Iodine-Biofortified Lettuce Can Promote Mitochondrial Dependent Pathway of Apoptosis in Human Gastrointestinal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Lettuce Extracts Induce Apoptosis in AGS and HT-29 Cells
2.2. Lettuce Extracts Reduce Mitochondrial Membrane Potential
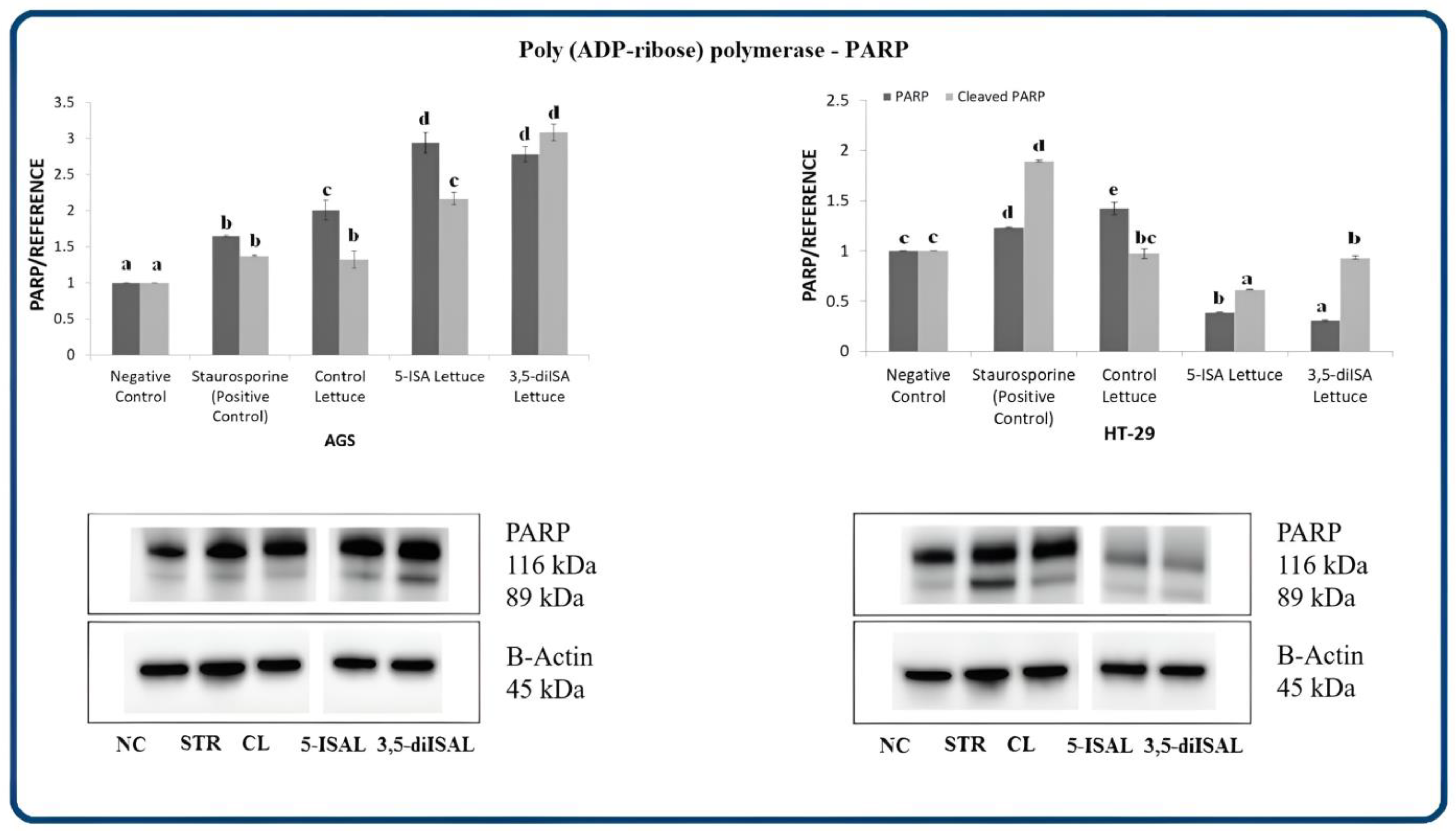
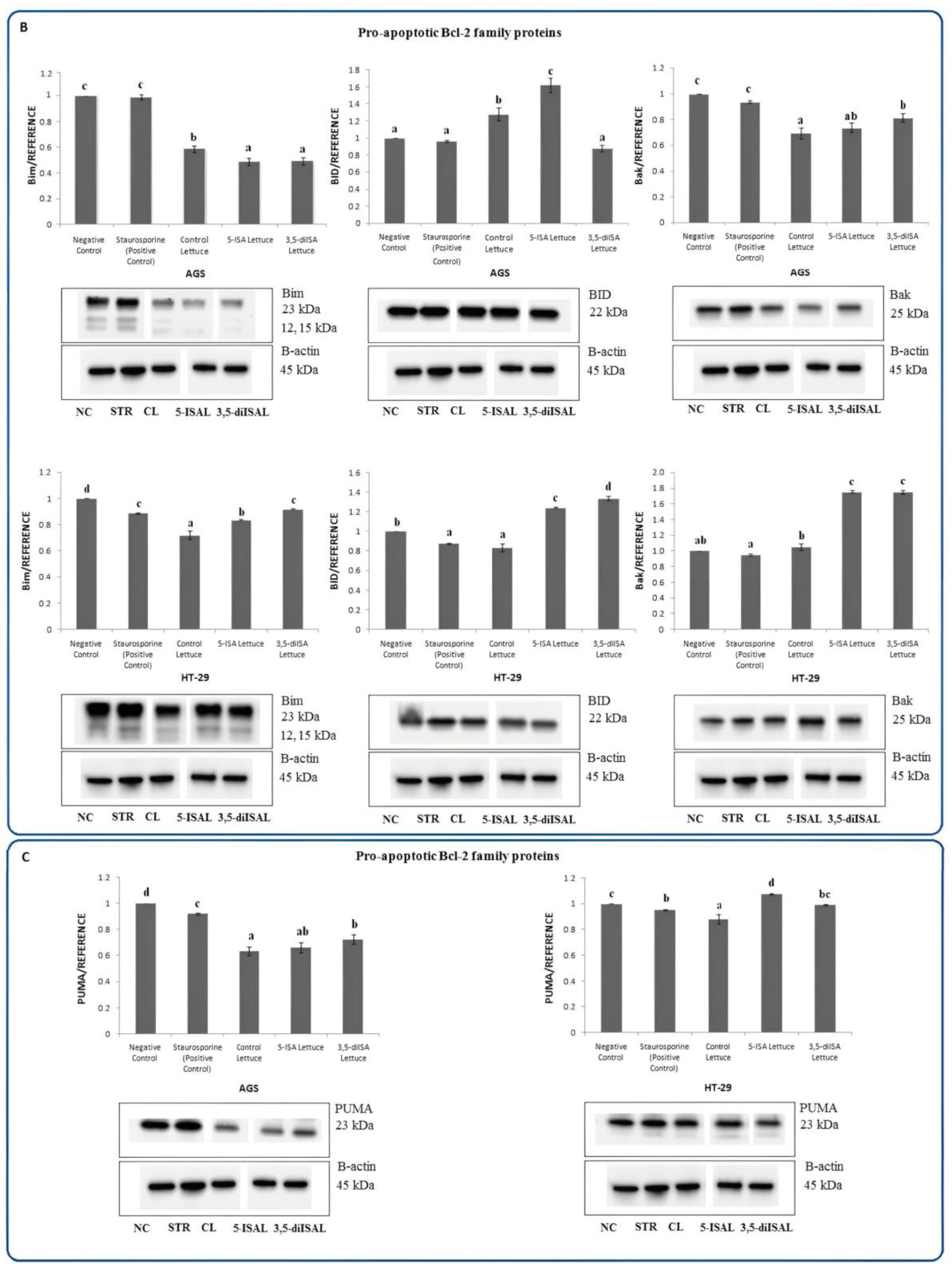
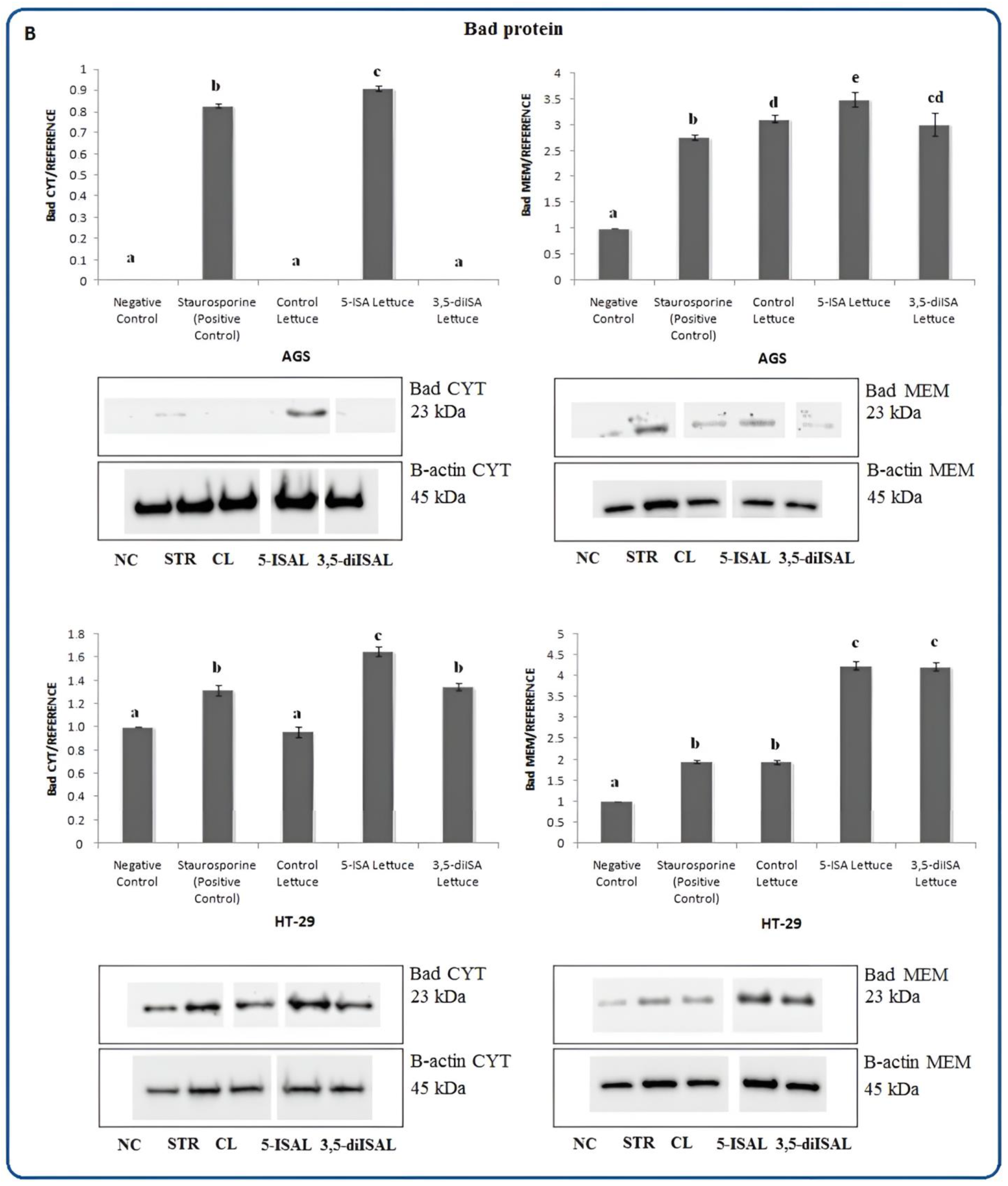
2.3. Lettuce Extracts Impact Expression of Apoptosis-Associated Proteins
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Cell Culture
4.3. Cell Treatment
4.4. Apoptosis Analysis
4.5. Mitochondrial Membrane Potential Analysis
4.6. Protein Expression Analysis
4.7. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Fernald, K.; Kurokawa, M. Evading Apoptosis in Cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Fulda, S. Targeting Apoptosis Signaling Pathways for Anticancer Therapy. Front. Oncol. 2011, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pu, X.; Pu, X.; Li, X.; Liu, Z.; Mei, M.; Wang, X.; Zhang, F.; Qiu, B.; Yu, J. Extracts of Knoxia Roxburghii (Spreng.) M. A. Rau Induce Apoptosis in Human MCF-7 Breast Cancer Cells via Mitochondrial Pathways. Molecules 2022, 27, 6435. [Google Scholar] [CrossRef]
- Fouzat, A.; Hussein, O.J.; Gupta, I.; Al-Farsi, H.F.; Khalil, A.; Al Moustafa, A.E. Elaeagnus Angustifolia Plant Extract Induces Apoptosis via P53 and Signal Transducer and Activator of Transcription 3 Signaling Pathways in Triple-Negative Breast Cancer Cells. Front. Nutr. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Lem, F.F.; Cheong, B.E.; Teoh, P.L. Ruellia Tuberosa Ethyl Acetate Leaf Extract Induces Apoptosis and Cell Cycle Arrest in Human Breast Cancer Cell Line, MCF-7. Sci. Pharm. 2022, 90, 44. [Google Scholar] [CrossRef]
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Varì, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory Effects of Polyphenols on Apoptosis Induction: Relevance for Cancer Prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar] [CrossRef]
- Mao, H.; Wen, Y.; Yu, Y.; Li, H.; Wang, J.; Sun, B. Ignored Role of Polyphenol in Boosting Reactive Oxygen Species Generation for Polyphenol/Chemodynamic Combination Therapy. Mater. Today Bio 2022, 16, 100436. [Google Scholar] [CrossRef]
- Elena-Real, C.A.; Díaz-Quintana, A.; González-Arzola, K.; Velázquez-Campoy, A.; Orzáez, M.; López-Rivas, A.; Gil-Caballero, S.; De La Rosa, M.; Díaz-Moreno, I. Cytochrome c Speeds up Caspase Cascade Activation by Blocking 14-3-3ϵ-Dependent Apaf-1 Inhibition Article. Cell Death Dis. 2018, 9, 365. [Google Scholar] [CrossRef]
- Nava-Villalba, M.; Aceves, C. 6-Iodolactone, Key Mediator of Antitumoral Properties of Iodine. Prostaglandins Other Lipid Mediat. 2014, 112, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Aranda, N.; Sosa, S.; Delgado, G.; Aceves, C.; Anguiano, B. Uptake and Antitumoral Effects of Iodine and 6-Iodolactone in Differentiated and Undifferentiated Human Prostate Cancer Cell Lines. Prostate 2013, 73, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Tiwari, M.; Sinha, R.A.; Kumar, A.; Balapure, A.K.; Bajpai, V.K.; Sharma, R.; Mitra, K.; Tandon, A.; Godbole, M.M. Molecular Iodine Induces Caspase-Independent Apoptosis in Human Breast Carcinoma Cells Involving the Mitochondria-Mediated Pathway. J. Biol. Chem. 2006, 281, 19762–19771. [Google Scholar] [CrossRef] [PubMed]
- Aceves, C.; García-Solís, P.; Arroyo-Helguera, O.; Vega-Riveroll, L.; Delgado, G.; Anguiano, B. Antineoplastic Effect of Iodine in Mammary Cancer: Participation of 6-Iodolactone (6-IL) and Peroxisome Proliferator-Activated Receptors (PPAR). Mol. Cancer 2009, 8, 33. [Google Scholar] [CrossRef]
- Arroyo-Helguera, O.; Rojas, E.; Delgado, G.; Aceves, C. Signaling Pathways Involved in the Antiproliferative Effect of Molecular Iodine in Normal and Tumoral Breast Cells: Evidence That 6-Iodolactone Mediates Apoptotic Effects. Endocr. Relat. Cancer 2008, 15, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Rösner, H.; Torremante, P.; Möller, W.; Gärtner, R. Antiproliferative/Cytotoxic Activity of Molecular Iodine and Iodolactones in Various Human Carcinoma Cell Lines. No Interfering with EGF-Signaling, but Evidence for Apoptosis. Exp. Clin. Endocrinol. Diabetes 2009, 118, 410–419. [Google Scholar] [CrossRef]
- Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I.; Leszczyńska, T. Chemical Composition of Lettuce (Lactuca sativa L.) Biofortified with Iodine by KIO3, 5-Iodo-, and 3.5-Diiodosalicylic Acid in a Hydroponic Cultivation. Agronomy 2020, 10, 1022. [Google Scholar] [CrossRef]
- Sularz, O.; Koronowicz, A.; Smoleń, S.; Kowalska, I.; Skoczylas, Ł.; Liszka-Skoczylas, M.; Tabaszewska, M.; Pitala, J. Anti- And pro-Oxidant Potential of Lettuce (Lactuca sativa L.) Biofortified with Iodine by KIO3, 5-Iodo- And 3,5-Diiodosalicylic Acid in Human Gastrointestinal Cancer Cell Lines. RSC Adv. 2021, 11, 27547–27560. [Google Scholar] [CrossRef]
- Sularz, O.; Koronowicz, A.; Boycott, C.; Smoleń, S.; Stefanska, B. Molecular Effects of Iodine-Biofortified Lettuce in Human Gastrointestinal Cancer Cells. Nutrients 2022, 14, 4287. [Google Scholar] [CrossRef]
- Hikisz, P.; Kiliańska, Z.M. Puma, a Critical Mediator of Cell Death—One Decade on from Its Discovery. Cell. Mol. Biol. Lett. 2012, 17, 646–669. [Google Scholar] [CrossRef]
- Pua, L.J.W.; Mai, C.W.; Chung, F.F.L.; Khoo, A.S.B.; Leong, C.O.; Lim, W.M.; Hii, L.W. Functional Roles of JNK and P38 MAPK Signaling in Nasopharyngeal Carcinoma. Int. J. Mol. Sci. 2022, 23, 1108. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Loba, A.; Manieri, E.; González-Terán, B.; Mora, A.; Leiva-Vega, L.; Santamans, A.M.; Romero-Becerra, R.; Rodríguez, E.; Pintor-Chocano, A.; Feixas, F.; et al. P38γ Is Essential for Cell Cycle Progression and Liver Tumorigenesis. Nature 2019, 568, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kisling, L.A.; Das, J.M. Prevention Strategies. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, K.; Yan, C.; Yin, Y.; He, S.; Qiu, L.; Li, G. Natural Polyphenols for Treatment of Colorectal Cancer. Molecules 2022, 27, 8810. [Google Scholar] [CrossRef]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Tonse, R. Emerging Evidence on the Effects of Dietary Factors on the Gut Microbiome in Colorectal Cancer. Front. Nutr. 2021, 8, 811. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Aceves, C.; Mendieta, I.; Anguiano, B.; Delgado-González, E. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int. J. Mol. Sci. 2021, 22, 1228. [Google Scholar] [CrossRef]
- Nuñez-Anita, R.E.; Arroyo-Helguera, O.; Cajero-Juárez, M.; López-Bojorquez, L.; Aceves, C. A Complex between 6-Iodolactone and the Peroxisome Proliferator-Activated Receptor Type Gamma May Mediate the Antineoplasic Effect of Iodine in Mammary Cancer. Prostaglandins Other Lipid Mediat. 2009, 89, 34–42. [Google Scholar] [CrossRef]
- Mendieta, I.; Nuñez-Anita, R.E.; Nava-Villalba, M.; Zambrano-Estrada, X.; Delgado-González, E.; Anguiano, B.; Aceves, C. Molecular Iodine Exerts Antineoplastic Effects by Diminishing Proliferation and Invasive Potential and Activating the Immune Response in Mammary Cancer Xenografts. BMC Cancer 2019, 19, 261. [Google Scholar] [CrossRef]
- Wang, D.; Ning, W.; Xie, D.; Guo, L.; DuBois, R.N. Peroxisome Proliferator-Activated Receptor δ Confers Resistance to Peroxisome Proliferator-Activated Receptor γ-Induced Apoptosis in Colorectal Cancer Cells. Oncogene 2012, 23, 1013–1023. [Google Scholar] [CrossRef]
- Nava-Villalba, M.; Nuñez-Anita, R.E.; Bontempo, A.; Aceves, C. Activation of Peroxisome Proliferator-Activated Receptor Gamma Is Crucial for Antitumoral Effects of 6-Iodolactone. Mol. Cancer 2015, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, D.M.; Thelen, M.; Thornberry, N.A.; Casciola-Rosen, L.; Rosen, A. Caspase-Mediated Proteolysis during Apoptosis: Insights from Apoptotic Neutrophils. FEBS Lett. 1998, 422, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.D.; Schmitz, I.; Chen, M.; Wang, J. Promotion of Caspase Activation by Caspase-9-Mediated Feedback Amplification of Mitochondrial Damage. J. Clin. Cell. Immunol. 2012, 9, 1000126. [Google Scholar] [CrossRef] [PubMed]
- Puig, B.; Tortosa, A.; Ferrer, I. Cleaved Caspase-3, Caspase-7 and Poly (ADP-Ribose) Polymerase Are Complementarily but Differentially Expressed in Human Medulloblastomas. Neurosci. Lett. 2001, 306, 85–88. [Google Scholar] [CrossRef]
- Soriano, O.; Delgado, G.; Anguiano, B.; Petrosyan, P.; Molina-Servín, E.D.; Gonsebatt, M.E.; Aceves, C. Antineoplastic Effect of Iodine and Iodide in Dimethylbenz[a]Anthracene- Induced Mammary Tumors: Association between Lactoperoxidase and Estrogen-Adduct Production. Endocr. Relat. Cancer 2011, 18, 529–539. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Li, J.; Yang, X.; Sun, J.; Wu, Y.; Qiao, H. High Iodine Effects on the Proliferation, Apoptosis, and Migration of Papillary Thyroid Carcinoma Cells as a Result of Autophagy Induced by BRAF Kinase. Biomed. Pharmacother. 2019, 120, 109476. [Google Scholar] [CrossRef]
- Inoue, S.; Browne, G.; Melino, G.; Cohen, G.M. Ordering of Caspases in Cells Undergoing Apoptosis by the Intrinsic Pathway. Cell Death Differ. 2009, 16, 1053–1061. [Google Scholar] [CrossRef]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner Caspase-3, -6, and -7 Perform Distinct, Non-Redundant Roles during the Demolition Phase of Apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin Inhibits Human Breast Cancer Cell Proliferation and Induces Apoptosis via Bcl-2 and Bax Regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar] [CrossRef]
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol Exerts Anti-Proliferative Effects on Human Ovarian Cancer Cells by Inducing Apoptosis, G0/G1 Cell Cycle Arrest and Modulation of MEK/ERK and STAT3 Pathways. J. Balk. Union Oncol. 2019, 24, 975–981. [Google Scholar]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 Regulated Apoptosis in Cancer. Open Biol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, K.; O’neill, K.L.; Pang, X.; Luo, X. Bax/Bak Activation in the Absence of Bid, Bim, Puma, and P53. Cell Death Dis. 2016, 7, e2266. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, Y.; Huang, H.; Yi, N.; Chen, Y. Emerging Role of BAD and DAD1 as Potential Targets and Biomarkers in Cancer (Review). Oncol. Lett. 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An Inhibitor of Bcl-2 Family Proteins Induces Regression of Solid Tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, B.; Zhang, L.; Mu, W.; Wang, C. ROS / P38 / P53 / Puma Signaling Pathway Is Involved in Emodin-Induced Apoptosis of Human Colorectal Cancer Cells. Int. J. Clin. Exp. Med. 2015, 8, 15413–15422. [Google Scholar]
- Song, Y.; Li, X.; Li, Y.; Li, N.; Shi, X.; Ding, H.; Zhang, Y.; Li, X.; Liu, G.; Wang, Z. Non-Esterified Fatty Acids Activate the ROS-P38-P53/Nrf2 Signaling Pathway to Induce Bovine Hepatocyte Apoptosis in Vitro. Apoptosis 2014, 19, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Gräb, J.; Rybniker, J. The Expanding Role of P38 Mitogen-Activated Protein Kinase in Programmed Host Cell Death. Microbiol. Insights 2019, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. P38 MAP-Kinases Pathway Regulation, Function and Role in Human Diseases. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Pranteda, A.; Piastra, V.; Stramucci, L.; Fratantonio, D.; Bossi, G. The P38 Mapk Signaling Activation in Colorectal Cancer upon Therapeutic Treatments. Int. J. Mol. Sci. 2020, 21, 2773. [Google Scholar] [CrossRef] [PubMed]
- Losa, J.H.; Cobo, C.P.; Viniegra, J.G.; Sánchez-Arevalo Lobo, V.J.; Ramón y Cajal, S.; Sánchez-Prieto, R. Role of the P38 MAPK Pathway in Cisplatin-Based Therapy. Oncogene 2003, 22, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Chuang, Y.C.; Lo, Y.S.; Lin, C.C.; Hsi, Y.T.; Hsieh, M.J.; Chen, M.K. Asiatic Acid, Extracted from Centella Asiatica and Induces Apoptosis Pathway through the Phosphorylation P38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. Biomolecules 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Kanmura, S.; Oda, K.; Arima, S.; Kumagai, K.; Mawatari, S.; Tanoue, S.; Sasaki, F.; Hashimoto, S.; Ido, A. Extract of Lactobacillus Plantarum Strain 06CC2 Induces JNK/P38 MAPK Pathway-Mediated Apoptosis through Endoplasmic Reticulum Stress in Caco2 Colorectal Cancer Cells. Biochem. Biophys. Rep. 2019, 20, 100691. [Google Scholar] [CrossRef]
- Whitaker, R.H.; Cook, J.G. Stress Relief Techniques: P38 MAPK Determines the Balance of Cell Cycle and Apoptosis Pathways. Biomolecules 2021, 11, 1444. [Google Scholar] [CrossRef]
- Zhuang, S.; Demirs, J.T.; Kochevar, I.E. P38 Mitogen-Activated Protein Kinase Mediates Bid Cleavage, Mitochondrial Dysfunction, and Caspase-3 Activation During Apoptosis Induced By Singlet Oxygen But Not By Hydrogen Peroxide. J. Biol. Chem. 2000, 275, 25939–25948. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Igea, A.; Canovas, B.; Dolado, I.; Nebreda, A.R. Inhibition of P38 MAPK Sensitizes Tumour Cells to Cisplatin-Induced Apoptosis Mediated by Reactive Oxygen Species and JNK. EMBO Mol. Med. 2013, 5, 1759–1774. [Google Scholar] [CrossRef]
- Sudo, T.; Maruyama, M.; Osada, H. Stress Meets Development in P38 MAP Kinase. Prog. Biotechnol. 2002, 22, 3–11. [Google Scholar] [CrossRef]
- Rouse, J.; Cohen, P.; Trigon, S.; Morange, M.; Alonso-Llamazares, A.; Zamanillo, D.; Hunt, T.; Nebreda, A.R. A Novel Kinase Cascade Triggered by Stress and Heat Shock That Stimulates MAPKAP Kinase-2 and Phosphorylation of the Small Heat Shock Proteins. Cell 1994, 78, 1027–1037. [Google Scholar] [CrossRef]
- Zheng, C.; Lin, Z.; Zhao, Z.J.; Yang, Y.; Niu, H.; Shen, X. MAPK-Activated Protein Kinase-2 (MK2)-Mediated Formation and Phosphorylation-Regulated Dissociation of the Signal Complex Consisting of P38, MK2, Akt, and Hsp27. J. Biol. Chem. 2006, 281, 37215–37226. [Google Scholar] [CrossRef]
- Ricci, J.E.; Maulon, L.; Battaglione-Hofman, V.; Bertolotto, C.; Luciano, F.; Mari, B.; Hofman, P.; Auberger, P. A Jurkat T Cell Variant Resistant to Death Receptor-Induced Apoptosis. Correlation with Heat Shock Protein (Hsp) 27 and 70 Levels. Eur. Cytokine Netw. 2001, 12, 126–134. [Google Scholar]
- Seul-Ki, C.; Kam, H.; Kye-Young, K.; In Park, S.; Yun-Sil, L. Targeting Heat Shock Protein 27 in Cancer: A Druggable Target for Cancer Treatment? Cancers 2019, 11, 1195. [Google Scholar] [CrossRef]
- Concannon, C.G.; Orrenius, S.; Samali, A. Hsp27 Inhibits Cytochrome C-Mediated Caspase Activation by Sequestering Both pro-Caspase-3 and Cytochrome c. Gene Expr. 2001, 9, 195–201. [Google Scholar] [CrossRef]
- Umar, H.I.; Ajayi, A.T.; Mukerjee, N.; Aborode, A.T.; Hasan, M.M.; Maitra, S.; Bello, R.O.; Alabere, H.O.; Sanusi, A.A.; Awolaja, O.O.; et al. Discovery of Novel HSP27 Inhibitors as Prospective Anti-Cancer Agents Utilizing Computer-Assisted Therapeutic Discovery Approaches. Cells 2022, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, H.; Deng, W.; Zhao, S.; Li, K.; Wang, Y.; Wei, Q.; Du, J. Activation of P38/HSP27 Pathway Counters Melatonin-Induced Inhibitory Effect on Proliferation of Human Gastric Cancer Cells. J. Biomed. Res. 2019, 33, 317–324. [Google Scholar] [CrossRef]
- Li, X.; Miao, X.; Wang, H.; Xu, Z.; Li, B. The Tissue Dependent Interactions between P53 and Bcl-2 in Vivo. Oncotarget 2015, 6, 35699–35709. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Green, D.R. Dissecting P53-Dependent Apoptosis. Cell Death Differ. 2006, 13, 994–1002. [Google Scholar] [CrossRef]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-Only Proteins Noxa and Puma Are Key Regulators of Induced Apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef]
- Kondratovskii, P.M.; Dubikov, A.I.; Doroshevskaya, A.Y.; Eliseikina, M.G. PUMA Protein in P53 Regulatory Molecule Pattern Determines the Prognosis for Patients with Lymphoproliferative Diseases. Bull. Exp. Biol. Med. 2014, 156, 849–853. [Google Scholar] [CrossRef]
- Ray, R.M.; Bhattacharya, S.; Johnson, L.R. Mdm2 Inhibition Induces Apoptosis in P53 Deficient Human Colon Cancer Cells by Activating P73- and E2F1-Mediated Expression of PUMA and Siva-1. Apoptosis 2011, 16, 35–44. [Google Scholar] [CrossRef]
- Faruq, F.; Zhao, D.; Wu, J.; George William, J.N.; Zhang, M.; Chang, H. Downregulation of MDM2 Leads to Anti-Proliferative Effects through Activation of P53-Associated Pathway Mediated By Both Dual Inhibitor MX69 and Mir-548c-3p in Multiple Myeloma. Blood 2019, 134, 4419. [Google Scholar] [CrossRef]
- Taylor, C.A.; Zheng, Q.; Liu, Z.; Thompson, J.E. Role of P38 and JNK MAPK Signaling Pathways and Tumor Suppressor P53 on Induction of Apoptosis in Response to Ad-EIF5A1 in A549 Lung Cancer Cells. Mol. Cancer 2013, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.; Sibilia, M.; Wagner, E.F. Amino-Terminal Phosphorylation of c-Jun Regulates Stress-Induced Apoptosis and Cellular Proliferation. Nat. Genet. 1999, 21, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.; Leech, J.T.; Kad, N.M.; Mason, J.M. Selective Antagonism of CJun for Cancer Therapy. J. Exp. Clin. Cancer Res. 2020, 39, 184. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Raab, M.S.; Tonon, G.; Sattler, M.; Barilà, D.; Zhang, J.; Tai, Y.T.; Yasui, H.; Raje, N.; DePinho, R.A.; et al. Up-Regulation of c-Jun Inhibits Proliferation and Induces Apoptosis via Caspase-Triggered c-Abl Cleavage in Human Multiple Myeloma. Cancer Res. 2007, 67, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Verna, L.; Hardy, S.; Zhu, Y.; Ma, K.S.; Birrer, M.J.; Stemerman, M.B. C-Jun Triggers Apoptosis in Human Vascular Endothelial Cells. Circ. Res. 1997, 85, 387–393. [Google Scholar] [CrossRef]
- Humar, M.; Loop, T.; Schmidt, R.; Hoetzel, A.; Roesslein, M.; Andriopoulos, N.; Pahl, H.L.; Geiger, K.K.; Pannen, B.H.J. The Mitogen-Activated Protein Kinase P38 Regulates Activator Protein 1 by Direct Phosphorylation of c-Jun. Int. J. Biochem. Cell Biol. 2007, 39, 2278–2288. [Google Scholar] [CrossRef]
- Sánchez-Pérez, I.; Perona, R. Lack of C-Jun Activity Increases Survival to Cisplatin. FEBS Lett. 1999, 453, 151–158. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sularz, O.; Koronowicz, A.; Smoleń, S.; Boycott, C.; Stefanska, B. Iodine-Biofortified Lettuce Can Promote Mitochondrial Dependent Pathway of Apoptosis in Human Gastrointestinal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 9869. https://doi.org/10.3390/ijms24129869
Sularz O, Koronowicz A, Smoleń S, Boycott C, Stefanska B. Iodine-Biofortified Lettuce Can Promote Mitochondrial Dependent Pathway of Apoptosis in Human Gastrointestinal Cancer Cells. International Journal of Molecular Sciences. 2023; 24(12):9869. https://doi.org/10.3390/ijms24129869
Chicago/Turabian StyleSularz, Olga, Aneta Koronowicz, Sylwester Smoleń, Cayla Boycott, and Barbara Stefanska. 2023. "Iodine-Biofortified Lettuce Can Promote Mitochondrial Dependent Pathway of Apoptosis in Human Gastrointestinal Cancer Cells" International Journal of Molecular Sciences 24, no. 12: 9869. https://doi.org/10.3390/ijms24129869
APA StyleSularz, O., Koronowicz, A., Smoleń, S., Boycott, C., & Stefanska, B. (2023). Iodine-Biofortified Lettuce Can Promote Mitochondrial Dependent Pathway of Apoptosis in Human Gastrointestinal Cancer Cells. International Journal of Molecular Sciences, 24(12), 9869. https://doi.org/10.3390/ijms24129869







