Total Neoadjuvant Treatment for Locally Advanced Rectal Cancer Patients: Where Do We Stand?
Abstract
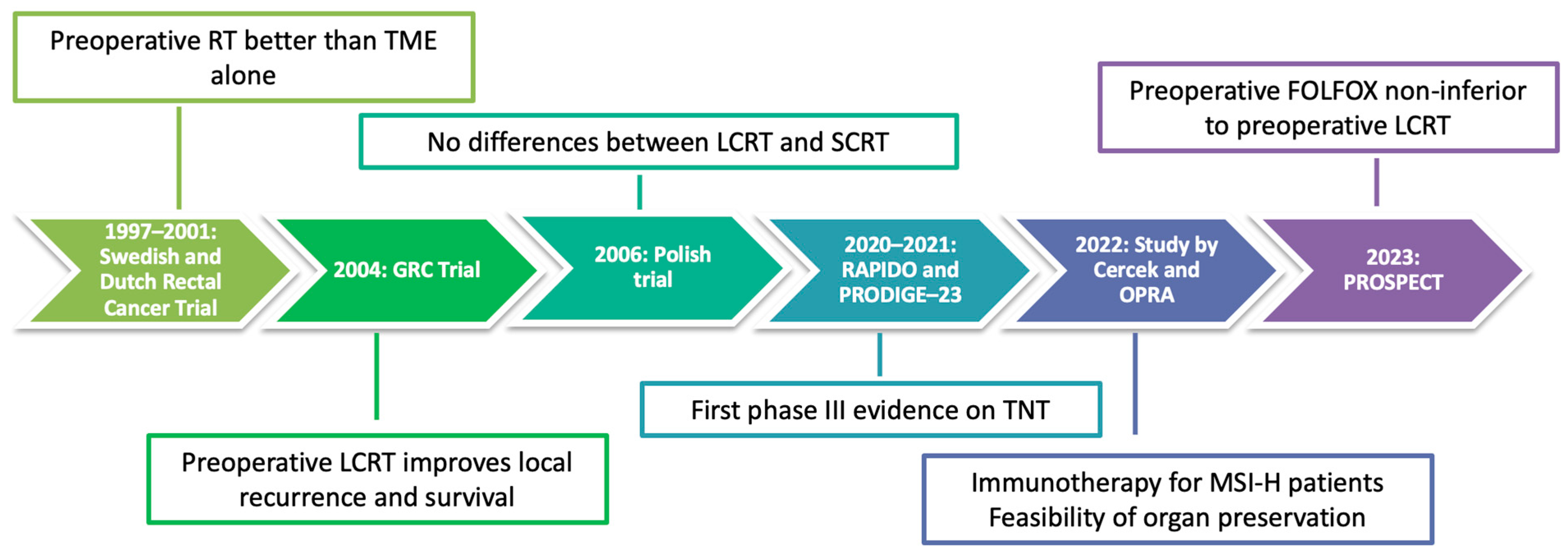
:1. Introduction
2. Phase III Practice-Changing Trials
2.1. RAPIDO
2.2. PRODIGE-23
2.3. PROSPECT
2.4. Considerations
3. How to Decide Optimal Treatment?
3.1. Patient Selection Criteria
3.2. Alternative Schemes of TNT in Phase II Trials
3.2.1. GRC-3
3.2.2. CAO/ARO/AIO-12
3.2.3. OPRA
3.3. Induction vs. Consolidation Chemotherapy
3.4. Risk of Overtreament
3.5. The Role of Surgery
4. Discussion and Future Perspectives
4.1. Role of Microsatellite Instability (MSI)
4.2. Nonoperative Management
4.3. Role of Circulating Tumor DNA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Kokelaar, R.F.; Evans, M.D.; Davies, M.; Harris, D.A.; Beynon, J. Locally advanced rectal cancer: Management challenges. Onco Targets Ther. 2016, 9, 6265–6272. [Google Scholar] [CrossRef] [Green Version]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; Van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Folkesson, J.; Birgisson, H.; Pahlman, L.; Cedermark, B.; Glimelius, B.; Gunnarsson, U. Swedish Rectal Cancer Trial: Long Lasting Benefits From Radiotherapy on Survival and Local Recurrence Rate. J. Clin. Oncol. 2005, 23, 5644–5650. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.; Goldstein, D.; Joseph, D.; Ackland, S.P.; Schache, D.; McClure, B.; McLachlan, S.-A.; et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.-F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.-J.; Bardet, E.; Beny, A.; Ollier, J.-C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sainato, A.; Nunzia, V.C.L.; Valentini, V.; De Paoli, A.; Maurizi, E.R.; Lupattelli, M.; Aristei, C.; Vidali, C.; Conti, M.; Galardi, A.; et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother. Oncol. 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, Å.; Broek, C.B.M.v.D.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.H.; Marijnen, C.A.M.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.; Bos, A.C.; Lemmens, V.E.; Tanis, P.J.; Hugen, N.; Nagtegaal, I.D.; de Wilt, J.H.; Verhoeven, R.H. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Nilsson, P.J.; Hospers, G.A.; Bahadoer, R.R.; Kranenbarg, E.M.-K.; Roodvoets, A.G.; Putter, H.; Berglund, Å.; Cervantes, A.; Crolla, R.M.; et al. Locoregional Failure During and After Short-course Radiotherapy followed by Chemotherapy and Surgery Compared to Long-course Chemoradiotherapy and Surgery—A Five-year Follow-up of the RAPIDO Trial. Ann. Surg. 2023, 10–109. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crinò, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III Trial of Infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (FOLFOXIRI) Compared with Infusional Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) As First-Line Treatment for Metastatic Colorectal Cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar] [CrossRef] [Green Version]
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507. [Google Scholar] [CrossRef]
- Conroy, T.; Etienne, P.-L.; Rio, E.; Evesque, L.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Boileve, A.; Delaye, M.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2023, 41, LBA3504. [Google Scholar] [CrossRef]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Dueck, A.C.; Mitchell, S.A.; Mamon, H.; Weiser, M.; Saltz, L.; Gollub, M.; Rogak, L.; Ginos, B.; Mazza, G.L.; et al. Patient-Reported Outcomes During and After Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048). J. Clin. Oncol. 2023, 41, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Glynne-Jones, R.; Appelt, A.; Beets-Tan, R.; Beets, G.; Haustermans, K.; Marijnen, C.; Minsky, B.D.; Ludmir, E.; Quirke, P.; et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol. 2020, 21, e252–e264. [Google Scholar] [CrossRef]
- Valentini, V.; van Stiphout, R.G.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.F.; Bujko, K.; Cionini, L.; et al. Selection of appropriate end-points (pCR vs 2yDFS) for tailoring treatments with prediction models in locally advanced rectal cancer. Radiother. Oncol. 2015, 114, 302–309. [Google Scholar] [CrossRef]
- Chua, Y.J.; Barbachano, Y.; Cunningham, D.; Oates, J.R.; Brown, G.; Wotherspoon, A.; Tait, D.; Massey, A.; Tebbutt, N.C.; Chau, I. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: A phase 2 trial. Lancet Oncol. 2010, 11, 241–248. [Google Scholar] [CrossRef]
- Dewdney, A.; Cunningham, D.; Tabernero, J.; Capdevila, J.; Glimelius, B.; Cervantes, A.; Tait, D.; Brown, G.; Wotherspoon, A.; de Castro, D.G.; et al. Multicenter Randomized Phase II Clinical Trial Comparing Neoadjuvant Oxaliplatin, Capecitabine, and Preoperative Radiotherapy with or without Cetuximab Followed by Total Mesorectal Excision in Patients with High-Risk Rectal Cancer (EXPERT-C). J. Clin. Oncol. 2012, 30, 1620–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, I.; Allen, M.; Cunningham, D.; Tait, D.; Brown, G.; Hill, M.; Sumpter, K.; Rhodes, A.; Wotherspoon, A.; Norman, A.R.; et al. Neoadjuvant systemic fluorouracil and mitomycin C prior to synchronous chemoradiation is an effective strategy in locally advanced rectal cancer. Br. J. Cancer 2003, 88, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Martos, C.; Pericay, C.; Aparicio, J.; Salud, A.; Safont, M.; Massuti, B.; Vera, R.; Escudero, P.; Maurel, J.; Marcuello, E.; et al. Phase II, Randomized Study of Concomitant Chemoradiotherapy Followed by Surgery and Adjuvant Capecitabine Plus Oxaliplatin (CAPOX) Compared with Induction CAPOX Followed by Concomitant Chemoradiotherapy and Surgery in Magnetic Resonance Imaging–Defined, Locally Advanced Rectal Cancer: Grupo Cáncer de Recto 3 Study. J. Clin. Oncol. 2010, 28, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martos, C.; Pericay, C.; Aparicio, J.; Salud, A.; Safont, M.; Massuti, B.; Vera, R.; Escudero, P.; Maurel, J.; Marcuello, E.; et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long-term results of the Spanish GCR-3 phase II randomized trial. Ann. Oncol. 2015, 26, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Giunta, E.; Bregni, G.; Pretta, A.; Deleporte, A.; Liberale, G.; Bali, A.; Moretti, L.; Troiani, T.; Ciardiello, F.; Hendlisz, A.; et al. Total neoadjuvant therapy for rectal cancer: Making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat. Rev. 2021, 96, 102177. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.-L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients with Locally Advanced Rectal Cancer. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Verheij, F.S.; Omer, D.M.R.; Williams, H.; Buckley, J.T.; Lin, S.T.; Qin, L.-X.; Thompson, H.M.; Yuval, J.B.; Gollub, M.J.; Wu, A.J.-C.; et al. Sustained organ preservation in patients with rectal cancer treated with total neoadjuvant therapy: Long-term results of the OPRA trial. J. Clin. Oncol. 2023, 41, 3520. [Google Scholar] [CrossRef]
- Goffredo, P.; Quezada-Diaz, F.F.; Garcia-Aguilar, J.; Smith, J.J. Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers 2022, 14, 3204. [Google Scholar] [CrossRef]
- Lord, A.C.; Corr, A.; Chandramohan, A.; Hodges, N.; Pring, E.; Airo-Farulla, C.; Moran, B.; Jenkins, J.T.; Di Fabio, F.; Brown, G. Assessment of the 2020 NICE criteria for preoperative radiotherapy in patients with rectal cancer treated by surgery alone in comparison with proven MRI prognostic factors: A retrospective cohort study. Lancet Oncol. 2022, 23, 793–801. [Google Scholar] [CrossRef]
- NICE Colorectal Cancer. Available online: https://WwwNiceOrgUk/Guidance/Ng151n.d (accessed on 20 June 2023).
- Ruppert, R.; Kube, R.; Strassburg, J.; Lewin, A.; Baral, J.; Maurer, C.A.; Sauer, J.; Junginger, T.; Hermanek, P.; Merkel, S.; et al. Avoidance of Overtreatment of Rectal Cancer by Selective Chemoradiotherapy: Results of the Optimized Surgery and MRI-Based Multimodal Therapy Trial. J. Am. Coll. Surg. 2020, 231, 413–413.e2. [Google Scholar] [CrossRef]
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009, 373, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heald, R.J.; Moran, B.J.; Ryall, R.D.; Sexton, R.; MacFarlane, J.K. Rectal cancer: The Basingstoke experience of total mesorectal excision, 1978–1997. Arch. Surg. 1998, 133, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foppa, C.; Carvello, M.; Maroli, A.; Sacchi, M.; Gramellini, M.; Montorsi, M.; Spinelli, A. Single-stapled anastomosis is associated with a lower anastomotic leak rate than double-stapled technique after minimally invasive total mesorectal excision for MRI-defined low rectal cancer. Surgery 2023, 173, 1367–1373. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Tian, G.; Liu, Y.; Jiang, Y.; Li, X.; Song, M. Meta-Analysis on the Efficacy of Indocyanine Green Fluorescence Angiography for Reduction of Anastomotic Leakage After Rectal Cancer Surgery. Am. Surg. 2021, 87, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.; Croft, J.; Corrigan, N.; Brown, J.M.; Goh, V.; Quirke, P.; Hulme, C.; Tolan, D.; Kirby, A.; Cahill, R.; et al. IntAct: Intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: A randomized controlled trial. Color. Dis. 2018, 20, O226–O234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Ogino, S.; Goel, A. Molecular classification and correlates in colorectal cancer. J. Mol. Diagn. 2008, 10, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Hoeijmakers, J.H. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Cabezón-Gutiérrez, L.; Custodio-Cabello, S.; Palka-Kotlowska, M.; Díaz-Pérez, D.; Mateos-Dominguez, M.; Galindo-Jara, P. Neoadjuvant immunotherapy for dMMR/MSI-H locally advanced rectal cancer: The future new standard approach? Eur. J. Surg. Oncol. 2023, 49, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Cercek, A.; Fernandes, G.D.S.; Roxburgh, C.S.; Ganesh, K.; Ng, S.; Sanchez-Vega, F.; Yaeger, R.; Segal, N.H.; Reidy-Lagunes, D.L.; Varghese, A.M.; et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin. Cancer Res. 2020, 26, 3271–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokas, E.; Appelt, A.; Glynne-Jones, R.; Beets, G.; Perez, R.; Garcia-Aguilar, J.; Rullier, E.; Smith, J.J.; Marijnen, C.; Peters, F.P.; et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat. Rev. Clin. Oncol. 2021, 18, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Strombom, P.; Chow, O.S.; Roxburgh, C.S.; Lynn, P.; Eaton, A.; Widmar, M.; Ganesh, K.; Yaeger, R.; Cercek, A.; et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients with a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019, 5, e185896. [Google Scholar] [CrossRef] [PubMed]
- Lynn, P.; Strombom, P.; Garcia-Aguilar, J. Organ-Preserving Strategies for the Management of Near-Complete Responses in Rectal Cancer after Neoadjuvant Chemoradiation. Clin. Colon. Rectal Surg. 2017, 30, 395–403. [Google Scholar] [CrossRef]
- Maas, M.; Lambregts, D.M.J.; Nelemans, P.J.; Heijnen, L.A.; Martens, M.H.; Leijtens, J.W.A.; Sosef, M.; Hulsewé, K.W.E.; Hoff, C.; Breukink, S.O.; et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann. Surg. Oncol. 2015, 22, 3873–3880. [Google Scholar] [CrossRef] [Green Version]
- Smith, F.M.; Wiland, H.; Mace, A.; Pai, R.K.; Kalady, M.F. Depth and lateral spread of microscopic residual rectal cancer after neoadjuvant chemoradiation: Implications for treatment decisions. Color. Dis. 2014, 16, 610–615. [Google Scholar] [CrossRef]
- Duldulao, M.P.M.; Lee, W.B.; Streja, L.D.; Chu, P.M.; Li, W.B.; Chen, Z.; Kim, J.M.; Garcia-Aguilar, J.M. Distribution of Residual Cancer Cells in the Bowel Wall After Neoadjuvant Chemoradiation in Patients with Rectal Cancer. Dis. Colon. Rectum 2013, 56, 142–149. [Google Scholar] [CrossRef] [Green Version]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Kranenbarg, E.M.-K.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.A.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Schøler, L.V.; Reinert, T.; Ørntoft, M.-B.W.; Kassentoft, C.G.; Árnadóttir, S.S.; Vang, S.; Nordentoft, I.K.; Knudsen, M.; Lamy, P.; Andreasen, D.; et al. Clinical Implications of Monitoring Circulating Tumor DNA in Patients with Colorectal Cancer. Clin. Cancer Res. 2017, 23, 5437–5445. [Google Scholar] [CrossRef] [Green Version]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- van Rees, J.M.; Wullaert, L.; Grüter, A.A.J.; Derraze, Y.; Tanis, P.J.; Verheul, H.M.W.; Martens, J.W.M.; Wilting, S.M.; Vink, G.; van Vugt, J.L.A.; et al. Circulating tumour DNA as biomarker for rectal cancer: A systematic review and meta-analyses. Front. Oncol. 2023, 13, 1083285. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.-J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.T.; Heneghan, H.M.; Winter, D.C. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br. J. Surg. 2012, 99, 918–928. [Google Scholar] [CrossRef] [PubMed]

| Patients Characteristics | RAPIDO (Exp vs. Std) | PRODIGE-23 (Exp vs. Std) | PROSPECT (Exp vs. Std) |
|---|---|---|---|
| Number of patients | 462 vs. 450 | 231 vs. 230 | 585 vs. 543 |
| Median age (yrs) | 61 vs. 61 | 61 vs. 62 | 57 vs. 57 |
| Elderly patients (≥65 yrs) | 39% vs. 40% | 32% vs. 37% | |
| Sex | |||
| Male | 65% vs. 69% | 65% vs. 68% | 63% vs. 68% |
| Female | 35% vs. 31% | 35% vs. 32% | 37% vs. 32% |
| Performance Status | |||
| ECOG PS 0 | 80% vs. 81% | 78% vs. 81% | 99% vs. 99% |
| ECOG PS 1 | 20% vs. 19% | 22% vs. 19% | |
| Clinical T stage | |||
| T2 | 3% vs. 3% | 1% vs. 1% | 11% vs. 7% |
| T3 | 65% vs. 66% | 81% vs. 84% | 89% vs. 93% |
| cT4 | 31.8% vs. 30.4% | 18% vs. 16% | / |
| Clinical N stage | |||
| cN2 | 68% vs. 68% | 26% vs. 23% | / |
| Distance from anal verge | |||
| <5 cm | 22% vs. 26% | 38% vs. 36% | 14% vs. 17% |
| 5–10 cm | 39% vs. 34% | 49% vs. 51% | 64% vs. 63% |
| 10–15 cm | 32% vs. 34% | 13% vs. 13% | 22% vs. 20% |
| Unknown | 7% vs. 7% | / | / |
| High-risk features | |||
| EMVI+ | 32% vs. 28% | Not stated | / |
| MRF+ | 60% vs. 62% | 26% vs. 27% | / |
| Lateral N+ | 14% vs. 15% | Not stated | / |
| Clinical Outcomes | RAPIDO (Exp vs. Std) | PRODIGE-23 (Exp vs. Std) | PROSPECT (Exp vs. Std) |
|---|---|---|---|
| Median follow-up | 4.6 yrs | 4.6 yrs | 4.8 yrs |
| Primary endpoint | 3-yrs DrTF | 3-yrs DFS | 5-yrs DFS |
| 3-yrs Primary event (Δ%) * | 23.7% vs. 30.4% (6.7%) | 76% vs. 69% (7%) | n/a |
| 5-yrs | 27.8% vs. 34% (7%) | 73.1% vs. 65.5% (7.6%) | 80.8% vs. 78.6% (2.2%) |
| 7-yrs | n/a | 67.6% vs. 62.5% (5.1%) | n/a |
| * HR (95% CI); p value | 0.75 [0.60–0.96]; p = 0.019 | 0.69 [0.49–0.97]; p = 0.034 | 0.92 [0.72–1.14]; p = 0.005 for noninferiority |
| 3-yrs MFS | 80% vs. 73.2% | 79% vs. 72% | n/a |
| 5-yrs | 77% vs. 69.6% | 77.6% vs. 67.7% | n/a |
| 7-yrs | n/a | 73.6% vs. 65.4% | n/a |
| pCR rate | 28.4% vs. 14.3% | 27.5% vs. 11.7% | 21.9% vs. 24.3% |
| Local relapse rate | 12% vs. 8% at 5 yrs | 5.3% vs. 8.1% at 7 yrs | 1.8% vs. 1.6% at 5 yrs |
| Distant relapse rate | 23% vs. 30.4% at 5 yrs | 20.7% vs. 27.7% at 7 yrs | n/a |
| 3-yrs OS | 89.1% vs. 88.8% | 91% vs. 88% | n/a |
| 5-yrs OS | 81.7% vs. 80.2% | 86.9% vs. 80% | 89.5% vs. 90.2% |
| 7-yrs OS | n/a | 81.9% vs. 76.1% | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daprà, V.; Airoldi, M.; Bartolini, M.; Fazio, R.; Mondello, G.; Tronconi, M.C.; Prete, M.G.; D’Agostino, G.; Foppa, C.; Spinelli, A.; et al. Total Neoadjuvant Treatment for Locally Advanced Rectal Cancer Patients: Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 12159. https://doi.org/10.3390/ijms241512159
Daprà V, Airoldi M, Bartolini M, Fazio R, Mondello G, Tronconi MC, Prete MG, D’Agostino G, Foppa C, Spinelli A, et al. Total Neoadjuvant Treatment for Locally Advanced Rectal Cancer Patients: Where Do We Stand? International Journal of Molecular Sciences. 2023; 24(15):12159. https://doi.org/10.3390/ijms241512159
Chicago/Turabian StyleDaprà, Valentina, Marco Airoldi, Michela Bartolini, Roberta Fazio, Giuseppe Mondello, Maria Chiara Tronconi, Maria Giuseppina Prete, Giuseppe D’Agostino, Caterina Foppa, Antonino Spinelli, and et al. 2023. "Total Neoadjuvant Treatment for Locally Advanced Rectal Cancer Patients: Where Do We Stand?" International Journal of Molecular Sciences 24, no. 15: 12159. https://doi.org/10.3390/ijms241512159








