Phosphoproteome Reveals Extracellular Regulated Protein Kinase Phosphorylation Mediated by Mitogen-Activated Protein Kinase Kinase-Regulating Granulosa Cell Apoptosis in Broody Geese
Abstract
:1. Introduction
2. Results
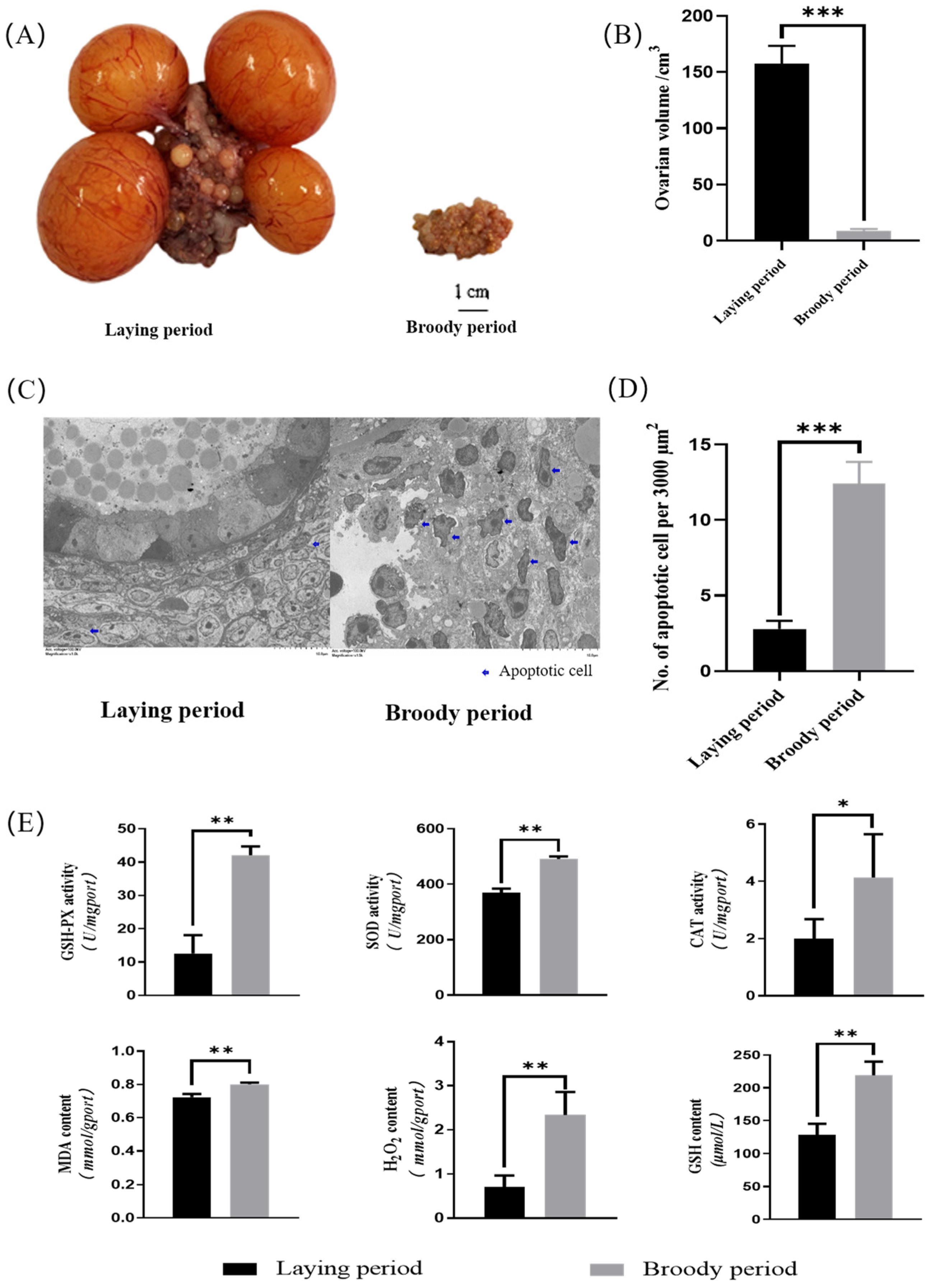
2.1. Morphological Detection and Antioxidant Activity of Ovaries in Broody Geese
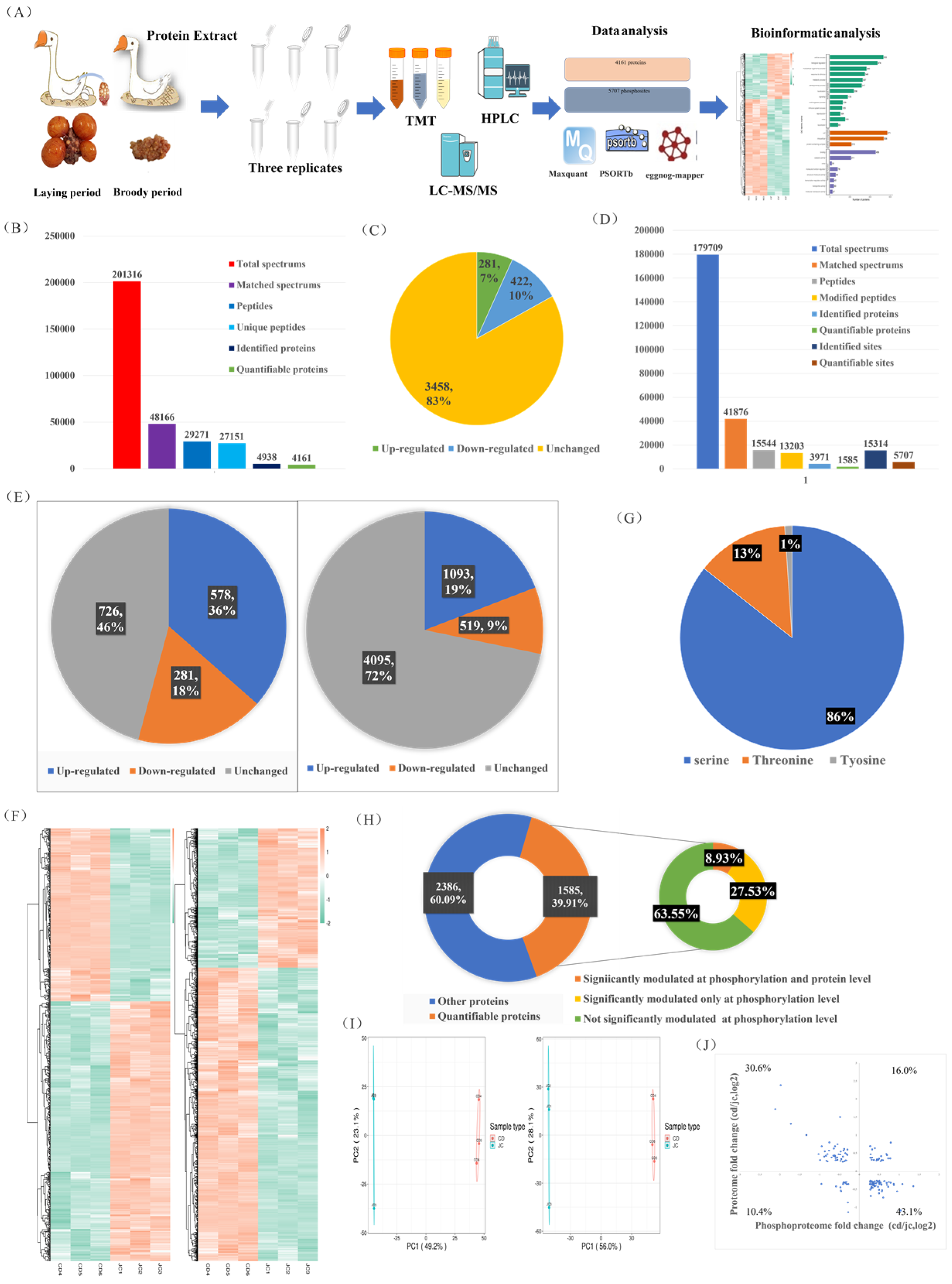
2.2. MS-Based Quantitative Proteomic and Phosphoproteomic Characterizations of the Ovaries of Laying and Broody Geese
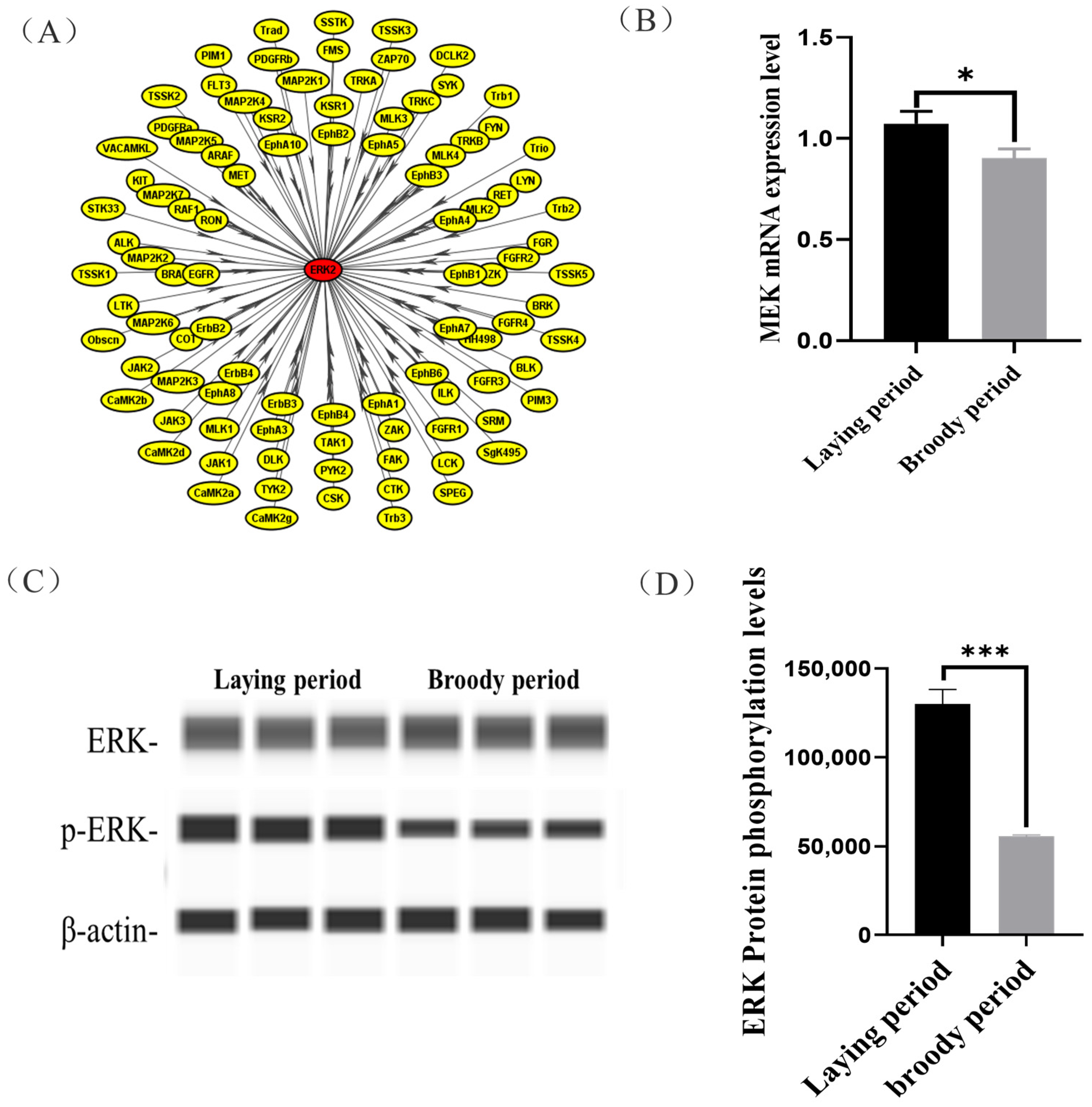
2.3. Identification of Signaling Pathways and Key Substrate of Differential Abundance Phosphoproteins between Laying and Broody Geese
2.4. Functional Validation of Specific Kinase (MEK)
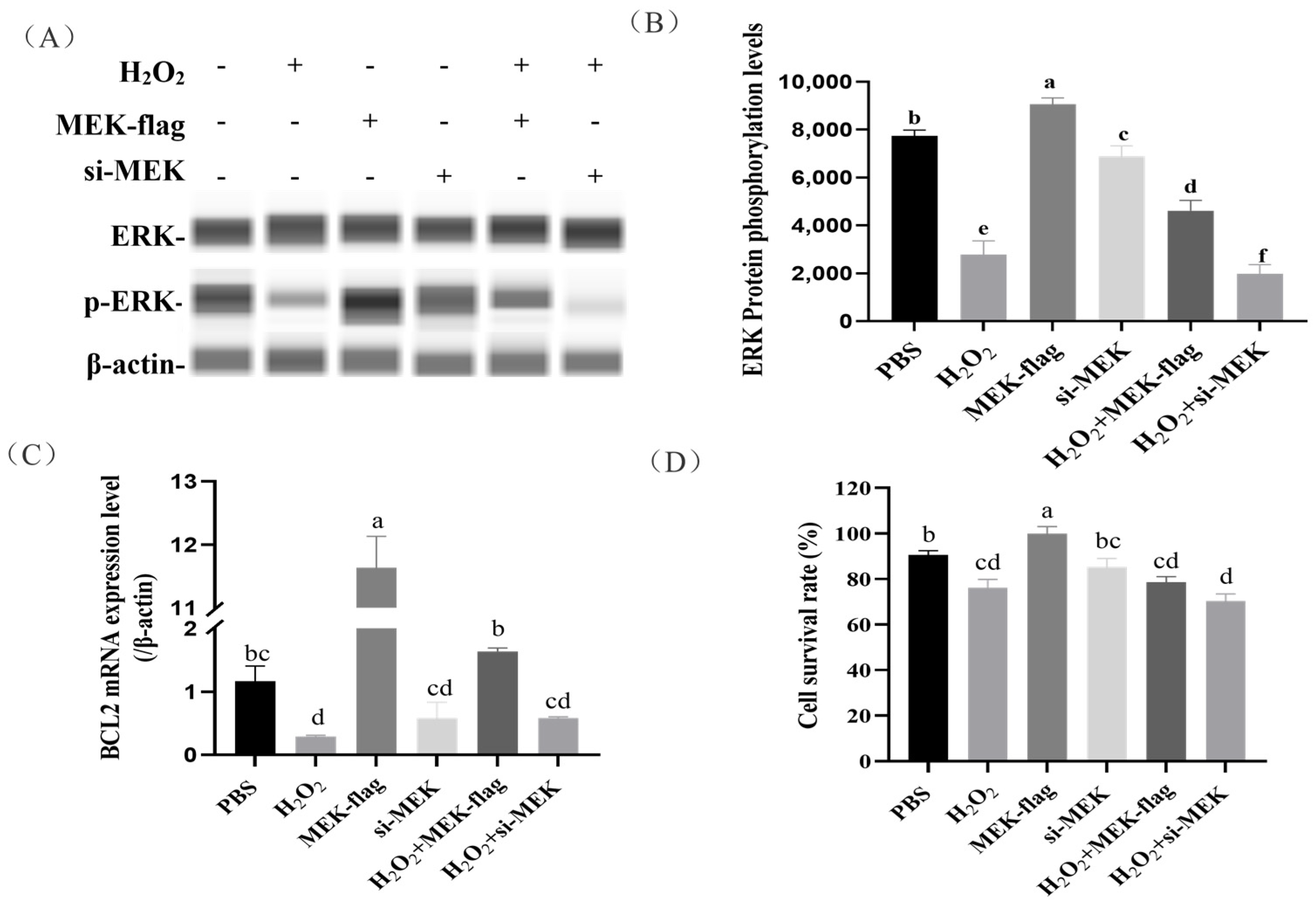
2.5. Regulation of Granulosa Cell Apoptosis by MEK-Mediated Phosphorylation of ERK
3. Discussion
4. Materials and Methods
4.1. Animal and Sample Collection
4.2. Morphological Detection and Antioxidant Activity of Ovaries
4.3. Proteome and Phosphoproteome Analyses
4.3.1. Protein Extraction
4.3.2. Protein Digestion and TMT Labeling
4.3.3. LC-MS/MS Analysis and Database Search
4.3.4. Bioinformatics Analysis
4.4. Isolation and Identification of Goose Ovarian Granulosa Cells
4.5. Obtaining Overexpression Vectors and MEK siRNA, and Transfecting Them
4.6. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
4.7. Capillary Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkar, P. Broodiness and Broody Hen Management During Egg Incubation. Rev. Agric. Sci. 2022, 10, 337–343. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Y.Z.; Gu, T.T.; Cao, Z.F.; Zhao, W.M.; Qin, H.R.; Xu, Q.; Chen, G.H. Comparison of the broody behavior characteristics of different breeds of geese. Poult. Sci. 2019, 98, 5226–5233. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Bai, S.; Zeng, Q.; Su, Z.; Zhuo, Y.; Mao, X.; Yin, H.; Feng, B.; Liu, J.; et al. Dietary tributyrin improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult. Sci. 2021, 100, 101429. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Q.; Zhao, D.H.; Cheng, H.Z.; Wang, S.B.; Yang, J.; Cui, H.X.; Lu, M.Y.; Wu, H.Z.; Xu, L.; Liu, G.J. Effects of dietary Enteromorpha powder on reproduction-related hormones and genes during the late laying period of Zi geese. Anim. Biosci. 2021, 34, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Gu, T.; Weng, K.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effects of Oxidative Stress on the Autophagy and Apoptosis of Granulosa Cells in Broody Geese. Int. J. Mol. Sci. 2023, 24, 2154. [Google Scholar] [CrossRef]
- Lim, S.-C.; Duong, H.-Q.; Parajuli, K.R.; Han, S.I. Pro-apoptotic role of the MEK/ERK pathway in ursodeoxycholic acid-induced apoptosis in SNU601 gastric cancer cells. Oncol. Rep. 2012, 28, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Schweyer, S.; Soruri, A.; Meschter, O.; Heintze, A.; Zschunke, F.; Miosge, N.; Thelen, P.; Schlott, T.; Radzun, H.J.; Fayyazi, A. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. Br. J. Cancer 2004, 91, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Woessmann, W.; Chen, X.; Borkhardt, A. Ras-mediated activation of ERK by cisplatin induces cell death independently of p53 in osteosarcoma and neuroblastoma cell lines. Cancer Chemother. Pharmacol. 2002, 50, 397–404. [Google Scholar] [CrossRef]
- Liu, H.; Jian, Q.; Xue, K.; Ma, C.; Xie, F.; Wang, R.; Liao, W.; Liu, Y.; Chi, S.; Li, C. The MEK/ERK signalling cascade is required for sonic hedgehog signalling pathway-mediated enhancement of proliferation and inhibition of apoptosis in normal keratinocytes. Exp. Dermatol. 2014, 23, 896–901. [Google Scholar] [CrossRef]
- Cosulich, S.C.; Savory, P.J.; Clarke, P.R. Bcl-2 regulates amplification of caspase activation by cytochrome c. Curr. Biol. CB 1999, 9, 147–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewari, R.; Sharma, V.; Koul, N.; Sen, E. Involvement of miltefosine-mediated ERK activation in glioma cell apoptosis through Fas regulation. J. Neurochem. 2008, 107, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Guo, Z.; Tang, Z.; Ai, X.; Qi, Q.; Liu, X.; Huang, D.; Li, Z.; Ji, S.; Guo, Q. Mesenchymal Stem Cells Activate the MEK/ERK Signaling Pathway and Enhance DNA Methylation via DNMT1 in PBMC from Systemic Lupus Erythematosus. BioMed Res. Int. 2020, 2020, 4174082. [Google Scholar] [CrossRef] [PubMed]
- Chwang, W.B.; O’Riordan, K.J.; Levenson, J.M.; Sweatt, J.D. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn. Mem. (Cold Spring Harb. N.Y.) 2006, 13, 322–328. [Google Scholar] [CrossRef] [Green Version]
- MacInnis, B.L.; Campenot, R.B. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol. Cell. Neurosci. 2005, 28, 430–439. [Google Scholar] [CrossRef]
- Li, F.; Omori, N.; Sato, K.; Jin, G.; Nagano, I.; Manabe, Y.; Shoji, M.; Abe, K. Coordinate expression of survival p-ERK and proapoptotic cytochrome c signals in rat brain neurons after transient MCAO. Brain Res. 2002, 958, 83–88. [Google Scholar] [CrossRef]
- Oh, U.H.; Kim, D.-H.; Lee, J.; Han, S.-I.; Kim, J.-H. Eriodictyol induces apoptosis via regulating phosphorylation of JNK, ERK, and FAK/AKT in pancreatic cancer cells. J. Appl. Biol. Chem. 2022, 65, 83–88. [Google Scholar] [CrossRef]
- Li, Q.; Chen, M.; Liu, H.; Yang, L.; Yang, T.; Yang, G.; He, G. The dual role of ERK signaling in the apoptosis of neurons. Front. Biosci. -Landmark 2014, 19, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Hayashi-Takanaka, Y.; Stasevich, T.J.; Sato, Y. Visualizing posttranslational and epigenetic modifications of endogenous proteins in vivo. Histochem. Cell Biol. 2015, 144, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Pi, Y.; Fang, C.-L.; Su, Z.-Y. Protein phosphorylation: A potential target in glioma development. Ibrain 2022, 8, 176–189. [Google Scholar] [CrossRef]
- Berndsen, K.; Lis, P.; Yeshaw, W.M.; Wawro, P.S.; Nirujogi, R.S.; Wightman, M.; Macartney, T.; Dorward, M.; Knebel, A.; Tonelli, F.; et al. PPM1H phosphatase counteracts LRRK2 signaling by selectively dephosphorylating Rab proteins. eLife 2019, 8, e50416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Zhang, Y.; Yao, Y.; Zhao, W.; Xu, Q.; Chen, G. Characterization of ovarian morphology and reproductive hormones in Zhedong white geese (Anser cygnoides domesticus) during the reproductive cycle. J. Anim. Physiol. Anim. Nutr. 2021, 105, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiao, Q.; Gilbert, E.R.; Cui, Z.; Zhao, X.; Wang, Y.; Yin, H.; Li, D.; Zhang, H.; Zhu, Q. Whole-transcriptome analysis of atrophic ovaries in broody chickens reveals regulatory pathways associated with proliferation and apoptosis. Sci. Rep. 2018, 8, 7231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, A.; Yoshimura, Y.; Okamoto, T. Changes in the Populations of Mitotic and Apoptotic Cells in White Follicles During Atresia in Hens. Poult. Sci. 2002, 81, 408–413. [Google Scholar] [CrossRef]
- Zhang, B.; Arany, Z.; Mann, D.; Rhee, J.G.; Fenton, R.G. Partitioning apoptosis: A novel form of the execution phase of apoptosis. Apoptosis 2005, 10, 219–231. [Google Scholar] [CrossRef]
- Surai, P.F. Antioxidant Systems in Poultry Biology: Superoxide Dismutase. Nutrition 2016, 1, 8. [Google Scholar] [CrossRef]
- Cheng, S.B.; Li, X.Q.; Wang, J.X.; Wu, Y.; Li, P.; Pi, J.S. The effect of light on follicular development in laying hens. Anim. Biosci. 2021, 34, 1766–1775. [Google Scholar] [CrossRef]
- Sharma, K.; D’Souza, R.C.J.; Tyanova, S.; Schaab, C.; Wiśniewski, J.R.; Cox, J.; Mann, M. Ultradeep Human Phosphoproteome Reveals a Distinct Regulatory Nature of Tyr and Ser/Thr-Based Signaling. Cell Rep. 2014, 8, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- Sacco, F.; Seelig, A.; Humphrey, S.J.; Krahmer, N.; Volta, F.; Reggio, A.; Marchetti, P.; Gerdes, J.; Mann, M. Phosphoproteomics Reveals the GSK3-PDX1 Axis as a Key Pathogenic Signaling Node in Diabetic Islets. Cell Metab. 2019, 29, 1422–1432. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Xia, P.; Xu, X.Y. PI3K/Akt/mTOR signaling pathway in cancer stem cells: From basic research to clinical application. Am. J. Cancer Res. 2015, 5, 1602–1609. [Google Scholar] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawson, T.; Nash, P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000, 14, 1027–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Thakkar, H.; Tyan, F.; Gim, S.; Robinson, H.; Lee, C.; Pandey, S.K.; Nwokorie, C.; Onwudiwe, N.; Srivastava, R.K. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene 2001, 20, 6073–6083. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zeng, J.; Shen, K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch. Gynecol. Obstet. 2014, 290, 1067–1078. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Fan, Y.; Song, X.; Wu, J.; Fu, Z.; Li, T.; Huang, Y.; Tang, Z.; Meng, S.; et al. Tropoelastin improves adhesion and migration of intra-articular injected infrapatellar fat pad MSCs and reduces osteoarthritis progression. Bioact. Mater. 2022, 10, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.; Xu, X.; Hu, H.; Bai, Y.; Hai, J.; Cheng, L.; Zhu, R. Ion elemental-optimized layered double hydroxide nanoparticles promote chondrogenic differentiation and intervertebral disc regeneration of mesenchymal stem cells through focal adhesion signaling pathway. Bioact. Mater. 2023, 22, 75–90. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell–cell and cell–matrix adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Baribault, H.; Adamson, E.D. Vinculin knockout results in heart and brain defects during embryonic development. Development 1998, 125, 327–337. [Google Scholar] [CrossRef]
- Ezzell, R.M.; Goldmann, W.H.; Wang, N.; Parashurama, N.; Ingber, D.E. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp. Cell Res. 1997, 231, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Deming, D.; Geiger, P.; Chen, H.; Vaccaro, A.; Kunnimalaiyaan, M.; Holen, K. ZM336372, A Raf-1 Activator, Causes Suppression of Proliferation in a Human Hepatocellular Carcinoma Cell Line. J. Gastrointest. Surg. 2008, 12, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Piperdi, S.; Gorlick, R. Activation of the RAF/mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase pathway mediates apoptosis induced by chelerythrine in osteosarcoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 6396–6404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.E.; Jeong, J.Y.; Choi, H.; Chang, Y.K.; Ahn, M.S.; Ham, Y.R.; Na, K.R.; Lee, K.W. ERK phosphorylation plays an important role in the protection afforded by hypothermia against renal ischemia-reperfusion injury. Surgery 2017, 161, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Jo, S.K.; Cho, W.Y.; Sung, S.A.; Kim, H.K.; Won, N.H. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005, 67, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Casique, A.; Montes-Alvarado, J.B.; Barragán, M.; Larrauri-Rodríguez, K.A.; Perez-Gonzalez, A.; Delgado-Magallón, A.; Millán-Perez-Peña, L.; Rosas-Murrieta, N.H.; Maycotte, P. ERK activation modulates invasiveness and Reactive Oxygen Species (ROS) production in triple negative breast cancer cell lines. Cell. Signal. 2023, 101, 110487. [Google Scholar] [CrossRef]
- Choi, B.K.; Choi, C.H.; Oh, H.L.; Kim, Y.K. Role of ERK Activation in Cisplatin-Induced Apoptosis in A172 Human Glioma Cells. NeuroToxicology 2004, 25, 915–924. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A. The mode of cisplatin-induced cell death in CYP2E1-overexpressing HepG2 cells: Modulation by ERK, ROS, glutathione, and thioredoxin. Free. Radic. Biol. Med. 2007, 43, 1061–1075. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK Activation in Cisplatin-induced Apoptosis*. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef] [Green Version]
- Dumont, A.; Hehner, S.P.; Hofmann, T.G.; Ueffing, M.; Dröge, W.; Schmitz, M.L. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-κB. Oncogene 1999, 18, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.F.; Dietz, R.; von Harsdorf, R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells. Circulation 1997, 96, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Herlyn, M.; Yang, X. TRIM15 and CYLD regulate ERK activation via lysine-63-linked polyubiquitination. Nat. Cell Biol. 2021, 23, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Sulkshane, P.; Teni, T. Myeloid cell leukemia-1: A formidable barrier to anticancer therapeutics and the quest of targeting it. Explor. Target. Anti-Tumor Ther. 2022, 3, 278–296. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Gu, T.; Weng, K.; Zhang, Y.; Cao, Z.; Zhang, Y.; Zhao, W.; Chen, G.; Xu, Q. Phosphoproteome Reveals Extracellular Regulated Protein Kinase Phosphorylation Mediated by Mitogen-Activated Protein Kinase Kinase-Regulating Granulosa Cell Apoptosis in Broody Geese. Int. J. Mol. Sci. 2023, 24, 12278. https://doi.org/10.3390/ijms241512278
Zhao S, Gu T, Weng K, Zhang Y, Cao Z, Zhang Y, Zhao W, Chen G, Xu Q. Phosphoproteome Reveals Extracellular Regulated Protein Kinase Phosphorylation Mediated by Mitogen-Activated Protein Kinase Kinase-Regulating Granulosa Cell Apoptosis in Broody Geese. International Journal of Molecular Sciences. 2023; 24(15):12278. https://doi.org/10.3390/ijms241512278
Chicago/Turabian StyleZhao, Shuai, Tiantian Gu, Kaiqi Weng, Yu Zhang, Zhengfeng Cao, Yang Zhang, Wenming Zhao, Guohong Chen, and Qi Xu. 2023. "Phosphoproteome Reveals Extracellular Regulated Protein Kinase Phosphorylation Mediated by Mitogen-Activated Protein Kinase Kinase-Regulating Granulosa Cell Apoptosis in Broody Geese" International Journal of Molecular Sciences 24, no. 15: 12278. https://doi.org/10.3390/ijms241512278





