The Role of Bifidobacterium bifidum novaBBF7, Bifidobacterium longum novaBLG2 and Lactobacillus paracasei TJB8 to Improve Mechanisms Linked to Neuronal Cells Protection against Oxidative Condition in a Gut-Brain Axis Model
Abstract
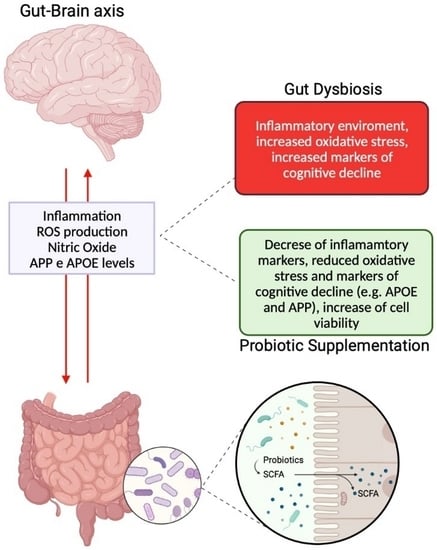
:1. Introduction
2. Results
2.1. The Effects of B. bifidum novaBBF7, B. longum novaBLG2 and L. paracasei TJB8 Supplementation on the In Vitro Barrier Model
2.2. The Effects of B. bifidum novaBBF7, B. longum novaBLG2 and and L. paracasei TJB8 on the Gut-Brain Axis
2.3. Analysis of the Mechanisms Underlying Cognitive Functions under Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Agents Preparation
4.3. Experimental Protocol
4.4. Gut-Brain Axis Model
4.5. MTT Test
4.6. Intestinal Integrity Analysis
4.7. Butyric Acid Quantification
4.8. ROS Production
4.9. TNFα Assay Kit
4.10. Mitochondrial Membrane Potential
4.11. iNOS ELISA Kit
4.12. ERK/MAPKS ELISA Kit
4.13. APOE 9 ELISA Kit
4.14. APP ELISA Kit
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADV DMEM | advanced Dulbecco’s Modified Eagle’s Medium |
| ADV DMEM-F12 | advanced Dulbecco’s Modified Eagle’s Medium/Nutrient F-12 Ham |
| APOE | apolipoprotein E |
| APP | β-amyloid analysis |
| BBB | blood-brain barrier |
| BDNF | brain-derived neurotrophic factor |
| CFU | colony-forming unit |
| CNS | central nervous system |
| EMA | European Medicines Agency |
| ERK | extracellular signal-regulated kinase |
| FBS | fetal bovine serum |
| FDA | Food and Drug Administration |
| GABA | gamma-aminobutyric acid |
| HRP | horseradish peroxidase |
| iNOS | inducible nitric oxide synthase |
| MAPK | mitogen-activated protein kinase |
| MCI | mild cognitive impairment |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PNS | peripheral nervous system |
| SCFAs | short chain fatty acid |
| TEER | transepithelial electrical resistance |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| TNFα | tumor necrosis factor α |
References
- Abdivalievna, A.N. Features of Cognitive Disorders. In Innovative Society: Problems, Analysis and Development Prospects (Spain). 2022, pp. 101–105. Available online: https://scholar.google.com/scholar_lookup?journal=Innovat.+Soc.+Probl.+Anal.+Dev.+Prospects&title=Features+of+cognitive+disorders&author=A.+N.+Abdivalievna&volume=2022&publication_year=2022&pages=101-105& (accessed on 24 June 2023).
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Campbell, N.L.; Unverzagt, F.; LaMantia, M.A.; Khan, B.A.; Boustani, M.A. Risk factors for the progression of mild cognitive impairment to dementia. Clin. Geriatr. Med. 2013, 29, 873–893. [Google Scholar] [CrossRef] [Green Version]
- Vassilaki, M.; Aakre, J.A.; Cha, R.H.; Kremers, W.K.; St. Sauver, J.L.; Mielke, M.M.; Roberts, R.O. Multimorbidity and risk of mild cognitive impairment. J. Am. Geriatr. Soc. 2015, 63, 1783–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geda, Y.E.; Roberts, R.O.; Knopman, D.S.; Christianson, T.J.; Pankratz, V.S.; Ivnik, R.J.; Rocca, W.A. Physical exercise, aging, and mild cognitive impairment: A population-based study. Arch. Neurol. 2010, 67, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, J.M.T.; Wong, G.T.C. Cerebral glutamate regulation and receptor changes in perioperative neuroinflammation and cognitive dysfunction. Biomolecules 2022, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W. Detection and prevention of cognitive decline. Am. J. Geriatr. Psychiatry 2016, 24, 1142–1150. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Abbasabadi, Z.; Braidy, N.; Nabavi, S.M. Role of green tea catechins in prevention of age-related cognitive decline: Pharmacological targets and clinical perspective. J. Cell. Physiol. 2019, 234, 2447–2459. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Rosshart, S.P.; Vassallo, B.G.; Angeletti, D.; Hutchinson, D.S.; Morgan, A.P.; Takeda, K.; Rehermann, B. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 2017, 171, 1015–1028. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay between the gut-brain axis, obesity and cognitive function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Raes, J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Matsuda, K. How microbes affect depression: Underlying mechanisms via the Gut–brain axis and the modulating role of probiotics. Int. J. Mol. Sci. 2022, 23, 1172. [Google Scholar] [CrossRef]
- Sikorska, M.; Antosik-Wójcińska, A.Z.; Dominiak, M. Probiotics as a Tool for Regulating Molecular Mechanisms in Depression: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Mol. Sci. 2023, 24, 3081. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tang, M.; Wu, X.; Kong, X.; Liu, Y.; Xu, X. Lactobacillus rhamnosus zz-1 exerts preventive effects on chronic unpredictable mild stress-induced depression in mice via regulating the intestinal microenvironment. Food Funct. 2022, 13, 4331–4343. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Savignac, H.M.; Tramullas, M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Sharma, H.; Bajwa, J. Approach of probiotics in mental health as a psychobiotics. Arch. Microbiol. 2022, 204, 30. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J. Clin. Diagn. Res. 2017, 11, KC01. [Google Scholar] [CrossRef]
- Ju, I.G.; Hong, S.M.; Yun, S.W.; Huh, E.; Kim, D.H.; Kim, S.Y.; Oh, M.S. CCL01, a novel formulation composed of Cuscuta seeds and Lactobacillus paracasei NK112, enhances memory function via nerve growth factor-mediated neurogenesis. Food Funct. 2021, 12, 10690–10699. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Burmester, M.; Langeheine, M.; Brehm, R.; Empl, M.T.; Seeger, B.; Breves, G. Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS ONE 2021, 16, e0257824. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Lin, J.H.; Kuo, Y.W.; Chiang, P.R.; Ho, H.H. Probiotics and their Metabolites Reduce Oxidative Stress in Middle-Aged Mice. Curr. Microbiol. 2022, 79, 104. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, C.B.; Bowen, J.M.; Gladman, M.A.; Lustberg, M.B.; Mayo, S.J.; Wardill, H.R. The microbiota-gut-brain axis: An emerging therapeutic target in chemotherapy-induced cognitive impairment. Neurosci. Biobehav. Rev. 2020, 116, 470–479. [Google Scholar] [CrossRef]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef]
- Bich, L.; Moreno, A. The role of regulation in the origin and synthetic modelling of minimal cognition. Biosystems 2016, 148, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Hanczyc, M.M.; Ikegami, T. Chemical basis for minimal cognition. Artif. Life 2010, 16, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Keijzer, F.A. Evolutionary convergence and biologically embodied cognition. Interface Focus 2017, 7, 20160123. [Google Scholar] [CrossRef] [Green Version]
- Van Duijn, M.; Keijzer, F.; Franken, D. Principles of minimal cognition: Casting cognition as sensorimotor coordination. Adapt. Behav. 2006, 14, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimer’s Dis. 2011, 26, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, S.; Yuan, Q.; Liu, M.; Jiang, N.; Zhuang, L.; Wang, P. A Cell Co-Culture Taste Sensor Using Different Proportions of Caco-2 and SH-SY5Y Cells for Bitterness Detection. Chemosensors 2022, 10, 173. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxidative Med. Cell. Longev. 2019, 2019, 2843121. [Google Scholar] [CrossRef] [Green Version]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials 2017, 129, 152–162. [Google Scholar] [CrossRef]
- Galla, R.; Ruga, S.; Aprile, S.; Ferrari, S.; Brovero, A.; Grosa, G.; Molinari, C.; Uberti, F. New Hyaluronic Acid from Plant Origin to Improve Joint Protection-An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8114. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Ruga, S.; Galla, R.; Farghali, M.; Notte, F.; Bozzo, C.; Magnani, C.; Nardone, A.; Molinari, C. Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients 2020, 12, 573. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, L.; Meloni, M. La valutazione dell’assorbimento intestinale in vitro. L’integratore Nutr. 2014, 17, 62–65. [Google Scholar]
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef] [Green Version]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in CaCo-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Fda.Gov. Available online: https://www.fda.gov/media/117974/download (accessed on 29 May 2023).
- Ema.Eu. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-2b-first-version_en.pdf (accessed on 29 May 2023).
- Wu, H.C.; Hu, Q.L.; Zhang, S.J.; Wang, Y.M.; Jin, Z.K.; Lv, L.F.; Zhang, S.; Liu, Z.L.; Wu, H.L.; Cheng, O.M. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen. Res. 2018, 13, 1375–1383. [Google Scholar]
- Jayashree, S.; Karthikeyan, R.; Nithyalakshmi, S.; Ranjani, J.; Gunasekaran, P.; Rajendhran, J. Anti-adhesion Property of the Potential Probiotic Strain Lactobacillus fermentum 8711 Against Methicillin-Resistant Staphylococcus aureus (MRSA). Front. Microbiol. 2018, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Rayner, B.S.; Duong, T.H.; Myers, S.J.; Witting, P.K. Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J. Neurochem. 2006, 97, 211–221. [Google Scholar] [CrossRef]
- Piletz, J.E.; Cooper, J.; Chidester, K.; Erson, K.; Melton, S.; Osemeka, A.; Patterson, M.; Strickland, K.; Wan, J.X.; Williams, K. Transepithelial Effect of Probiotics in a Novel Model of Gut Lumen to Nerve Signaling. Nutrients 2022, 14, 4856. [Google Scholar] [CrossRef] [PubMed]
- Ruga, S.; Galla, R.; Ferrari, S.; Invernizzi, M.; Uberti, F. Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study. Int. J. Mol. Sci. 2023, 24, 5503. [Google Scholar] [CrossRef] [PubMed]
- Allers, K.; Stahl-Hennig, C.; Fiedler, T.; Wibberg, D.; Hofmann, J.; Kunkel, D.; Schneider, T. The colonic mucosa-associated microbiome in SIV infection: Shift towards bacteroidetes coincides with mucosal CD4+ T cell depletion and enterocyte damage. Sci. Rep. 2020, 10, 10887. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Dong, Y.; Chen, Z.; Denton, K.R.; Duff, M.O.; Blackstone, C.; Li, X.J. Impaired lipid metabolism in astrocytes underlies degeneration of cortical projection neurons in hereditary spastic paraplegia. Acta Neuropathol. Commun. 2020, 8, 214. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, S.; Galla, R.; Mulè, S.; Rosso, G.; Brovero, A.; Macchi, V.; Ruga, S.; Uberti, F. The Role of Bifidobacterium bifidum novaBBF7, Bifidobacterium longum novaBLG2 and Lactobacillus paracasei TJB8 to Improve Mechanisms Linked to Neuronal Cells Protection against Oxidative Condition in a Gut-Brain Axis Model. Int. J. Mol. Sci. 2023, 24, 12281. https://doi.org/10.3390/ijms241512281
Ferrari S, Galla R, Mulè S, Rosso G, Brovero A, Macchi V, Ruga S, Uberti F. The Role of Bifidobacterium bifidum novaBBF7, Bifidobacterium longum novaBLG2 and Lactobacillus paracasei TJB8 to Improve Mechanisms Linked to Neuronal Cells Protection against Oxidative Condition in a Gut-Brain Axis Model. International Journal of Molecular Sciences. 2023; 24(15):12281. https://doi.org/10.3390/ijms241512281
Chicago/Turabian StyleFerrari, Sara, Rebecca Galla, Simone Mulè, Giorgia Rosso, Arianna Brovero, Valentina Macchi, Sara Ruga, and Francesca Uberti. 2023. "The Role of Bifidobacterium bifidum novaBBF7, Bifidobacterium longum novaBLG2 and Lactobacillus paracasei TJB8 to Improve Mechanisms Linked to Neuronal Cells Protection against Oxidative Condition in a Gut-Brain Axis Model" International Journal of Molecular Sciences 24, no. 15: 12281. https://doi.org/10.3390/ijms241512281
APA StyleFerrari, S., Galla, R., Mulè, S., Rosso, G., Brovero, A., Macchi, V., Ruga, S., & Uberti, F. (2023). The Role of Bifidobacterium bifidum novaBBF7, Bifidobacterium longum novaBLG2 and Lactobacillus paracasei TJB8 to Improve Mechanisms Linked to Neuronal Cells Protection against Oxidative Condition in a Gut-Brain Axis Model. International Journal of Molecular Sciences, 24(15), 12281. https://doi.org/10.3390/ijms241512281