; D’Antonio, M.; et al. Sox2 expression in Schwann cells inhibits myelination in vivo and induces influx of macrophages to the nerve. Development 2017, 144, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]





| Species | Name | Symbol | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|---|
| Mouse | chemokine (C-C motif) ligand 2 | Ccl2 | GTCCCAAAGAAGCTGTAGTTTTTG | ATGTATGTCTGGACCCATTCC |
| Mouse | chemokine (C-C motif) ligand 3 | Ccl3 | AGATTCCACGCCAATTCATC | CTCAAGCCCCTGCTCTACAC |
| Mouse | chemokine (C-X-C motif) ligand 2 | Cxcl2 | TCAACGGAAGAACCAAAGAG | AATAAGTGAACTCTCAGACAGC |
| Mouse | hypoxanthine guanine phosphoribosyl transferase | Hprt1 | GACTGAAGAGCTACTGTAATG | AGATCATCTCCACCAATAAC |
| Mouse | prostaglandin-endoperoxide synthase 2 | Ptgs2 | TGACCCCCAAGGCTCAAATAT | TGAACCCAGGTCCTCGCTTA |
| Mouse | ribosomal protein L19 | Rpl19 | TGACCTGGATGAGAAGGATGAG | CTGTGATACATATGGCGGTCAATC |
| Mouse | TATA box binding protein | Tbp | CCCTTCACCAATGACTCCTATG | ACAGCCAAGATTCACGGTAG |
| Mouse | tumor necrosis factor | Tnf | CTACTGAACTTCGGGGTGATC | TGAGTGTGAGGGTCTGGGC |
| Rat | beta-2 microglobulin | B2m | CGTGATCTTTCTGGTGCTTG | GGTGGAACTGAGACACGTAG |
| Rat | C-C motif chemokine ligand 2 | Ccl2 | GCAAGATGATCCCAATGAGTC | GCTTGGTGACAAATACTACAGC |
| Rat | glial cell derived neurotrophic factor | Gdnf | GCTGACCAGTGACTCCAATATG | TGCCGCCGCTTGTTTATC |
| Rat | hypoxanthine phosphoribosyltransferase 1 | Hprt1 | ACCTCTCGAAGTGTTGGATAC | GATTCAAATCCCTGAAGTGCTC |
| Rat | LIF, interleukin 6 family cytokine | Lif | CAAGAGTCAACTGGCTCAAC | GCATGGAAAGGTGGGAAATC |
| Rat | neural cell adhesion molecule 1 | Ncam1 | GTGAGGTCTTTGCCTACC | CCAGATAGCTCGCAGATG |
| Rat | roundabout guidance receptor 1 | Robo1 | CTGGACGGAAGACAAGTCAC | GGTGGAAGGTCTCGCTTTG |
| Rat | ribosomal protein S18 | Rps18 | CTTCGCTATCACTGCCATTAAG | GTGAGGTCAATGTCTGCTTTC |
| Rat | SRY-box transcription factor 2 | Sox2 | GCAGTACAACTCCATGACCAG | GCGAGTAGGACATGCTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillemot-Legris, O.; Girmahun, G.; Shipley, R.J.; Phillips, J.B. Local Administration of Minocycline Improves Nerve Regeneration in Two Rat Nerve Injury Models. Int. J. Mol. Sci. 2023, 24, 12085. https://doi.org/10.3390/ijms241512085
Guillemot-Legris O, Girmahun G, Shipley RJ, Phillips JB. Local Administration of Minocycline Improves Nerve Regeneration in Two Rat Nerve Injury Models. International Journal of Molecular Sciences. 2023; 24(15):12085. https://doi.org/10.3390/ijms241512085
Chicago/Turabian StyleGuillemot-Legris, Owein, Gedion Girmahun, Rebecca J. Shipley, and James B. Phillips. 2023. "Local Administration of Minocycline Improves Nerve Regeneration in Two Rat Nerve Injury Models" International Journal of Molecular Sciences 24, no. 15: 12085. https://doi.org/10.3390/ijms241512085
APA StyleGuillemot-Legris, O., Girmahun, G., Shipley, R. J., & Phillips, J. B. (2023). Local Administration of Minocycline Improves Nerve Regeneration in Two Rat Nerve Injury Models. International Journal of Molecular Sciences, 24(15), 12085. https://doi.org/10.3390/ijms241512085






