Delivery of Immunostimulatory Cargos in Nanocarriers Enhances Anti-Tumoral Nanovaccine Efficacy
Abstract
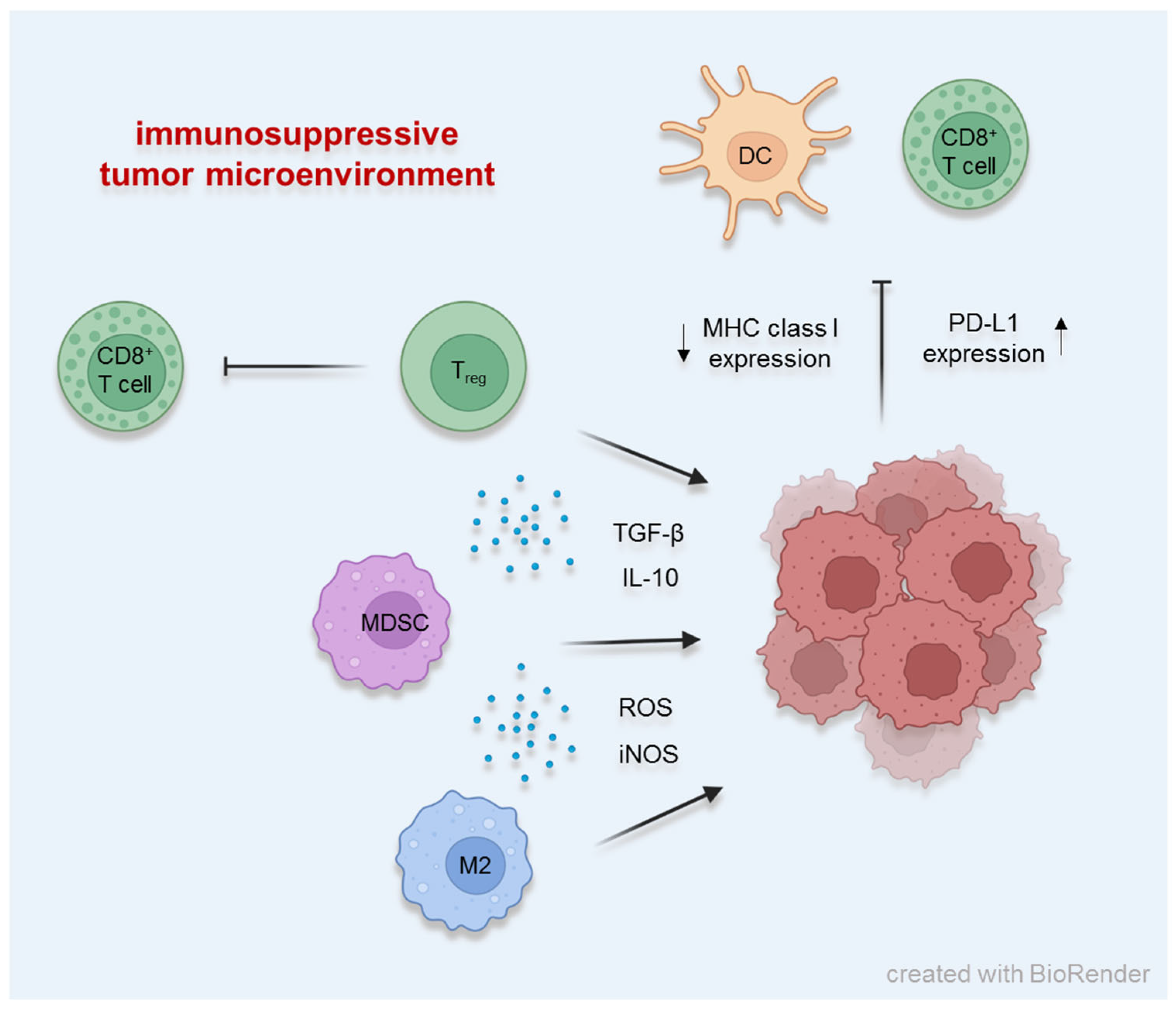
1. Different Factors Establishing the Immunosuppressive Tumor Microenvironment
2. Nucleic Acid-Based Vaccines: DNA and RNA Encoding for Tumor Antigens
3. Tumor Cell-Based Vaccines
4. Dendritic Cell-Based Vaccines
5. Adjuvants Play a Key Role in Enhancing Immune Responses to Vaccines
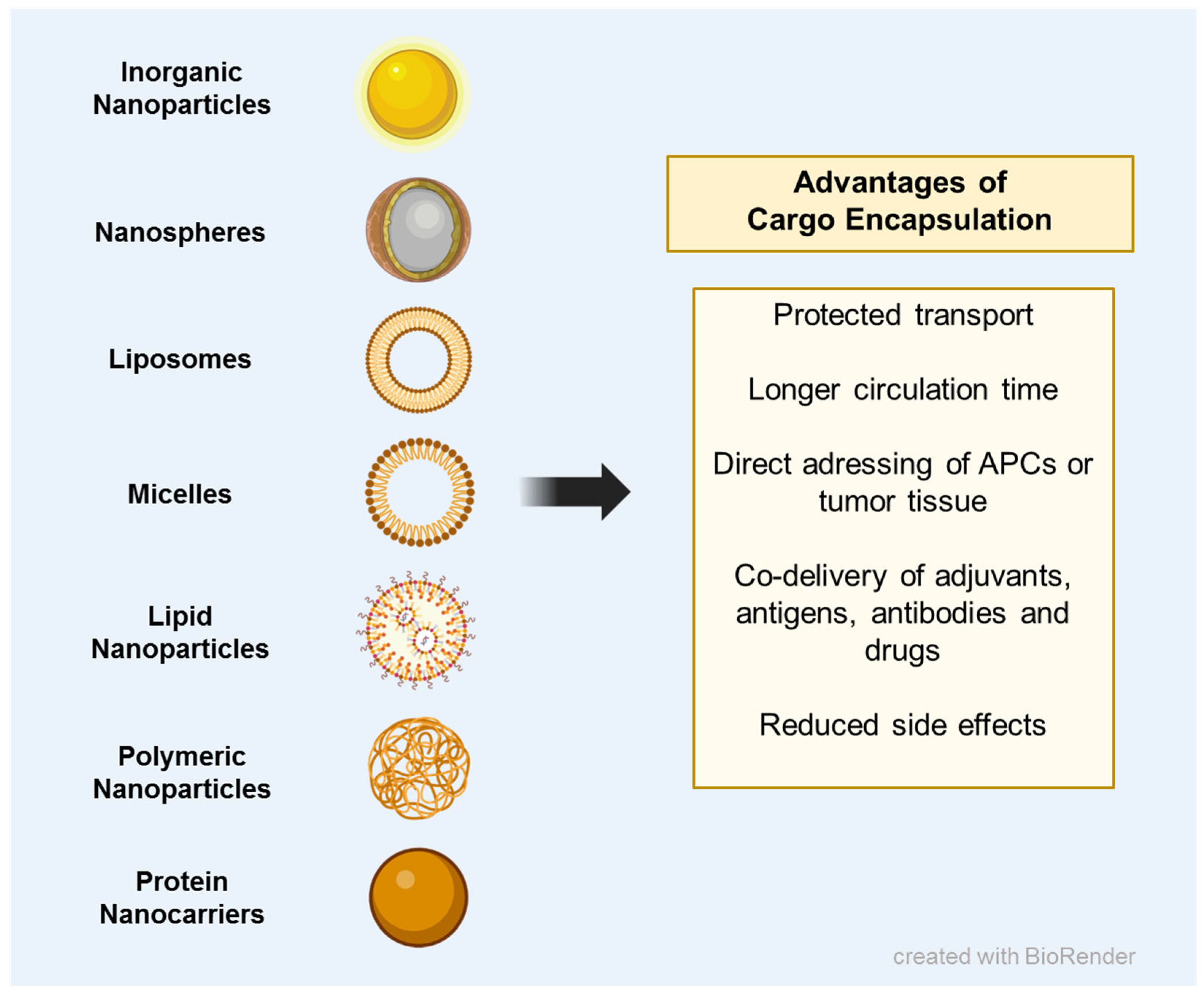
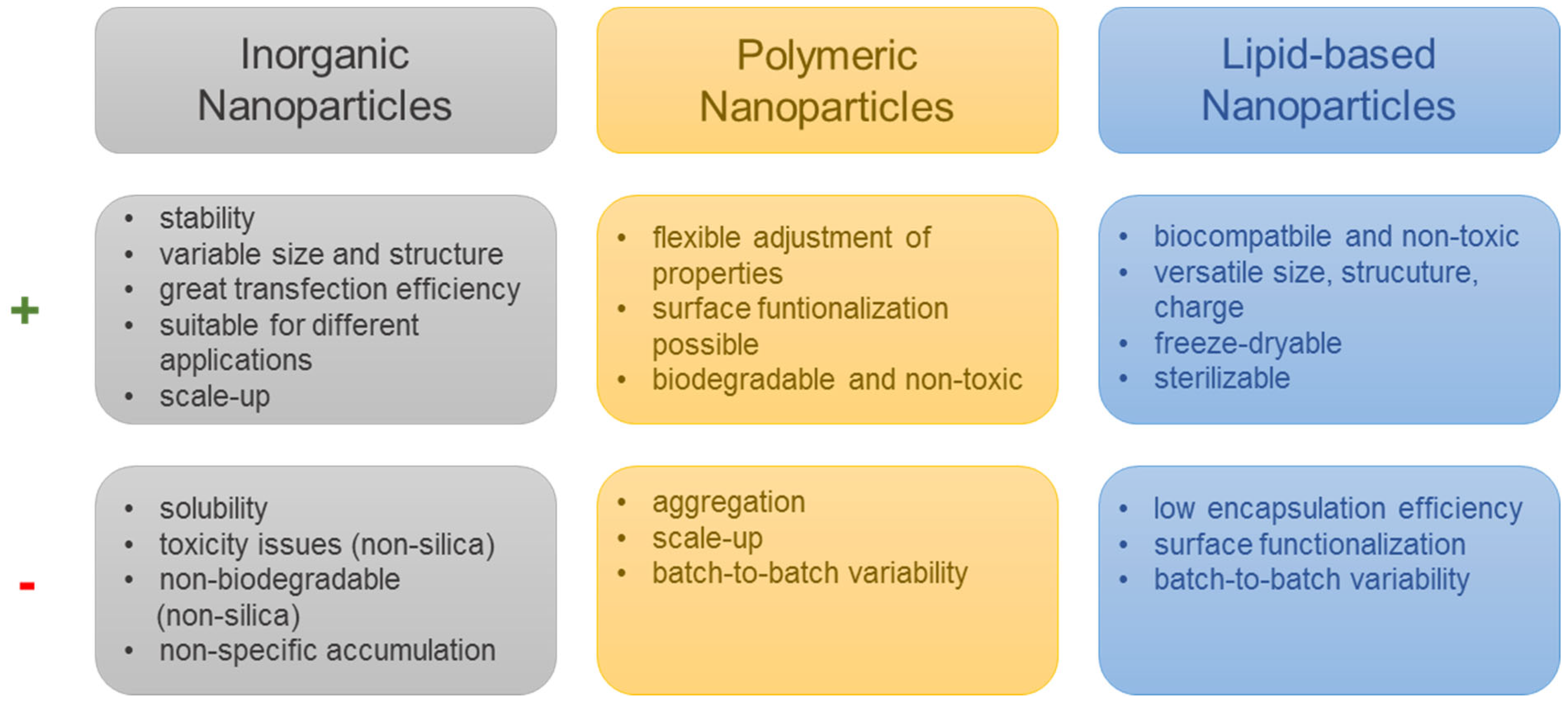
6. Nanomedicine Enables the Combined Delivery of Immunostimulatory Cargos and Reduces Side Effects Elicited by Chemotherapeutic Drugs
7. Combining Nanovaccines with Immune Checkpoint Therapy Enhances Anti-Tumoral Immune Responses
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrido, F.; Cabrera, T.; Aptsiauri, N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: Implications for immunotherapy. Int. J. Cancer 2010, 127, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lampen, M.H.; van Hall, T. Strategies to counteract MHC-I defects in tumors. Curr. Opin. Immunol. 2011, 23, 293–298. [Google Scholar] [CrossRef]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Lim, T.S.; Chew, V.; Sieow, J.L.; Goh, S.; Yeong, J.P.-S.; Soon, A.L.; Ricciardi-Castagnoli, P. PD-1 expression on dendritic cells suppresses CD8+T cell function and antitumor immunity. Oncoimmunology 2016, 5, e1085146. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Luo, Z.-L.; Xiong, Y.-L.; Yang, J.; Shi, A.-P.; Zheng, K.-F.; Liu, Y.-J.; Shu, C.; Ma, N.; Lu, Q.; et al. The Proliferative Role of Immune Checkpoints in Tumors: Double Regulation. Cancers 2022, 14, 5374. [Google Scholar] [CrossRef]
- Reschke, R.; Ziemer, M. Rechallenge with checkpoint inhibitors in metastatic melanoma. JDDG J. Der Dtsch. Dermatol. Ges. 2020, 18, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Gobert, M.; Treilleux, I.; Bendriss-Vermare, N.; Bachelot, T.; Goddard-Leon, S.; Arfi, V.; Biota, C.; Doffin, A.C.; Durand, I.; Olive, D.; et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009, 69, 2000–2009. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Baruch, K.; Rosenzweig, N.; Kertser, A.; Deczkowska, A.; Sharif, A.M.; Spinrad, A.; Tsitsou-Kampeli, A.; Sarel, A.; Cahalon, L.; Schwartz, M. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 2015, 6, 7967. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef]
- Yang, R.; Cai, Z.; Zhang, Y.; Yutzy, W.H., IV; Roby, K.F.; Roden, R.B. CD80 in Immune Suppression by Mouse Ovarian Carcinoma–Associated Gr-1+CD11b+ Myeloid Cells. Cancer Res. 2006, 66, 6807–6815. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pan, P.-Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.-H. Gr-1+CD115+ Immature Myeloid Suppressor Cells Mediate the Development of Tumor-Induced T Regulatory Cells and T-Cell Anergy in Tumor-Bearing Host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef]
- Tiainen, S.; Tumelius, R.; Rilla, K.; Hämäläinen, K.; Tammi, M.; Tammi, R.; Kosma, V.M.; Oikari, S.; Auvinen, P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015, 66, 873–883. [Google Scholar] [CrossRef]
- Jou, J.; Harrington, K.J.; Zocca, M.-B.; Ehrnrooth, E.; Cohen, E.E. The Changing Landscape of Therapeutic Cancer Vaccines—Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. Npj Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Pedersen, S.R.; Sørensen, M.R.; Buus, S.; Christensen, J.P.; Thomsen, A.R. Comparison of Vaccine-Induced Effector CD8 T Cell Responses Directed against Self- and Non–Self-Tumor Antigens: Implications for Cancer Immunotherapy. J. Immunol. 2013, 191, 3955–3967. [Google Scholar] [CrossRef]
- Osipov, A.; Murphy, A.; Zheng, L. From immune checkpoints to vaccines: The past, present and future of cancer immunotherapy. Adv. Cancer Res. 2019, 143, 63–144. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA vaccines for cancer immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Cancer vaccines: Between the idea and the reality. Nat. Rev. Immunol. 2003, 3, 630–641. [Google Scholar] [CrossRef]
- Rice, J.; Ottensmeier, C.H.; Stevenson, F.K. DNA vaccines: Precision tools for activating effective immunity against cancer. Nat. Rev. Cancer 2008, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Raviprakash, K. DNA Vaccine Delivery and Improved Immunogenicity. Curr. Issues Mol. Biol. 2017, 22, 129–138. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Buchan, S.; Grønevik, E.; Mathiesen, I.; King, C.A.; Stevenson, F.K.; Rice, J. Electroporation as a “Prime/Boost” Strategy for Naked DNA Vaccination against a Tumor Antigen. J. Immunol. 2005, 174, 6292–6298. [Google Scholar] [CrossRef]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Préat, V.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Sällberg, M.; Frelin, L.; Ahlén, G.; Sällberg-Chen, M. Electroporation for therapeutic DNA vaccination in patients. Med. Microbiol. Immunol. 2015, 204, 131–135. [Google Scholar] [CrossRef]
- Vasan, S.; Hurley, A.; Schlesinger, S.J.; Hannaman, D.; Gardiner, D.F.; Dugin, D.P.; Boente-Carrera, M.; Vittorino, R.; Caskey, M.; Andersen, J.; et al. In Vivo Electroporation Enhances the Immunogenicity of an HIV-1 DNA Vaccine Candidate in Healthy Volunteers. PLoS ONE 2011, 6, e19252. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. Toward rational vaccine engineering. Adv. Drug Deliv. Rev. 2022, 183, 114142. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hui, A.-M. The paradigm shift in treatment from Covid-19 to oncology with mRNA vaccines. Cancer Treat. Rev. 2022, 107, 102405. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L.M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Diken, M.; Kreiter, S.; Selmi, A.; Britten, C.M.; Huber, C.; Türeci, Ö.; Sahin, U. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011, 18, 702–708. [Google Scholar] [CrossRef]
- Bialkowski, L.; van Weijnen, A.; Van der Jeught, K.; Renmans, D.; Daszkiewicz, L.; Heirman, C.; Stangé, G.; Breckpot, K.; Aerts, J.L.; Thielemans, K. Intralymphatic mRNA vaccine induces CD8 T-cell responses that inhibit the growth of mucosally located tumours. Sci. Rep. 2016, 6, 22509. [Google Scholar] [CrossRef]
- Caruso, D.A.; Orme, L.M.; Neale, A.M.; Radcliff, F.J.; Amor, G.M.; Maixner, W.; Downie, P.; Hassall, T.E.; Tang, M.L.; Ashley, D.M. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro-Oncol. 2004, 6, 236–246. [Google Scholar] [CrossRef]
- Bonehill, A.; Tuyaerts, S.; Van Nuffel, A.M.; Heirman, C.; Bos, T.J.; Fostier, K.; Neyns, B.; Thielemans, K. Enhancing the T-cell Stimulatory Capacity of Human Dendritic Cells by Co-electroporation With CD40L, CD70 and Constitutively Active TLR4 Encoding mRNA. Mol. Ther. 2008, 16, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Wilgenhof, S.; Van Nuffel, A.M.T.; Benteyn, D.; Corthals, J.; Aerts, C.; Heirman, C.; Van Riet, I.; Bonehill, A.; Thielemans, K.; Neyns, B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann. Oncol. 2013, 24, 2686–2693. [Google Scholar] [CrossRef]
- De Keersmaecker, B.; Claerhout, S.; Carrasco, J.; Bar, I.; Corthals, J.; Wilgenhof, S.; Neyns, B.; Thielemans, K. TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: Link between T-cell activation and clinical responses in advanced melanoma. J. Immunother. Cancer 2020, 8, e000329. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Pardoll, D.M.; Jaffee, E.M. Cellular Vaccine Approaches. Cancer J. 2010, 16, 304–310. [Google Scholar] [CrossRef]
- Higano, C.S.; Corman, J.M.; Smith, D.C.; Centeno, A.S.; Steidle, C.P.; Gittleman, M.; Simons, J.W.; Sacks, N.; Aimi, J.; Small, E.J. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 2008, 113, 975–984. [Google Scholar] [CrossRef]
- Small, E.J.; Fratesi, P.; Reese, D.M.; Strang, G.; Laus, R.; Peshwa, M.V.; Valone, F.H. Immunotherapy of Hormone-Refractory Prostate Cancer With Antigen-Loaded Dendritic Cells. J. Clin. Oncol. 2000, 18, 3894–3903. [Google Scholar] [CrossRef]
- Morton, D.L.; Foshag, L.J.; Hoon, D.S.; Nizze, J.A.; Famatiga, E.; Wanek, L.A.; Chang, C.; Davtyan, D.G.; Gupta, R.K.; Elashoff, R.; et al. Prolongation of Survival in Metastatic Melanoma After Active Specific Immunotherapy With a New Polyvalent Melanoma Vaccine. Ann. Surg. 1992, 216, 463–482. [Google Scholar] [CrossRef]
- Hsueh, E.C.; Gupta, R.K.; Qi, K.; Morton, D.L. Correlation of specific immune responses with survival in melanoma patients with distant metastases receiving polyvalent melanoma cell vaccine. J. Clin. Oncol. 1998, 16, 2913–2920. [Google Scholar] [CrossRef]
- Sosman, J.A.; Unger, J.M.; Liu, P.-Y.; Flaherty, L.E.; Park, M.S.; Kempf, R.A.; Thompson, J.A.; Terasaki, P.I.; Sondak, V.K. Adjuvant Immunotherapy of Resected, Intermediate-Thickness, Node-Negative Melanoma With an Allogeneic Tumor Vaccine: Impact of HLA Class I Antigen Expression on Outcome. J. Clin. Oncol. 2002, 20, 2067–2075. [Google Scholar] [CrossRef]
- Sondak, V.K.; A Sosman, J. Results of clinical trials with an allogeneic melanoma tumor cell lysate vaccine: Melacine®. Semin. Cancer Biol. 2003, 13, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Butterfield, L.H. Dendritic Cell-Based Cancer Vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Dendritic cells in cancer immunotherapy clinical trials: Are we making progress? Front. Immunol. 2013, 4, 454. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G. Chemokines and the Homing of Dendritic Cells to the T Cell Areas of Lymphoid Organs. J. Exp. Med. 1999, 189, 447–450. [Google Scholar] [CrossRef]
- A Itano, A.; Jenkins, M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 2003, 4, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Théry, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef]
- Chen, L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Nakagawa, Y.; Yokomuro, K.; Berzofsky, J.A. Induction of CD8+ cytotoxic T lymphocytes by immunization with syngeneic irradiated HIV-1 envelope derived peptide-pulsed dendritic cells. Int. Immunol. 1993, 5, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Rosalia, R.A.; Quakkelaar, E.D.; Redeker, A.; Khan, S.; Camps, M.; Drijfhout, J.W.; Silva, A.L.; Jiskoot, W.; van Hall, T.; van Veelen, P.A.; et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur. J. Immunol. 2013, 43, 2554–2565. [Google Scholar] [CrossRef]
- Binder, R.J.; Anderson, K.M.; Basu, S.; Srivastava, P.K. Cutting Edge: Heat Shock Protein gp96 Induces Maturation and Migration of CD11c+ Cells In Vivo. J. Immunol. 2000, 165, 6029–6035. [Google Scholar] [CrossRef]
- Nestle, F.O.; Alijagic, S.; Gilliet, M.; Sun, Y.; Grabbe, S.; Dummer, R.; Burg, G.; Schadendorf, D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998, 4, 328–332. [Google Scholar] [CrossRef]
- Hsu, F.J.; Benike, C.; Fagnoni, F.; Liles, T.M.; Czerwinski, D.; Taidi, B.; Engleman, E.G.; Levy, R. Vaccination of patients with B–cell lymphoma using autologous antigen–pulsed dendritic cells. Nat. Med. 1996, 2, 52–58. [Google Scholar] [CrossRef]
- Van Tendeloo, V.F.; Van de Velde, A.; Van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA 2010, 107, 13824–13829. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Avivi, I.; Vasir, B.; Uhl, L.; Munshi, N.C.; Katz, T.; Dey, B.R.; Somaiya, P.; Mills, H.; Campigotto, F.; et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin. Cancer Res. 2013, 19, 3640–3648. [Google Scholar] [CrossRef]
- Schadendorf, D.; Ugurel, S.; Schuler-Thurner, B.; Nestle, F.O.; Enk, A.; Bröcker, E.-B.; Grabbe, S.; Rittgen, W.; Edler, L.; Sucker, A.; et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: A randomized phase III trial of the DC study group of the DeCOG. Ann. Oncol. 2006, 17, 563–570. [Google Scholar] [CrossRef]
- Niemi, J.V.L.; Sokolov, A.V.; Schiöth, H.B. Neoantigen Vaccines; Clinical Trials, Classes, Indications, Adjuvants and Combinatorial Treatments. Cancers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Gołoś, A.; Lutyńska, A. Aluminium-adjuvanted vaccines--a review of the current state of knowledge. Prz. Epidemiol. 2015, 69, 731–734. [Google Scholar]
- HogenEsch, H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 2002, 20 (Suppl. 3), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Mannhalter, J.W.; O Neychev, H.; Zlabinger, G.J.; Ahmad, R.; Eibl, M.M. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: Effect on antigen uptake and antigen presentation. Clin. Exp. Immunol. 1985, 61, 143–151. [Google Scholar]
- Ulanova, M.; Tarkowski, A.; Hahn-Zoric, M.; Hanson, L. The Common Vaccine Adjuvant Aluminum Hydroxide Up-Regulates Accessory Properties of Human Monocytes via an Interleukin-4-Dependent Mechanism. Infect. Immun. 2001, 69, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172. [Google Scholar] [CrossRef]
- Sultan, H.; Salazar, A.M.; Celis, E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin. Immunol. 2020, 49, 101414. [Google Scholar] [CrossRef]
- Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–259. [Google Scholar] [CrossRef]
- Smirnov, D.; Schmidt, J.J.; Capecchi, J.T.; Wightman, P.D. Vaccine adjuvant activity of 3M-052: An imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine 2011, 29, 5434–5442. [Google Scholar] [CrossRef]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88–dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Jurk, M.; Heil, F.; Vollmer, J.; Schetter, C.; Krieg, A.M.; Wagner, H.; Lipford, G.; Bauer, S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 2002, 3, 499. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Mills, B.N.; Qin, S.S.; Garrett-Larsen, J.; Murphy, J.D.; Uccello, T.P.; Han, B.J.; Vrooman, T.G.; Johnston, C.J.; Lord, E.M.; et al. Toll-like receptor 7/8 agonist R848 alters the immune tumor microenvironment and enhances SBRT-induced antitumor efficacy in murine models of pancreatic cancer. J. Immunother. Cancer 2022, 10, e004784. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Melis, M.H.M.; Wilkinson, R.W.; Adlard, A.L.; Stratford, I.J.; Honeychurch, J.; Illidge, T.M. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood 2013, 121, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Wang, Y.; Zhang, M.; Sun, W.; Dai, T.; Wang, A.; Wu, X.; Zhang, S.; Wang, S.; et al. A Dual Role of Type I Interferons in Antitumor Immunity. Adv. Biosyst. 2020, 4, e1900237. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef]
- Trinchieri, G.; Santoli, D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 1978, 147, 1314–1333. [Google Scholar] [CrossRef]
- Lee, C.-K.; Rao, D.T.; Gertner, R.; Gimeno, R.; Frey, A.B.; Levy, D.E. Distinct Requirements for IFNs and STAT1 in NK Cell Function. J. Immunol. 2000, 165, 3571–3577. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.; Schiavoni, G.; Mattei, F.; Gresser, I.; Belardelli, F.; Borrow, P.; Tough, D.F. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 2002, 99, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef]
- Bidwell, B.N.; Slaney, C.Y.; Withana, N.P.; Forster, S.; Cao, Y.; Loi, S.; Andrews, D.; Mikeska, T.; Mangan, N.E.; Samarajiwa, S.A.; et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 2012, 18, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Von Marschall, Z.; Scholz, A.; Cramer, T.; Schäfer, G.; Schirner, M.; Öberg, K.; Wiedenmann, B.; Höcker, M.; Rosewicz, S. Effects of Interferon Alpha on Vascular Endothelial Growth Factor Gene Transcription and Tumor Angiogenesis. Gynecol. Oncol. 2003, 95, 437–448. [Google Scholar] [CrossRef]
- Yıldırım, C.; Nieuwenhuis, S.; Teunissen, P.F.; Horrevoets, A.J.; van Royen, N.; Kraan, T.C.v.d.P. Interferon-Beta, a Decisive Factor in Angiogenesis and Arteriogenesis. J. Interf. Cytokine Res. 2015, 35, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.E.; Hermans, I.F.; Roberts, J.M.; Ching, L.M.; Ronchese, F. 5,6-Dimethylxanthenone-4-acetic acid treatment of a non-immunogenic tumour does not synergize with active or passive CD8+ T-cell immunotherapy. Immunol. Cell Biol. 2006, 84, 383–389. [Google Scholar] [CrossRef]
- Lemos, H.; Mohamed, E.; Huang, L.; Ou, R.; Pacholczyk, G.; Arbab, A.S.; Munn, D.; Mellor, A.L. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016, 76, 2076–2081. [Google Scholar] [CrossRef]
- McKeage, M.J.; Von Pawel, J.; Reck, M.; Jameson, M.B.; A Rosenthal, M.; Sullivan, R.; Gibbs, D.; Mainwaring, P.N.; Serke, M.; Lafitte, J.-J.; et al. Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br. J. Cancer 2008, 99, 2006–2012. [Google Scholar] [CrossRef]
- Lara, P.N., Jr.; Douillard, J.Y.; Nakagawa, K.; von Pawel, J.; McKeage, M.J.; Albert, I.; Losonczy, G.; Reck, M.; Heo, D.S.; Fan, X.; et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 2965–2971. [Google Scholar] [CrossRef]
- Shih, A.Y.; Damm-Ganamet, K.L.; Mirzadegan, T. Dynamic Structural Differences between Human and Mouse STING Lead to Differing Sensitivity to DMXAA. Biophys. J. 2018, 114, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sivick, K.E.; Desbien, A.L.; Glickman, L.H.; Reiner, G.L.; Corrales, L.; Surh, N.H.; Hudson, T.E.; Vu, U.T.; Francica, B.J.; Banda, T.; et al. Magnitude of Therapeutic STING Activation Determines CD8+ T Cell-Mediated Anti-tumor Immunity. Cell Rep. 2018, 25, 3074–3085.e5. [Google Scholar] [CrossRef] [PubMed]
- Francica, B.J.; Ghasemzadeh, A.; Desbien, A.L.; Theodros, D.; Sivick, K.E.; Reiner, G.L.; Glickman, L.H.; Marciscano, A.E.; Sharabi, A.B.; Leong, M.L.; et al. TNFα and Radioresistant Stromal Cells Are Essential for Therapeutic Efficacy of Cyclic Dinucleotide STING Agonists in Nonimmunogenic Tumors. Cancer Immunol. Res. 2018, 6, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Foote, J.B.; Kok, M.; Leatherman, J.M.; Armstrong, T.D.; Marcinkowski, B.C.; Ojalvo, L.S.; Kanne, D.B.; Jaffee, E.M.; Dubensky, T.W., Jr.; Emens, L.A. A STING Agonist Given with OX40 Receptor and PD-L1 Modulators Primes Immunity and Reduces Tumor Growth in Tolerized Mice. Cancer Immunol. Res. 2017, 5, 468–479. [Google Scholar] [CrossRef]
- Deng, Z.; Tian, Y.; Song, J.; An, G.; Yang, P. mRNA Vaccines: The Dawn of a New Era of Cancer Immunotherapy. Front. Immunol. 2022, 13, 887125. [Google Scholar] [CrossRef]
- Jekle, A.; Thatikonda, S.; Stevens, S.; Williams, C.; Kinkade, A.; Ren, S.; Jaisinghani, R.; Zhang, Q.; Misner, D.; Stoycheva, A.; et al. Abstract 4520: Preclinical characterization of ALG-031048, a novel STING agonist with potent anti-tumor activity in mice. Cancer Res. 2020, 80, 4520. [Google Scholar] [CrossRef]
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.-Y.; Tran, J.-L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 2018, 564, 439–443. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chelvanambi, M.; Filderman, J.N.; Storkus, W.J.; Luke, J.J. STING Agonists as Cancer Therapeutics. Cancers 2021, 13, 2695. [Google Scholar] [CrossRef]
- Burris, H.A.; Patel, M.R.; Cho, D.C.; Clarke, J.M.; Gutierrez, M.; Zaks, T.Z.; Frederick, J.; Hopson, K.; Mody, K.; Binanti-Berube, A.; et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J. Clin. Oncol. 2019, 37, 2523. [Google Scholar] [CrossRef]
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent Design to Treat Complex Disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Fichter, M.; Kuhn, G.; Brückner, M.; Kappel, C.; Schunke, J.; Klaus, T.; Grabbe, S.; Landfester, K.; Mailänder, V. Achieving dendritic cell subset-specific targeting in vivo by site-directed conjugation of targeting antibodies to nanocarriers. Nano Today 2022, 43, 101375. [Google Scholar] [CrossRef]
- Scherger, M.; Bolli, E.; Antunes, A.R.P.; Arnouk, S.; Stickdorn, J.; Van Driessche, A.; Schild, H.; Grabbe, S.; De Geest, B.G.; Van Ginderachter, J.A.; et al. Transient Multivalent Nanobody Targeting to CD206-Expressing Cells via PH-Degradable Nanogels. Cells 2020, 9, 2222. [Google Scholar] [CrossRef]
- Kalkanidis, M.; Pietersz, G.A.; Xiang, S.D.; Mottram, P.L.; Crimeen-Irwin, B.; Ardipradja, K.; Plebanski, M. Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods 2006, 40, 20–29. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Ow, H.; Larson, D.R.; Srivastava, M.; Baird, B.A.; Webb, W.W.; Wiesner, U. Bright and Stable Core−Shell Fluorescent Silica Nanoparticles. Nano Lett. 2005, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef]
- Jiang, S.; Prozeller, D.; Pereira, J.; Simon, J.; Han, S.; Wirsching, S.; Fichter, M.; Mottola, M.; Lieberwirth, I.; Morsbach, S.; et al. Controlling protein interactions in blood for effective liver immunosuppressive therapy by silica nanocapsules. Nanoscale 2020, 12, 2626–2637. [Google Scholar] [CrossRef]
- Moghadam, M.E.; Sadeghi, M.; Mansouri-Torshizi, H.; Saidifar, M. High cancer selectivity and improving drug release from mesoporous silica nanoparticles in the presence of human serum albumin in cisplatin, carboplatin, oxaliplatin, and oxalipalladium treatment. Eur. J. Pharm. Sci. 2023, 187, 106477. [Google Scholar] [CrossRef]
- Fan, J.; Fang, G.; Wang, X.; Zeng, F.; Xiang, Y.; Wu, S. Targeted anticancer prodrug with mesoporous silica nanoparticles as vehicles. Nanotechnology 2011, 22, 455102. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Yin, Y.; Yan, Y.; Fan, B.; Huang, W.; Zhang, J.; Hu, H.-Y.; Li, X.; Xiong, D.; Chou, S.-L.; Xiao, Y.; et al. Novel Combination Therapy for Triple-Negative Breast Cancer based on an Intelligent Hollow Carbon Sphere. Research 2023, 6, 0098. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zou, M.; Jiang, H.; Ji, Z.; Gao, P.; Cheng, G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur. J. Pharm. Sci. 2011, 44, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.D.; Lim, H.X.; Shameli, K.; Yeap, S.K.; Poh, C.L. Nano and Microparticles as Potential Oral Vaccine Carriers and Adjuvants Against Infectious Diseases. Front. Pharmacol. 2021, 12, 682286. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Allison, A.C.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252. [Google Scholar] [CrossRef]
- Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposome-based delivery system for vaccine candidates: Constructing an effective formulation. Nanomedicine 2012, 7, 1877–1893. [Google Scholar] [CrossRef]
- Nordly, P.; Madsen, H.B.; Nielsen, H.M.; Foged, C.; Pharm, M. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin. Drug Deliv. 2009, 6, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ma, Y.; Wang, C.; Hai, L.; Yan, C.; Zhang, Y.; Liu, F.; Cai, L. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: Role of lymphatic trafficking and biodistribution. J. Control. Release 2012, 159, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Salomon, N.; Vascotto, F.; Selmi, A.; Vormehr, M.; Quinkhardt, J.; Bukur, T.; Schrörs, B.; Löewer, M.; Diken, M.; Türeci, Ö.; et al. A liposomal RNA vaccine inducing neoantigen-specific CD4+ T cells augments the antitumor activity of local radiotherapy in mice. Oncoimmunology 2020, 9, 1771925. [Google Scholar] [CrossRef] [PubMed]
- Grabbe, S.; Haas, H.; Diken, M.; Kranz, L.M.; Langguth, P.; Sahin, U. Translating nanoparticulate-personalized cancer vaccines into clinical applications: Case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine 2016, 11, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Na Kong, N.; Zhang, X.; Cao, Y.; Langer, R.; Tao, W. The landscape of mRNA nanomedicine. Nat. Med. 2022, 28, 2273–2287. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, e2303261. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, R.; Moharil, P.; Liu, Y.; Chen, J.; Sun, R.; Song, X.; Ao, Q. Polymeric Micelles in Cancer Immunotherapy. Molecules 2021, 26, 1220. [Google Scholar] [CrossRef]
- Li, X.; Aldayel, A.M.; Cui, Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J. Control. Release 2014, 173, 148–157. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, H.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.; Sun, X. Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017, 122, 105–113. [Google Scholar] [CrossRef]
- Mukai, H.; Kogawa, T.; Matsubara, N.; Naito, Y.; Sasaki, M.; Hosono, A. A first-in-human Phase 1 study of epirubicin-conjugated polymer micelles (K-912/NC-6300) in patients with advanced or recurrent solid tumors. Investig. New Drugs 2017, 35, 307–314. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.-C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B.; et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Werner, M.E.; Cummings, N.D.; Sethi, M.; Wang, E.C.; Sukumar, R.; Moore, D.T.; Wang, A.Z. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 463–468. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. [Google Scholar] [CrossRef]
- Diwan, M.; Elamanchili, P.; Lane, H.; Gainer, A.; Samuel, J. Biodegradable Nanoparticle Mediated Antigen Delivery to Human Cord Blood Derived Dendritic Cells for Induction of Primary T Cell Responses. J. Drug Target. 2003, 11, 495–507. [Google Scholar] [CrossRef]
- Kempf, M.; Mandal, B.; Jilek, S.; Thiele, L.; Vörös, J.; Textor, M.; Merkle, H.P.; Walter, E. Improved Stimulation of Human Dendritic Cells by Receptor Engagement with Surface-modified Microparticles. J. Drug Target. 2003, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Waeckerlemen, Y.; Groettrup, M. PLGA microspheres for improved antigen delivery to dendritic cells as cellular vaccines. Adv. Drug Deliv. Rev. 2005, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Josephson, L.; Tung, C.-H.; Moore, A.; Weissleder, R. High-Efficiency Intracellular Magnetic Labeling with Novel Superparamagnetic-Tat Peptide Conjugates. Bioconjugate Chem. 1999, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.F.; Hioe, C.; Chen, P.-D.; Bian, Z.; Kuebler, P.; Li, M.-L.; Qiu, H.; Li, X.-M.; Singh, M.; Richardson, J.; et al. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine 1996, 14, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Warger, T.; Osterloh, P.; Rechtsteiner, G.; Fassbender, M.; Heib, V.; Schmid, B.; Schmitt, E.; Schild, H.; Radsak, M.P. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood 2006, 108, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Diwan, M.; Elamanchili, P.; Cao, M.; Samuel, J. Dose Sparing of CpG Oligodeoxynucleotide Vaccine Adjuvants by Nanoparticle Delivery. Curr. Drug Deliv. 2004, 1, 405–412. [Google Scholar] [CrossRef]
- Hamdy, S.; Elamanchili, P.; Alshamsan, A.; Molavi, O.; Satou, T.; Samuel, J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly(D,L-lactic-co-glycolic acid) nanoparticles. J. Biomed. Mater. Res. Part A 2006, 81A, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Elamanchili, P.; Lutsiak, C.M.E.; Hamdy, S.; Diwan, M.; Samuel, J. “Pathogen-Mimicking” Nanoparticles for Vaccine Delivery to Dendritic Cells. J. Immunother. 2007, 30, 378–395. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Hamdy, S.; Molavi, O.; Ma, Z.; Haddadi, A.; Alshamsan, A.; Gobti, Z.; Elhasi, S.; Samuel, J.; Lavasanifar, A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine 2008, 26, 5046–5057. [Google Scholar] [CrossRef]
- Levit, M.; Vdovchenko, A.; Dzhuzha, A.; Zashikhina, N.; Katernyuk, E.; Gostev, A.; Sivtsov, E.; Lavrentieva, A.; Tennikova, T.; Korzhikova-Vlakh, E. Self-Assembled Nanoparticles Based on Block-Copolymers of Poly(2-Deoxy-2-methacrylamido-d-glucose)/Poly(N-Vinyl Succinamic Acid) with Poly(O-Cholesteryl Methacrylate) for Delivery of Hydrophobic Drugs. Int. J. Mol. Sci. 2021, 22, 11457. [Google Scholar] [CrossRef]
- Piradashvili, K.; Fichter, M.; Mohr, K.; Gehring, S.; Wurm, F.R.; Landfester, K. Biodegradable Protein Nanocontainers. Biomacromolecules 2015, 16, 815–821. [Google Scholar] [CrossRef]
- Han, J.; Wang, Q.; Zhang, Z.; Gong, T.; Sun, X. Cationic Bovine Serum Albumin Based Self-Assembled Nanoparticles as siRNA Delivery Vector for Treating Lung Metastatic Cancer. Small 2013, 10, 524–535. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.-W. Protein-based nanoparticles in cancer vaccine development. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 164–174. [Google Scholar] [CrossRef]
- Dalmau, M.; Lim, S.; Chen, H.C.; Ruiz, C.; Wang, S.-W. Thermostability and molecular encapsulation within an engineered caged protein scaffold. Biotechnol. Bioeng. 2008, 101, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Molino, N.M.; Neek, M.; Tucker, J.A.; Nelson, E.L.; Wang, S.-W. Display of DNA on Nanoparticles for Targeting Antigen Presenting Cells. ACS Biomater. Sci. Eng. 2017, 3, 496–501. [Google Scholar] [CrossRef]
- Molino, N.M.; Anderson, A.K.L.; Nelson, E.L.; Wang, S.-W. Biomimetic Protein Nanoparticles Facilitate Enhanced Dendritic Cell Activation and Cross-Presentation. ACS Nano 2013, 7, 9743–9752. [Google Scholar] [CrossRef]
- Molino, N.M.; Neek, M.; Tucker, J.A.; Nelson, E.L.; Wang, S.-W. Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials 2016, 86, 83–91. [Google Scholar] [CrossRef]
- Hüppe, N.; Schunke, J.; Fichter, M.; Mailänder, V.; Wurm, F.R.; Landfester, K. Multicomponent encapsulation into fully degradable protein nanocarriers via interfacial azide–alkyne click reaction in miniemulsion allows the co-delivery of immunotherapeutics. Nanoscale Horiz. 2022, 7, 908–915. [Google Scholar] [CrossRef]
- Aswathy, R.G.; Sivakumar, B.; Brahatheeswaran, D.; Fukuda, T.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Biocompatible fluorescent zein nanoparticles for simultaneous bioimaging and drug delivery application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025006. [Google Scholar] [CrossRef]
- Zhen, X.; Wang, X.; Xie, C.; Wu, W.; Jiang, X. Cellular uptake, antitumor response and tumor penetration of cisplatin-loaded milk protein nanoparticles. Biomaterials 2013, 34, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Golla, K.; Bhaskar, C.; Ahmed, F.; Kondapi, A.K. A Target-Specific Oral Formulation of Doxorubicin-Protein Nanoparticles: Efficacy and Safety in Hepatocellular Cancer. J. Cancer 2013, 4, 644–652. [Google Scholar] [CrossRef]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.; Ke, H.; Ding, D.; Deng, Y.; Chen, H. Serum protein-based nanoparticles for cancer diagnosis and treatment. J. Control. Release 2020, 329, 997–1022. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women with Breast Cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Liu, Y.; Crowe, W.N.; Wang, L.; Petty, W.J.; Habib, A.A.; Zhao, D. Aerosolized immunotherapeutic nanoparticle inhalation potentiates PD-L1 blockade for locally advanced lung cancer. Nano Res. 2022, 16, 5300–5310. [Google Scholar] [CrossRef]
- Gao, X.; Lei, G.; Wang, B.; Deng, Z.; Karges, J.; Xiao, H.; Tan, D. Encapsulation of Platinum Prodrugs into PC7A Polymeric Nanoparticles Combined with Immune Checkpoint Inhibitors for Therapeutically Enhanced Multimodal Chemotherapy and Immunotherapy by Activation of the STING Pathway. Adv. Sci. 2022, 10, e2205241. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, C.; Wang, H.; Zhang, Z.; Yang, X.; Dong, Y.; Ma, L.; Luo, J. Combination of an autophagy inhibitor with immunoadjuvants and an anti-PD-L1 antibody in multifunctional nanoparticles for enhanced breast cancer immunotherapy. BMC Med. 2022, 20, 411. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Xu, Z.; Miao, L.; Huang, L. mRNA Vaccine with Antigen-Specific Checkpoint Blockade Induces an Enhanced Immune Response against Established Melanoma. Mol. Ther. 2017, 26, 420–434. [Google Scholar] [CrossRef]
- Gao, S.; Wang, J.; Zhu, Z.; Fang, J.; Zhao, Y.; Liu, Z.; Qin, H.; Wei, Y.; Xu, H.; Dan, X.; et al. Effective personalized neoantigen vaccine plus anti-PD-1 in a PD-1 blockade-resistant lung cancer patient. Immunotherapy 2023, 15, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Gazouli, M.; Pispas, S. Recent Advances and Future Perspectives in Polymer-Based Nanovaccines. Vaccines 2021, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.M.G.; Darroudi, M. Nanovaccine: A novel approach in immunization. J. Cell. Physiol. 2019, 234, 12530–12536. [Google Scholar] [CrossRef] [PubMed]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schunke, J.; Mailänder, V.; Landfester, K.; Fichter, M. Delivery of Immunostimulatory Cargos in Nanocarriers Enhances Anti-Tumoral Nanovaccine Efficacy. Int. J. Mol. Sci. 2023, 24, 12174. https://doi.org/10.3390/ijms241512174
Schunke J, Mailänder V, Landfester K, Fichter M. Delivery of Immunostimulatory Cargos in Nanocarriers Enhances Anti-Tumoral Nanovaccine Efficacy. International Journal of Molecular Sciences. 2023; 24(15):12174. https://doi.org/10.3390/ijms241512174
Chicago/Turabian StyleSchunke, Jenny, Volker Mailänder, Katharina Landfester, and Michael Fichter. 2023. "Delivery of Immunostimulatory Cargos in Nanocarriers Enhances Anti-Tumoral Nanovaccine Efficacy" International Journal of Molecular Sciences 24, no. 15: 12174. https://doi.org/10.3390/ijms241512174
APA StyleSchunke, J., Mailänder, V., Landfester, K., & Fichter, M. (2023). Delivery of Immunostimulatory Cargos in Nanocarriers Enhances Anti-Tumoral Nanovaccine Efficacy. International Journal of Molecular Sciences, 24(15), 12174. https://doi.org/10.3390/ijms241512174





