Knockdown of PTK7 Reduces the Oncogenic Potential of Breast Cancer Cells by Impeding Receptor Tyrosine Kinase Signaling
Abstract
:1. Introduction
2. Results
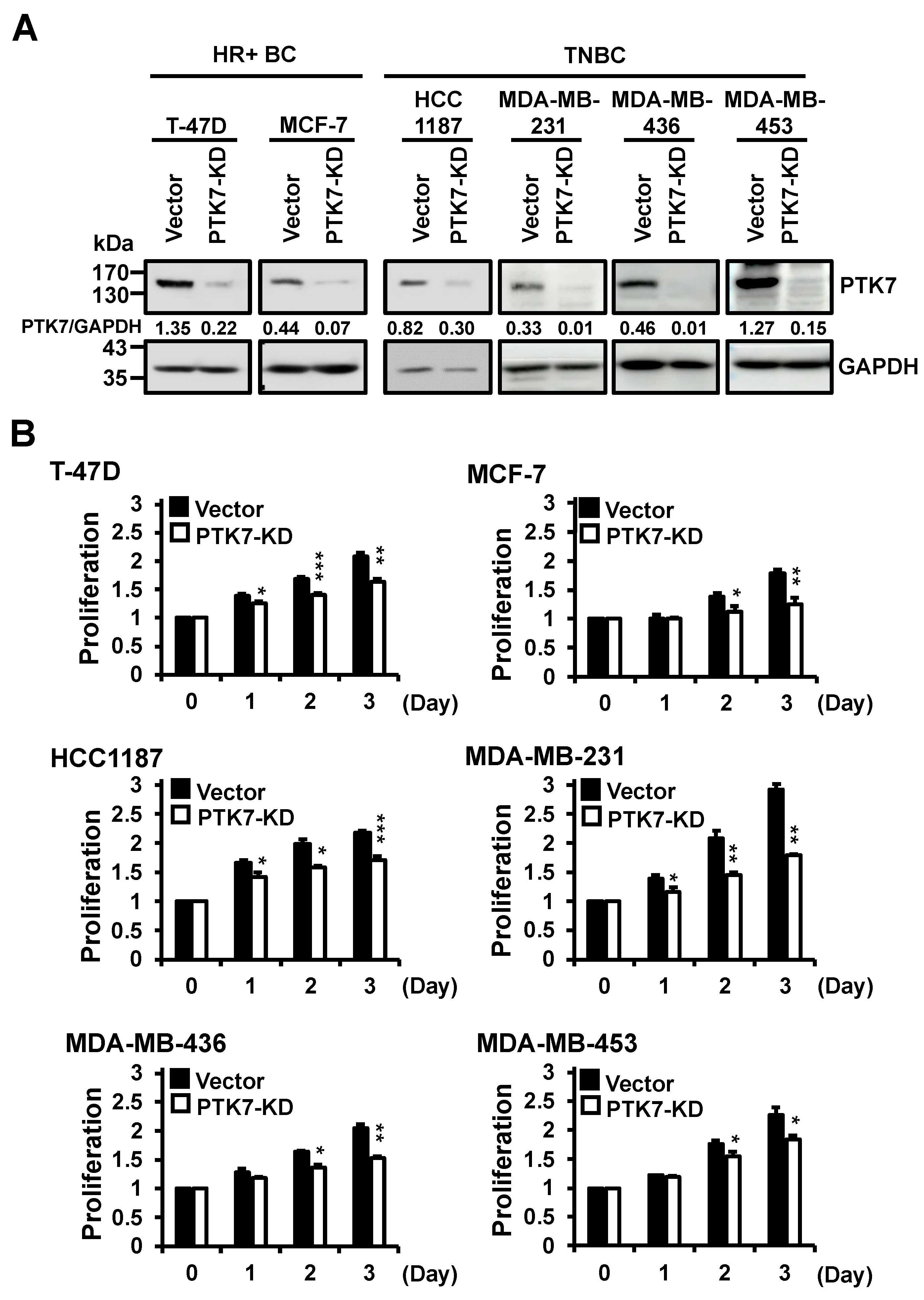
2.1. PTK7 Expression Is Upregulated in BC Tissues and Cell Lines
2.2. Knockdown of PTK7 Reduces Proliferation of BC Cells
2.3. Knockdown of PTK7 Decreases Cell Adhesion, Migration, and Invasion of TNBC Cells
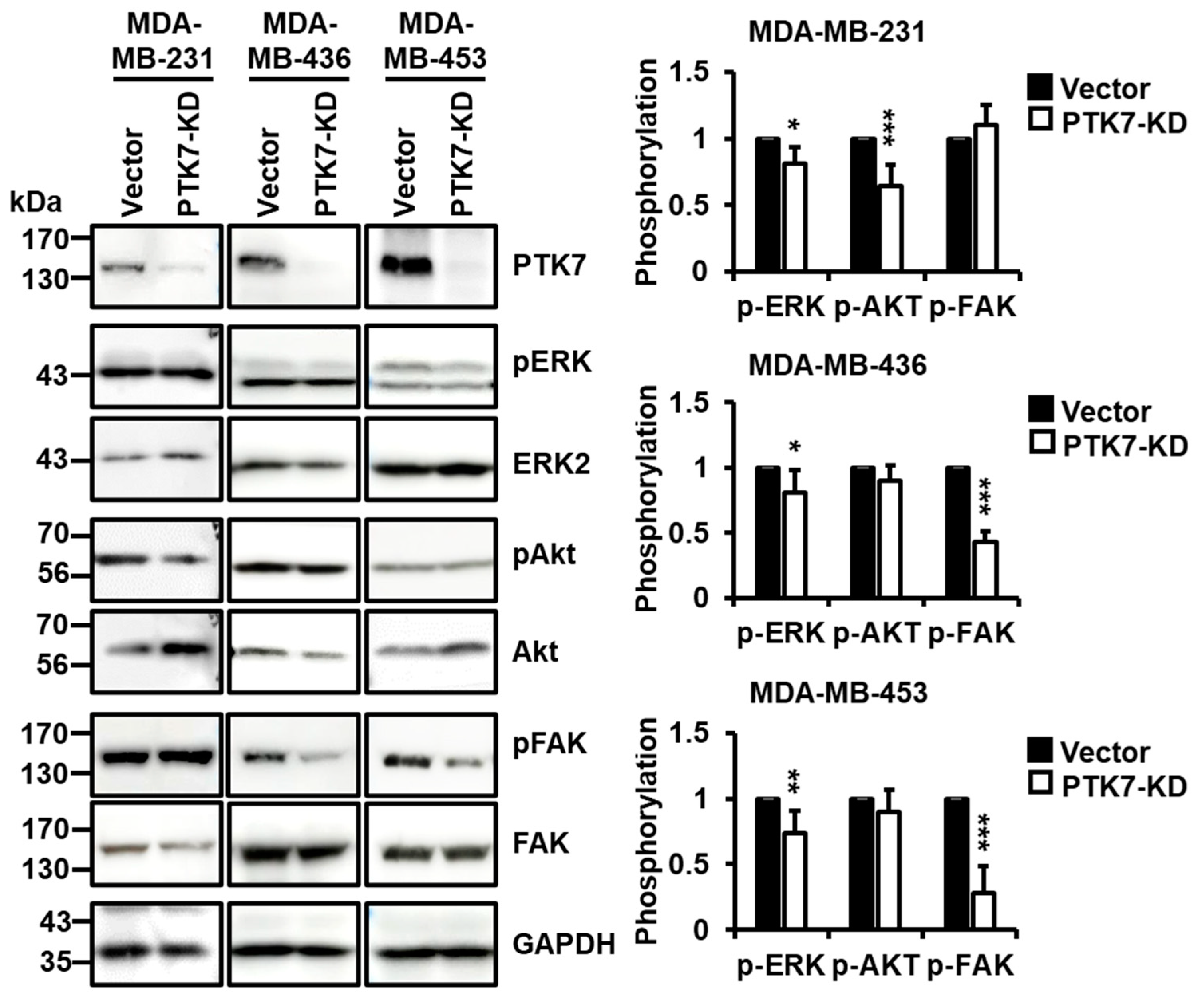
2.4. PTK7 Knockdown Inhibits the Activation of ERK, Akt, and FAK in TNBC Cells
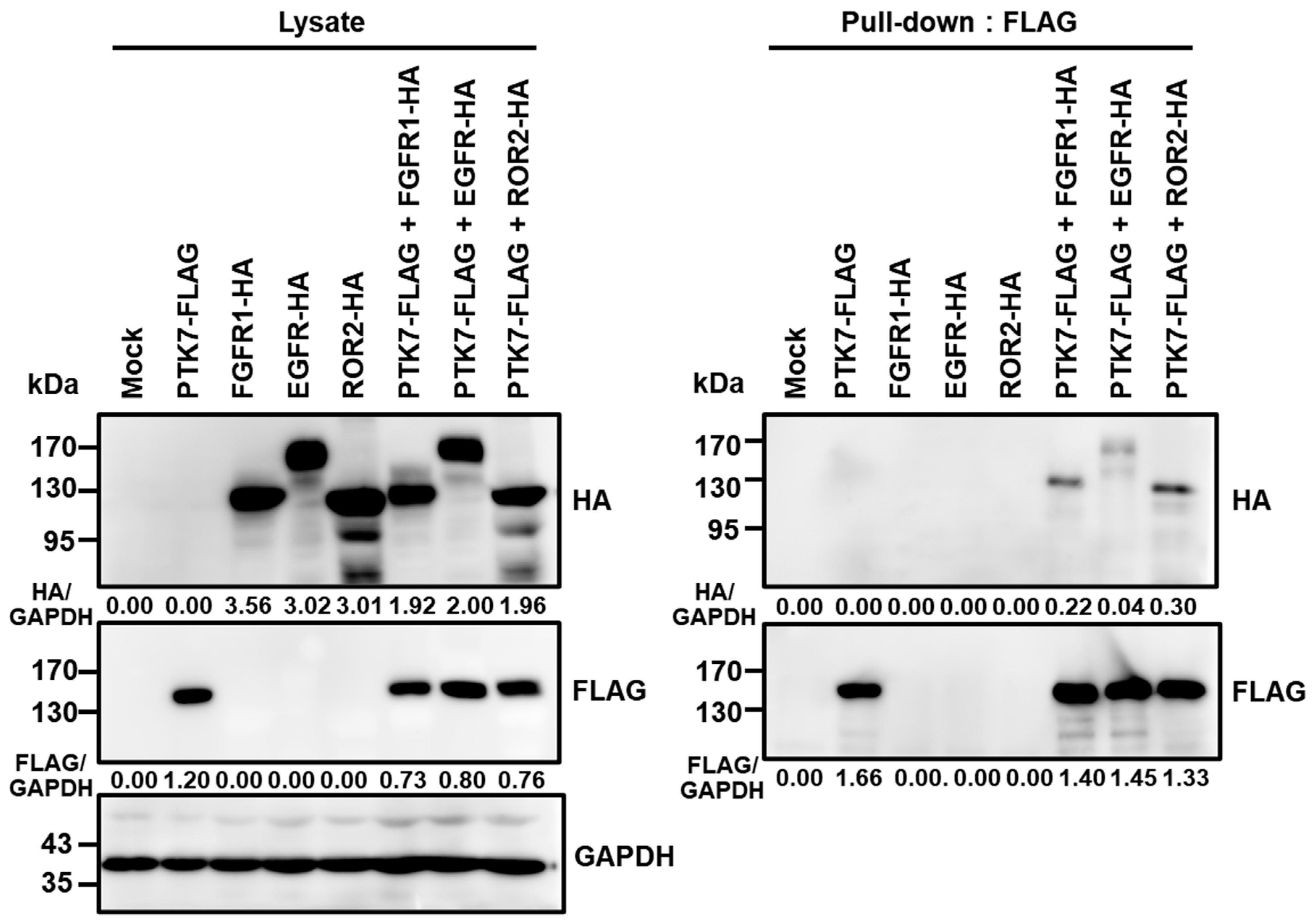
2.5. PTK7 Interacts with FGFR1, EGFR, and ROR2 in HEK293 Cells
2.6. PTK7 Knockdown Inhibits the Activation of FGFR1 and EGFR in MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Analysis of PTK7 mRNA Levels in BC Tissues from the TCGA Database
4.2. Cell Culture
4.3. Western Blot Analysis
4.4. Generation of PTK7-Knockdown Lentivirus and Infection of BC Cells
4.5. Cell Proliferation Assay
4.6. Cell Adhesion Assay
4.7. Chemotactic Migration Assay
4.8. Invasion Assay
4.9. Constructs Expressing PTK7-FLAG, FGFR1-HA, EGFR-HA, and ROR2-HA
4.10. Binding Analysis of PTK7 with FGFR1, EGFR, and ROR2
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, S. A historic and scientific review of breast cancer: The next global healthcare challenge. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. 1), S36–S39. [Google Scholar]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar]
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K.; et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer 2007, 110, 876–884. [Google Scholar]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [PubMed] [Green Version]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer 2008, 113, 2638–2645. [Google Scholar] [PubMed] [Green Version]
- Lee, S.T.; Strunk, K.M.; Spritz, R.A. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene 1993, 8, 3403–3410. [Google Scholar]
- Mossie, K.; Jallal, B.; Alves, F.; Sures, I.; Plowman, G.D.; Ullrich, A. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene 1995, 11, 2179–2184. [Google Scholar]
- Park, S.K.; Lee, H.S.; Lee, S.T. Characterization of the human full-length PTK7 cDNA encoding a receptor protein tyrosine kinase-like molecule closely related to chick KLG. J. Biochem. 1996, 119, 235–239. [Google Scholar]
- Lu, X.; Borchers, A.G.; Jolicoeur, C.; Rayburn, H.; Baker, J.C.; Tessier-Lavigne, M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 2004, 430, 93–98. [Google Scholar] [CrossRef]
- Shnitsar, I.; Borchers, A. PTK7 recruits dsh to regulate neural crest migration. Development 2008, 135, 4015–4024. [Google Scholar] [PubMed] [Green Version]
- Puppo, F.; Thome, V.; Lhoumeau, A.C.; Cibois, M.; Gangar, A.; Lembo, F.; Belotti, E.; Marchetto, S.; Lecine, P.; Prebet, T.; et al. Protein tyrosine kinase 7 has a conserved role in Wnt/beta-catenin canonical signalling. EMBO Rep. 2011, 12, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Bin-Nun, N.; Lichtig, H.; Malyarova, A.; Levy, M.; Elias, S.; Frank, D. PTK7 modulates Wnt signaling activity via LRP6. Development 2014, 141, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Prebet, T.; Lhoumeau, A.C.; Arnoulet, C.; Aulas, A.; Marchetto, S.; Audebert, S.; Puppo, F.; Chabannon, C.; Sainty, D.; Santoni, M.J.; et al. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood 2010, 116, 2315–2323. [Google Scholar]
- Meng, L.; Sefah, K.; O’Donoghue, M.B.; Zhu, G.; Shangguan, D.; Noorali, A.; Chen, Y.; Zhou, L.; Tan, W. Silencing of PTK7 in colon cancer cells: Caspase-10-dependent apoptosis via mitochondrial pathway. PLoS ONE 2010, 5, e14018. [Google Scholar]
- Gobble, R.M.; Qin, L.X.; Brill, E.R.; Angeles, C.V.; Ugras, S.; O’Connor, R.B.; Moraco, N.H.; Decarolis, P.L.; Antonescu, C.; Singer, S. Expression profiling of liposarcoma yields a multigene predictor of patient outcome and identifies genes that contribute to liposarcomagenesis. Cancer Res. 2011, 71, 2697–2705. [Google Scholar] [PubMed] [Green Version]
- Shin, W.S.; Kwon, J.; Lee, H.W.; Kang, M.C.; Na, H.W.; Lee, S.T.; Park, J.H. Oncogenic role of protein tyrosine kinase 7 in esophageal squamous cell carcinoma. Cancer Sci 2013, 104, 1120–1126. [Google Scholar]
- Shin, W.S.; Hong, Y.; Lee, H.W.; Lee, S.T. Catalytically defective receptor protein tyrosine kinase PTK7 enhances invasive phenotype by inducing MMP-9 through activation of AP-1 and NF-kappaB in esophageal squamous cell carcinoma cells. Oncotarget 2016, 7, 73242–73256. [Google Scholar]
- Shin, W.S.; Lee, H.W.; Lee, S.T. Catalytically inactive receptor tyrosine kinase PTK7 activates FGFR1 independent of FGF. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 12960–12971. [Google Scholar]
- Shin, W.S.; Maeng, Y.S.; Jung, J.W.; Min, J.K.; Kwon, Y.G.; Lee, S.T. Soluble PTK7 inhibits tube formation, migration, and invasion of endothelial cells and angiogenesis. Biochem. Biophys. Res. Commun. 2008, 371, 793–798. [Google Scholar]
- Shin, W.S.; Na, H.W.; Lee, S.T. Biphasic effect of PTK7 on KDR activity in endothelial cells and angiogenesis. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 2251–2260. [Google Scholar] [CrossRef] [Green Version]
- Gartner, S.; Gunesch, A.; Knyazeva, T.; Wolf, P.; Hogel, B.; Eiermann, W.; Ullrich, A.; Knyazev, P.; Ataseven, B. PTK 7 is a transforming gene and prognostic marker for breast cancer and nodal metastasis involvement. PLoS ONE 2014, 9, e84472. [Google Scholar]
- Ataseven, B.; Angerer, R.; Kates, R.; Gunesch, A.; Knyazev, P.; Hogel, B.; Becker, C.; Eiermann, W.; Harbeck, N. PTK7 expression in triple-negative breast cancer. Anticancer Res. 2013, 33, 3759–3763. [Google Scholar]
- Damelin, M.; Bankovich, A.; Bernstein, J.; Lucas, J.; Chen, L.; Williams, S.; Park, A.; Aguilar, J.; Ernstoff, E.; Charati, M.; et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci. Transl. Med. 2017, 9, 372. [Google Scholar]
- Shin, W.-S.; Park, M.-K.; Kim, J.H.; Oh, S.W.; Jang, J.-Y.; Lee, H.; Lee, S.-T. PTK7, a Catalytically Inactive Receptor Tyrosine Kinase, Increases Oncogenic Phenotypes in Xenograft Tumors of Esophageal Squamous Cell Carcinoma KYSE-30 Cells. Int. J. Mol. Sci. 2022, 23, 2391. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, W.-S.; Lee, S.-R.; Kim, S.; Choi, S.-Y.; Lee, S.-T. Anti-PTK7 Monoclonal Antibodies Exhibit Anti-Tumor Activity at the Cellular Level and in Mouse Xenograft Models of Esophageal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 12195. [Google Scholar] [PubMed]
- Oh, S.W.; Shin, W.S.; Lee, S.T. Anti-PTK7 Monoclonal Antibodies Inhibit Angiogenesis by Suppressing PTK7 Function. Cancers 2022, 14, 4463. [Google Scholar] [CrossRef]
- Shin, W.S.; Gim, J.; Won, S.; Lee, S.T. Biphasic regulation of tumorigenesis by PTK7 expression level in esophageal squamous cell carcinoma. Sci. Rep. 2018, 8, 8519. [Google Scholar]
- Saini, K.S.; Loi, S.; de Azambuja, E.; Metzger-Filho, O.; Saini, M.L.; Ignatiadis, M.; Dancey, J.E.; Piccart-Gebhart, M.J. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat. Rev. 2013, 39, 935–946. [Google Scholar] [PubMed]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [PubMed]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Cui, N.P.; Qiao, S.; Jiang, S.; Hu, J.L.; Wang, T.T.; Liu, W.W.; Qin, Y.; Wang, Y.N.; Zheng, L.S.; Zhang, J.C.; et al. Protein Tyrosine Kinase 7 Regulates EGFR/Akt Signaling Pathway and Correlates With Malignant Progression in Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 699889. [Google Scholar]
- Ataseven, B.; Gunesch, A.; Eiermann, W.; Kates, R.E.; Högel, B.; Knyazev, P.; Ullrich, A.; Harbeck, N. PTK7 as a potential prognostic and predictive marker of response to adjuvant chemotherapy in breast cancer patients, and resistance to anthracycline drugs. Onco Targets Ther. 2014, 7, 1723–1731. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Yan, L.; Zhang, D.; Guan, X.; Dong, B.; Zhao, M.; Hao, C. PTK7 overexpression in colorectal tumors: Clinicopathological correlation and prognosis relevance. Oncol. Rep. 2016, 36, 1829–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.J.; Li, H.L.; Guo, S.J.; Ma, H.; Liu, S.J.; Liu, D.; Xue, F.X. The Increased PTK7 Expression Is a Malignant Factor in Cervical Cancer. Dis. Markers 2019, 2019, 5380197. [Google Scholar]
- Duan, F.; Tang, J.; Kong, F.L.; Zou, H.W.; Ni, B.L.; Yu, J.C. Identification of PTK7 as a promising therapeutic target for thyroid cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6809–6817. [Google Scholar]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Wong, T.L.M.; Wong, T.L.; Zhou, L.; Man, K.; Purcell, J.; Lee, T.K.; Yun, J.P.; Ma, S. Protein Tyrosine Kinase 7 (PTK7) Promotes Metastasis in Hepatocellular Carcinoma via SOX9 Regulation and TGF-β Signaling. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Raivola, J.; Dini, A.; Karvonen, H.; Piki, E.; Salokas, K.; Niininen, W.; Kaleva, L.; Zhang, K.; Arjama, M.; Gudoityte, G.; et al. Multiomics characterization implicates PTK7 in ovarian cancer EMT and cell plasticity and offers strategies for therapeutic intervention. Cell Death Dis. 2022, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, B.; Valverius, E.M.; Wosikowski, K.; Saceda, M.; Pearson, J.W.; Martin, M.B.; Bates, S.E. Increased epidermal growth factor receptor in an estrogen-responsive, adriamycin-resistant MCF-7 cell line. J. Cell Physiol. 1993, 157, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.E.; Valverius, E.M.; Ennis, B.W.; Bronzert, D.A.; Sheridan, J.P.; Stampfer, M.R.; Mendelsohn, J.; Lippman, M.E.; Dickson, R.B. Expression of the transforming growth factor-alpha/epidermal growth factor receptor pathway in normal human breast epithelial cells. Endocrinology 1990, 126, 596–607. [Google Scholar] [CrossRef]
- McLeskey, S.W.; Ding, I.Y.; Lippman, M.E.; Kern, F.G. MDA-MB-134 breast carcinoma cells overexpress fibroblast growth factor (FGF) receptors and are growth-inhibited by FGF ligands. Cancer Res. 1994, 54, 523–530. [Google Scholar]
- Nelson, J.; McGivern, M.; Walker, B.; Bailie, J.R.; Murphy, R.F. Growth-inhibitory and growth-stimulatory effects of epidermal growth factor on human breast cancer cell line, MDA.MB.436: Dependence on culture conditions. Eur. J. Cancer Clin. Oncol. 1989, 25, 1851–1855. [Google Scholar]
- Liu, D.; Guo, P.; McCarthy, C.; Wang, B.; Tao, Y.; Auguste, D. Peptide density targets and impedes triple negative breast cancer metastasis. Nat. Commun. 2018, 9, 2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podleschny, M.; Grund, A.; Berger, H.; Rollwitz, E.; Borchers, A. A PTK7/Ror2 Co-Receptor Complex Affects Xenopus Neural Crest Migration. PLoS ONE 2015, 10, e0145169. [Google Scholar]
- Martinez, S.; Scerbo, P.; Giordano, M.; Daulat, A.M.; Lhoumeau, A.C.; Thome, V.; Kodjabachian, L.; Borg, J.P. The PTK7 and ROR2 Protein Receptors Interact in the Vertebrate WNT/Planar Cell Polarity (PCP) Pathway. J. Biol. Chem. 2015, 290, 30562–30572. [Google Scholar] [CrossRef] [Green Version]
- Sung, V.Y.C.; Knight, J.F.; Johnson, R.M.; Stern, Y.E.; Saleh, S.M.; Savage, P.; Monast, A.; Zuo, D.; Duhamel, S.; Park, M. Co-dependency for MET and FGFR1 in basal triple-negative breast cancers. NPJ Breast Cancer 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nejad-Ariani, H.; Althagafi, E.; Kaur, K. Small Peptide Ligands for Targeting EGFR in Triple Negative Breast Cancer Cells. Sci. Rep. 2019, 9, 2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.H.; Guy, G.R.; Hadari, Y.R.; Laks, S.; Gotoh, N.; Schlessinger, J.; Lax, I. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell Biol. 2000, 20, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Sobhani, N.; Fan, C.; Flores-Villanueva, P.O.; Generali, D.; Li, Y. The Fibroblast Growth Factor Receptors in Breast Cancer: From Oncogenesis to Better Treatments. Int. J. Mol. Sci. 2020, 21, 2011. [Google Scholar]
- Luqmani, Y.A.; Graham, M.; Coombes, R.C. Expression of basic fibroblast growth factor, FGFR1 and FGFR2 in normal and malignant human breast, and comparison with other normal tissues. Br. J. Cancer 1992, 66, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.L.; Thike, A.A.; Tan, S.Y.; Chua, P.J.; Bay, B.H.; Tan, P.H. Expression of FGFR1 is an independent prognostic factor in triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 151, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.A.; Naz, S.; Hashmi, S.K.; Irfan, M.; Hussain, Z.F.; Khan, E.Y.; Asif, H.; Faridi, N. Epidermal growth factor receptor (EGFR) overexpression in triple-negative breast cancer: Association with clinicopathologic features and prognostic parameters. Surg. Exp. Pathol. 2019, 2, 6. [Google Scholar]
- Hopper-Borge, E.A.; Nasto, R.E.; Ratushny, V.; Weiner, L.M.; Golemis, E.A.; Astsaturov, I. Mechanisms of tumor resistance to EGFR-targeted therapies. Expert Opin. Ther. Targets 2009, 13, 339–362. [Google Scholar] [CrossRef] [Green Version]
- Shahriyari, L. Effect of normalization methods on the performance of supervised learning algorithms applied to HTSeq-FPKM-UQ data sets: 7SK RNA expression as a predictor of survival in patients with colon adenocarcinoma. Brief. Bioinform. 2019, 20, 985–994. [Google Scholar]
- Wigler, M.; Sweet, R.; Sim, G.K.; Wold, B.; Pellicer, A.; Lacy, E.; Maniatis, T.; Silverstein, S.; Axel, R. Transformation of mammalian cells with genes from prokaryotes and eukaryotes. 1979. Biotechnology 1992, 24, 444–452. [Google Scholar] [PubMed]
- Shin, W.S.; Shim, H.J.; Lee, Y.H.; Pyo, M.; Park, J.S.; Ahn, S.Y.; Lee, S.T. PTK6 Localized at the Plasma Membrane Promotes Cell Proliferation and MigratiOn Through Phosphorylation of Eps8. J. Cell Biochem. 2017, 118, 2887–2895. [Google Scholar]
- Shin, W.S.; Park, M.K.; Lee, Y.H.; Kim, K.W.; Lee, H.; Lee, S.T. The catalytically defective receptor protein tyrosine kinase EphA10 promotes tumorigenesis in pancreatic cancer cells. Cancer Sci. 2020, 111, 3292–3302. [Google Scholar] [CrossRef]
- Kang, S.-A.; Lee, E.-S.; Yoon, H.-Y.; Randazzo, P.A.; Lee, S.-T. PTK6 Inhibits Down-regulation of EGF Receptor through Phosphorylation of ARAP1. J. Biol. Chem. 2010, 285, 26013–26021. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Levitzki, A.; Zhu, H.-J.; Walker, F.; Burgess, A.; Maruta, H. Platelet-derived Growth Factor Requires Epidermal Growth Factor Receptor to Activate p21-activated Kinase Family Kinases. J. Biol. Chem. 2001, 276, 26741–26744. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, W.-S.; Oh, S.W.; Park, H.N.; Kim, J.H.; Lee, S.-T. Knockdown of PTK7 Reduces the Oncogenic Potential of Breast Cancer Cells by Impeding Receptor Tyrosine Kinase Signaling. Int. J. Mol. Sci. 2023, 24, 12173. https://doi.org/10.3390/ijms241512173
Shin W-S, Oh SW, Park HN, Kim JH, Lee S-T. Knockdown of PTK7 Reduces the Oncogenic Potential of Breast Cancer Cells by Impeding Receptor Tyrosine Kinase Signaling. International Journal of Molecular Sciences. 2023; 24(15):12173. https://doi.org/10.3390/ijms241512173
Chicago/Turabian StyleShin, Won-Sik, Si Won Oh, Han Na Park, Jae Hoon Kim, and Seung-Taek Lee. 2023. "Knockdown of PTK7 Reduces the Oncogenic Potential of Breast Cancer Cells by Impeding Receptor Tyrosine Kinase Signaling" International Journal of Molecular Sciences 24, no. 15: 12173. https://doi.org/10.3390/ijms241512173
APA StyleShin, W.-S., Oh, S. W., Park, H. N., Kim, J. H., & Lee, S.-T. (2023). Knockdown of PTK7 Reduces the Oncogenic Potential of Breast Cancer Cells by Impeding Receptor Tyrosine Kinase Signaling. International Journal of Molecular Sciences, 24(15), 12173. https://doi.org/10.3390/ijms241512173






