Analysis of Novel Immunological Biomarkers Related to Rheumatoid Arthritis Disease Severity
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Recruited Patients
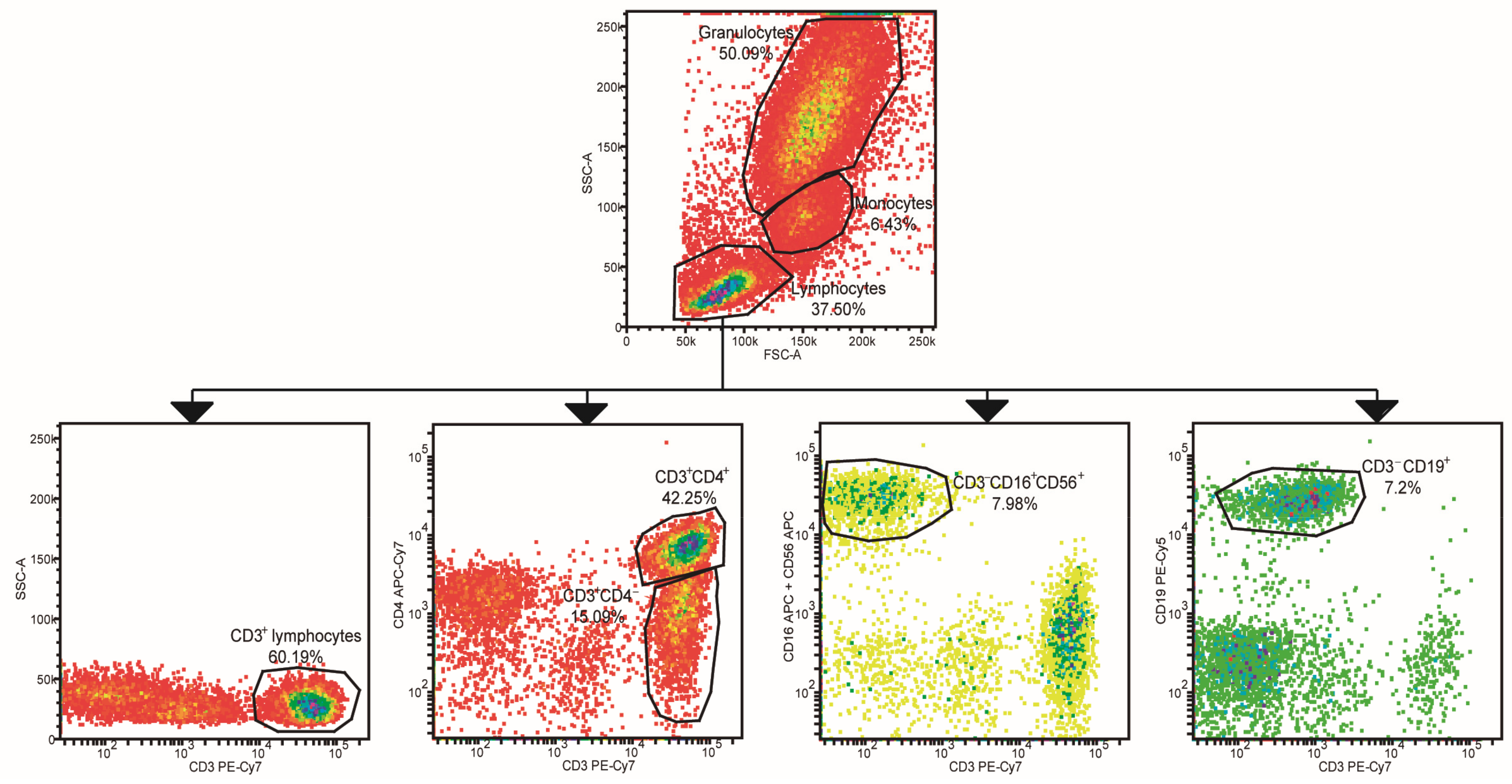
2.2. Leukocyte Populations Phenotyping
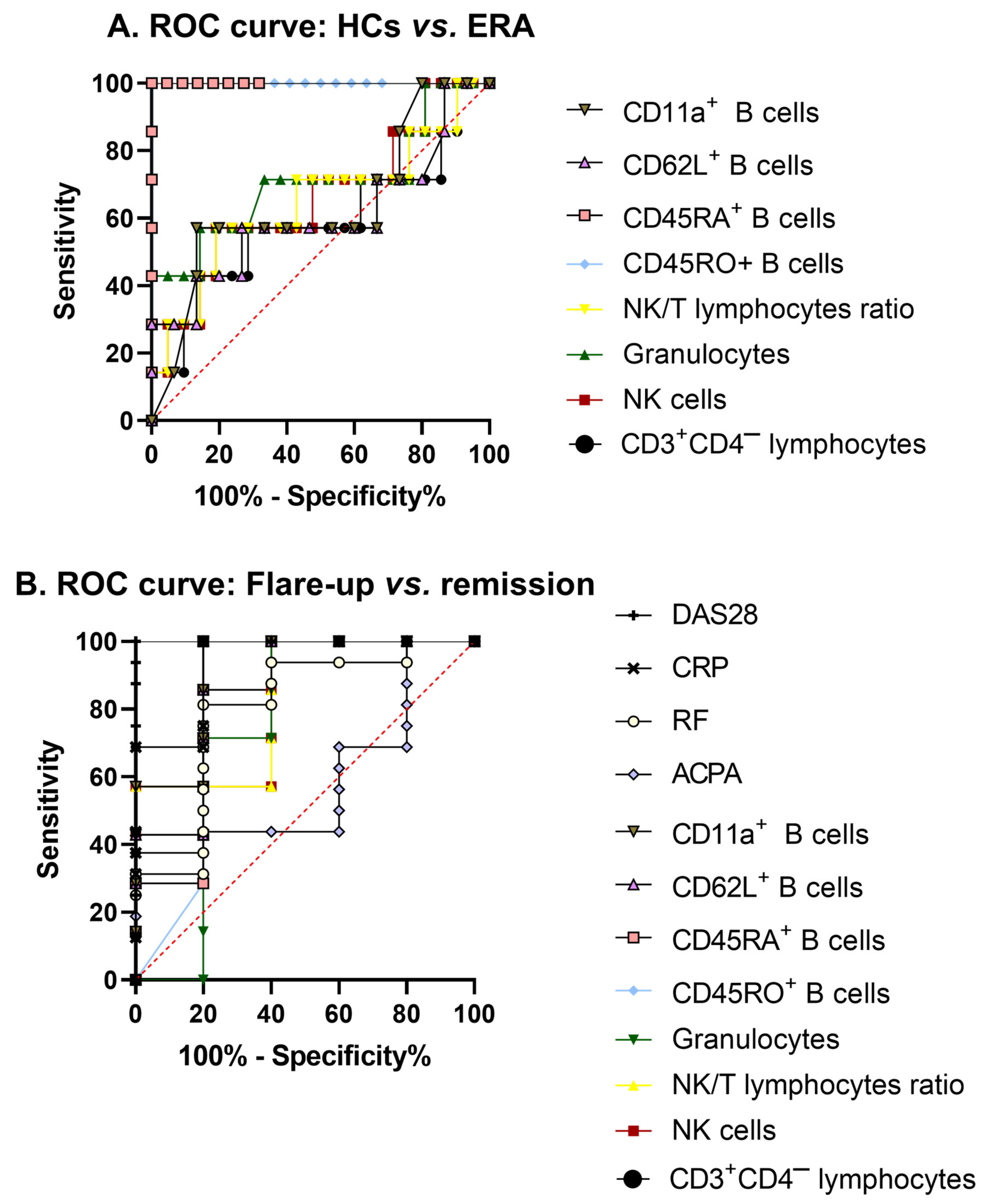
2.3. Analysis of Biomarkers Expression by PCA
2.4. Treg Cell Population Analysis
2.5. Analysis of Lymphocyte Proliferation
2.6. Serum Th1/Th2/Th17 Cytokine Profile
2.7. Supernatant Th1/Th2/Th17 Cytokine Profile
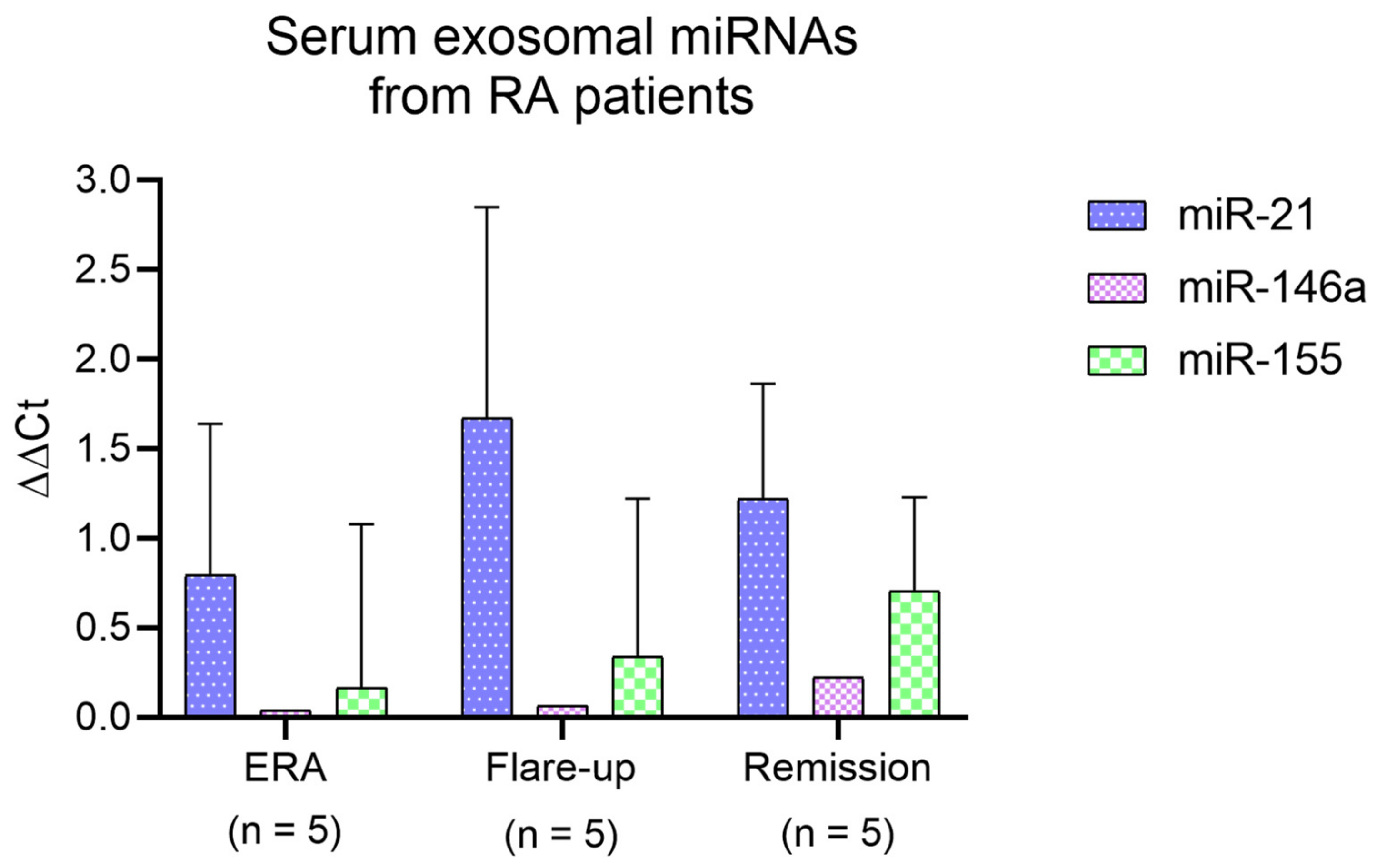
2.8. Serum Exosomal miRNA Analysis
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Isolation and Culture of PBMCs
4.3. Membrane/Intracellular Leukocyte Analysis
4.4. Proliferation Assay and Treg Analysis
4.5. Analysis of Cytokines
4.6. Isolation of Exosomes
Analysis of CD63 and CD9 Expression by Flow Cytometry
4.7. Study of Exosomal miRNA Cargo
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carbonell, J.; Cobo, T.; Balsa, A.; Descalzo, M.Á.; Carmona, L.; SERAP Study Group. The incidence of rheumatoid arthritis in Spain: Results from a nationwide primary care registry. Rheumatology 2008, 47, 1088–1092. [Google Scholar] [CrossRef] [Green Version]
- Dick, B.; Eccleston, C.; Crombez, G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002, 47, 639–644. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Balsa, A.; Cabezón, A.; Orozco, G.; Cobo, T.; Miranda-Carus, E.; López-Nevot, M.Á.; Vicario, J.L.; Martín-Mola, E.; Martín, J.; Pascual-Salcedo, D. Influence of HLA DRB1 alleles in the susceptibility of rheumatoid arthritis and the regulation of antibodies against citrullinated proteins and rheumatoid factor. Arthritis Res. Ther. 2010, 12, R62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, L.C.; Brock, M.; Hemmatazad, H.; Giger, O.T.; Moritz, F.; Trenkmann, M.; Distler, J.H.W.; Gay, R.E.; Kolling, C.; Moch, H.; et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007, 56, 1087–1093. [Google Scholar] [CrossRef]
- Rantapää-Dahlqvist, S.; De Jong, B.A.W.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies Against Cyclic Citrullinated Peptide and IgA Rheumatoid Factor Predict the Development of Rheumatoid Arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Ortells, J.M.; Pérez-García, V.; Marín-Alberca, G.; Peris-Pertusa, A.; Benito, J.M.; Marco, F.M.; Zubcoff, J.J.; Navarro-Blasco, F.J. Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to Disease Activity Score-28. Autoimmunity 2009, 42, 636–645. [Google Scholar] [CrossRef]
- Bugatti, S.; Manzo, A.; Montecucco, C.; Caporali, R. The clinical value of autoantibodies in rheumatoid arthritis. Front. Med. 2018, 5, 339. [Google Scholar] [CrossRef]
- Wei, S.-T.; Sun, Y.-H.; Zong, S.-H.; Xiang, Y.-B. Serum levels of IL-6 and TNF-α may correlate with activity and severity of rheumatoid arthritis. Med. Sci. Monit. 2015, 21, 4030–4038. [Google Scholar] [CrossRef] [Green Version]
- Nick, J.A.; Coldren, C.D.; Geraci, M.W.; Poch, K.R.; Fouty, B.W.; O’Brien, J.; Gruber, M.; Zarini, S.; Murphy, R.C.; Kuhn, K.; et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood 2004, 104, 3878–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.-J.; Liu, Y.; Deng, S.-H.; Liao, L.-H.; Lin, T.-L.; Ning, Q.; Luo, X.-P. Neuroprotective effects of activated protein C on intrauterine inflammation-induced neonatal white matter injury are associated with the downregulation of fibrinogen-like protein 2/fibroleukin prothrombinase and the inhibition of pro-inflammatory cytokine. Int. J. Mol. Med. 2015, 35, 1199–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Shen, K.; McKelvey, K.; Li, J.; Chan, Y.-K.A.; Hatzis, V.; March, L.; Little, C.B.; Tonkin, M.; Jackson, C.J. Endothelial protein C receptor-associated invasiveness of rheumatoid synovial fibroblasts is likely driven by group V secretory phospholipase A2. Arthritis Res. Ther. 2014, 16, R44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-M.; Chen, H.-H.; Chen, D.-C.; Huang, Y.-C.; Liu, S.-P.; Lin, Y.-J.; Chang, Y.-Y.; Lin, H.-W.; Chen, S.-Y.; Tsai, F.-J. Rheumatoid arthritis is associated with rs17337023 polymorphism and increased serum level of the EGFR protein. PLoS ONE 2017, 12, e0180604. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Xiao, P.; Li, X.U.E.; Deng, Z.; Qing, C.; Su, R.; Xu, J.; Guo, Y.; Huang, Z.; Li, J. Overexpression of CD64 on CD14++CD16- and CD14++CD16+ monocytes of rheumatoid arthritis patients correlates with disease activity. Exp. Ther. Med. 2018, 16, 2703–2711. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, A.; Mueller, Y.M.; Thomas, P.; Bojczuk, P.; O’Connors, C.; Katsikis, P.D. Decreased effector memory CD45RA+ CD62L- CD8+ T cells and increased central memory CD45RA- CD62L+ CD8+ T cells in peripheral blood of rheumatoid arthritis patients. Arthritis Res. Ther. 2003, 5, 91–96. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Wu, L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.; Stanley, P.; Jones, K.; Svensson, L.; McDowall, A.; Hogg, N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol. Rev. 2007, 218, 135–146. [Google Scholar] [CrossRef]
- Lv, L.-L.; Cao, Y.-H.; Ni, H.; Xu, M.; Liu, D.; Liu, H.; Chen, P.-S.; Liu, B.-C. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Ren. Physiol. 2013, 305, F1220–F1227. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Peng, W.; Ouyang, X.; Li, W.; Dai, Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl. Res. 2012, 160, 198–206. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Wade, S.; Floudas, A.; Orr, C.; McGarry, T.; Wade, S.; Cregan, S.; Fearon, U.; Veale, D.J. Serum miRNA Signature in Rheumatoid Arthritis and “At-Risk Individuals”. Front. Immunol. 2021, 12, 633201. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, T.; Duan, S.; Shi, Y.; Li, S.; Zhang, X.; Zhang, L. miR-155 promotes fibroblast-like synoviocyte proliferation and inflammatory cytokine secretion in rheumatoid arthritis by targeting FOXO3a. Exp. Ther. Med. 2019, 19, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Roos, J.; Enlund, E.; Funcke, J.B.; Tews, D.; Holzmann, K.; Debatin, K.M.; Wabitsch, M.; Fischer-Posovszky, P. MiR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci. Rep. 2016, 6, 38339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Su, L.; Duan, X.; Chen, X.; Hays, A.; Upadhyayula, S.; Shivde, J.; Wang, H.; Li, Y.; Huang, D.; et al. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol. Immunol. 2018, 101, 608–614. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, O.; Lee, A.C.; Cameron, F.; Tarbox, R.; Vafadar-Isfahani, N.; Tufarelli, C.; Lund, J.N. Inflammation and miR-21 pathways functionally interact to downregulate PDCD4 in colorectal cancer. PLoS ONE 2014, 9, e110267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, S685–S699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symmons, D.P.M.; Farr, M.; Salmon, M.; Bacon, P.A. Lymphopenia in rheumatoid arthritis. J. R. Soc. Med. 1989, 82, 462–463. [Google Scholar] [CrossRef]
- Carvalheiro, H.; Duarte, C.; Silva-Cardoso, S.; Da Silva, J.A.P.; Souto-Carneiro, M.M. CD8+ T cell profiles in patients with rheumatoid arthritis and their relationship to disease activity. Arthritis Rheumatol. 2015, 67, 363–371. [Google Scholar] [CrossRef]
- Lo, C.K.C.; Lam, Q.L.K.; Sun, L.; Wang, S.; Ko, K.H.; Xu, H.; Wu, C.-Y.; Zheng, B.-J.; Lu, L. Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthritis Rheum. 2008, 58, 2700–2711. [Google Scholar] [CrossRef] [Green Version]
- Aramaki, T.; Ida, H.; Izumi, Y.; Fujikawa, K.; Huang, M.; Arima, K.; Tamai, M.; Kamachi, M.; Nakamura, H.; Kawakami, A.; et al. A significantly impaired natural killer cell activity due to a low activity on a per-cell basis in rheumatoid arthritis. Mod. Rheumatol. 2009, 19, 245–252. [Google Scholar] [CrossRef]
- Mimpen, M.; Rolf, L.; Muris, A.H.; Gerlach, O.; Poelmans, G.; Hupperts, R.; Smolders, J.; Damoiseaux, J. NK/T cell ratios associate with interleukin-2 receptor alpha chain expression and shedding in multiple sclerosis. J. Neuroimmunol. 2021, 353, 577499. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, M.; Muris, A.H.; Rolf, L.; Gerlach, O.; Kuhle, J.; Hupperts, R.; Smolders, J.; Damoiseaux, J. Prognostic value of natural killer cell/T cell ratios for disease activity in multiple sclerosis. Eur. J. Neurol. 2021, 28, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; An, L.-L.; Chaerkady, R.; Mittereder, N.; Clarke, L.; Cohen, T.S.; Chen, B.; Hess, S.; Sims, G.P.; Mustelin, T. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci. Rep. 2018, 8, 15228. [Google Scholar] [CrossRef] [Green Version]
- Foulquier, C.; Sebbag, M.; Clavel, C.; Chapuy-Regaud, S.; Al Badine, R.; Méchin, M.C.; Vicent, C.; Nachat, R.; Yamada, M.; Takahara, H.; et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007, 56, 3541–3553. [Google Scholar] [CrossRef]
- Chen, S.Y.; Hsieh, J.L.; Wu, P.T.; Shiau, A.L.; Wu, C.L. MicroRNA-133 suppresses cell viability and migration of rheumatoid arthritis fibroblast-like synoviocytes by down-regulation of MET, EGFR, and FSCN1 expression. Mol. Cell. Biochem. 2022, 477, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Kondreddy, V.; Keshava, S.; Esmon, C.T.; Pendurthi, U.R.; Rao, L.V.M. A critical role of endothelial cell protein C receptor in the intestinal homeostasis in experimental colitis. Sci. Rep. 2020, 10, 20569. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.E.; Wennerberg, E.; Matt, P.; Lindqvist, U.; Kleinau, S. Dysregulated Fc receptor function in active rheumatoid arthritis. Immunol. Lett. 2014, 162, 200–206. [Google Scholar] [CrossRef]
- Torsteinsdóttir, I.; Arvidson, N.-G.; Hällgren, R.; Håkansson, L. Monocyte activation in rheumatoid arthritis (RA): Increased integrin, Fcgamma and complement receptor expression and the effect of glucocorticoids. Clin. Exp. Immunol. 1999, 115, 554–560. [Google Scholar] [CrossRef]
- Mkaddem, S.B.; Hayem, G.; Jönsson, F.; Rossato, E.; Boedec, E.; Boussetta, T.; El Benna, J.; Launay, P.; Goujon, J.-M.; Benhamou, M.; et al. Shifting FcγRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. J. Clin. Investig. 2014, 124, 3945–3959. [Google Scholar] [CrossRef]
- Camponeschi, A.; Gerasimcik, N.; Wang, Y.; Fredriksson, T.; Chen, D.; Farroni, C.; Thorarinsdottir, K.; Sjökvist Ottsjö, L.; Aranburu, A.; Cardell, S.; et al. Dissecting integrin expression and function on memory B cells in mice and humans in autoimmunity. Front. Immunol. 2019, 10, 534. [Google Scholar] [CrossRef]
- Zhang, X.I.A.; Zhang, X.; Zhuang, L.; Xu, C.; Li, T.A.O.; Zhang, G.; Liu, Y. Decreased regulatory t-cell frequency and interleukin-35 levels in patients with rheumatoid arthritis. Exp. Ther. Med. 2018, 16, 5366–5372. [Google Scholar] [CrossRef] [Green Version]
- Povoleri, G.A.M.; Lalnunhlimi, S.; Steel, K.J.A.; Agrawal, S.; O’Byrne, A.M.; Ridley, M.; Kordasti, S.; Frederiksen, K.S.; Roberts, C.A.; Taams, L.S. Anti-TNF treatment negatively regulates human CD4+ T-cell activation and maturation in vitro, but does not confer an anergic or suppressive phenotype. Eur. J. Immunol. 2020, 50, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Al-Saadany, H.M.; Hussein, M.S.; Gaber, R.A.; Zaytoun, H.A. Th-17 cells and serum IL-17 in rheumatoid arthritis patients: Correlation with disease activity and severity. Egypt. Rheumatol. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Abdel Meguid, M.H.; Hamad, Y.H.; Swilam, R.S.; Barakat, M.S. Relation of interleukin-6 in rheumatoid arthritis patients to systemic bone loss and structural bone damage. Rheumatol. Int. 2013, 33, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Aerts, N.E.; De Knop, K.J.; Leysen, J.; Ebo, D.G.; Bridts, C.H.; Weyler, J.J.; Stevens, W.J.; De Clerck, L.S. Increased IL-17 production by peripheral T helper cells after tumour necrosis factor blockade in rheumatoid arthritis is accompanied by inhibition of migration associated chemokine receptor expression. Rheumatology 2010, 49, 2264–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabaud, M.; Lubberts, E.; Joosten, L.; Van Den Berg, W.; Miossec, P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001, 3, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Lin, Y.C.; Wu, C.C.; Huang, M.Y.; Tsai, W.C.; Hung, C.H.; Kuo, P.-L. The immunomodulatory effects of TNF-α inhibitors on human Th17 cells via RORγt histone acetylation. Oncotarget 2017, 8, 7559–7571. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham III, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Parish, C.R.; Glidden, M.H.; Quah, B.J.; Warren, H.S. Use of the Intracellular Fluorescent Dye CFSE to Monitor Lymphocyte Migration and Proliferation. Curr. Protoc. Immunol. 2009, 84, 4.9.1–4.9.13. [Google Scholar] [CrossRef]
- Pelosi, A.; Lunardi, L.; Fiore, P.F.; Tinazzi, E.T.; Patuzzo, G.; Argentino, G.; Moretta, F.; Puccetti, A.; Dolcino, M. MicroRNA Expression Profiling in Psoriatic Arthritis. BioMed Res. Int. 2018, 2018, 7305380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poel, D.; Buffart, T.E.; Oosterling-Jansen, J.; Verheul, H.M.; Voortman, J. Evaluation of several methodological challenges in circulating miRNA qPCR studies in patients with head and neck cancer. Exp. Mol. Med. 2018, 50, e454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinnerthaler, G.; Hackl, H.; Hamacher, F.; Gampenrieder, S.P.; Hufnagl, C.; Romeder, F.; Monzo Fuentes, C.; Hauser-Kronberger, C.; Mlineritsch, B.; Greil, R. MiR-16 is the most stable-expressed housekeeping microRNA in breast cancer tissues from primary and metastatic sites. Int. J. Mol. Sci. 2016, 17, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Bai, Z.; Han, W.; Zhang, J.; Meng, H.; Bi, J.; Ma, X.; Han, S.; Zhang, Z. Identification of Suitable Reference Genes for qPCR Analysis of Serum microRNA in Gastric Cancer Patients. Dig. Dis. Sci. 2012, 57, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dong, H.; Zhang, Q.; Zhang, S. Combined assays for serum carcinoembryonic antigen and microRNA-17-3p offer improved diagnostic potential for stage I/II colon cancer. Mol. Clin. Oncol. 2015, 3, 1315–1318. [Google Scholar] [CrossRef] [Green Version]


| Treatments for RA Patients | |||
|---|---|---|---|
| ERA | Flare-Up | Remission | |
| Only methotrexate | 7 | - | - |
| NSAIDs or other drugs | 3 | - | - |
| JAK inhibitors | - | 1 | - |
| Methotrexate and anti-TNF-α antibodies | - | 3 | 3 |
| Methotrexate and anti-IL-6 receptor antibodies | - | - | 3 |
| Anti-TNF-α antibodies | - | 1 | 2 |
| Anti-IL-6 receptor antibodies | - | - | 8 |
| Clinical and Analytical Features of Study Subjects | |||||||
|---|---|---|---|---|---|---|---|
| Group | p-Values | ||||||
| HC | ERA | Flare-Up | Remission | ERA vs. Flare-Up | ERA vs. Remission | Flare-Up vs. Remission | |
| n | 39 | 10 | 5 | 16 | |||
| Ratio women/men | 27:12 | 8:2 | 4:1 | 10:6 | |||
| Age (years) | 53 ± 5.6 | 51 ± 4.3 | 51.6 ± 8.6 | 61.8 ± 3.7 | 0.58 | 0.07 | 0.41 |
| Disease duration (months) | - | 9.8 ± 6.3 | 87.4 ± 40.1 | 114 ± 39 | 0.03 * | 0.0001 * | 0.48 |
| Painful joints | - | 6.7 ± 1.3 | 6.6 ± 3.4 | 0.1 ± 0.1 | 0.89 | <0.0001 * | 0.0012 * |
| Swollen joints | - | 3.2 ± 1.1 | 1.4 ± 0.2 | 0.1 ± 0.1 | 0.85 | 0.0006 * | 0.0028 * |
| Level of discomfort (mm) | - | 35.6 ± 6.3 | 53 ± 8.7 | 6.6 ± 1.5 | 0.39 | 0.0011 * | 0.0003 * |
| Level of pain (mm) | - | 36.4 ± 6.6 | 53.6 ± 9.5 | 6.7 ± 1.5 | 0.54 | 0.0007 * | 0.0006 * |
| Evaluation of RA symptoms by the rheumatologist (mm) | - | 37.4 ± 6 | 45.3 ± 9.5 | 3.8 ± 1 | 0.69 | 0.0002 * | 0.0006 * |
| CRP (mg/L) | - | 10.4 ± 3.4 | 7.9 ± 2.7 | 1.2 ± 0.3 | 0.77 | 0.0035 * | 0.0069 * |
| RF (mg/L) | - | 137.8 ± 67 | 173.8 ± 79.1 | 58.7 ± 19.6 | 0.28 | 0.38 | 0.053 |
| ACPA (mg/L) | - | 298.9 ± 148.7 | 791.8 ± 590.5 | 357.6 ± 123.4 | 0.28 | 0.59 | 0.46 |
| DAS28 | - | 4.2 ± 0.4 | 3.8 ± 0.3 | 1.3 ± 0.1 | 0.31 | <0.0001 * | <0.0001 * |
| Cytokines Measured in Sera Samples (pg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | p-Values | |||||||||
| HC | ERA | FU | REM | HC vs. ERA | HC vs. FU | HC vs. REM | ERA vs. FU | ERA vs. REM | REM vs. FU | |
| n | 5 | 5 | 4 | 5 | ||||||
| IL-17A | 33.3 ± 8.9 | 19.6 ± 12 | 20.4 ± 15.5 | 55.8 ± 7.2 | 0.3728 | 0.4284 | 0.1523 | 0.9590 | 0.0283 * | 0.0410 * |
| IFN-γ | 11.5 ± 2 | 11.5 ± 3.1 | 15 ± 1.5 | 13.9 ± 2.2 | >0.9999 | 0.3260 | 0.4745 | 0.3260 | 0.4745 | 0.7506 |
| TNF-α | 11.2 ± 1.4 | 36.5 ± 8.1 | 24.4 ± 7.3 | 46.4 ± 18.5 | 0.0074 * | 0.1197 | 0.0111 * | 0.3317 | 0.8878 | 0.4022 |
| IL-2 | 32.3 ± 3.1 | 51.3 ± 8.4 | 76.2 ± 22.7 | 55 ± 11 | 0.6640 | 0.0986 | 0.5333 | 0.5046 | 0.9960 | 0.6271 |
| IL-6 | 12.3 ± 2.3 | 75.4 ± 36.5 | 31.4 ± 7.1 | 73.8 ± 26.1 | 0.0854 | 0.6351 | 0.0928 | 0.2824 | 0.9632 | 0.2996 |
| IL-4 | 17 ± 3.2 | 28.9 ± 7.4 | 56.9 ± 24 | 36.9 ± 12.1 | 0.4459 | 0.0579 | 0.1665 | 0.2388 | 0.5345 | 0.5537 |
| IL-10 | 4.6 ± 0.4 | 7.2 ± 2.9 | 11.5 ± 5.3 | 15 ± 6.5 | 0.3354 | 0.0963 | 0.0413 * | 0.4504 | 0.2816 | 0.7945 |
| A. Cytokines Measured in Non-Stimulated PBMC Supernatant (pg/mL) | ||||||
|---|---|---|---|---|---|---|
| Group | p-Values | |||||
| HC | RA | RA + Ab | HC vs. RA | HC vs. RA + Ab | RA vs. RA + Ab | |
| n | 5 | 12 | 12 | |||
| IL-17A | 3.7 ± 2.6 | 7.1 ± 1.9 | 4 ± 1.6 | 0.3582 | 0.9985 | 0.2299 |
| IFN-γ | ND | ND | ND | 0.1355 | 0.2690 | 0.5965 |
| TNF-α | 2.8 ± 0.6 | 4.2 ± 0.7 | 2.4 ± 0.5 | 0.1638 | 0.7260 | 0.0278 * |
| IL-2 | 0.4 ± 1.1 | 6.6 ± 3.1 | 1.2 ± 2 | >0.9999 | >0.9999 | >0.9999 |
| IL-6 | 1.6 ± 1.1 | 7.3 ± 2 | 8.7 ± 2.2 | 0.1171 | 0.0485 | 0.6003 |
| IL-4 | ND | 1.5 ± 1.5 | ND | 0.0840 | 0.3493 | 0.3371 |
| IL-10 | 2.4 ± 0.9 | 2.1 ± 0.4 | 1.9 ± 0.3 | 0.7339 | 0.9132 | 0.5701 |
| B. Cytokines Measured in Stimulated PBMC Supernatant (pg/mL) | ||||||
| Group | p-Values | |||||
| HC | RA | RA+ Ab | HC vs. RA | HC vs. RA + Ab | RA vs. RA + Ab | |
| n | 5 | 12 | 12 | |||
| IL-17A | 37.6 ± 12 | 58.3 ± 20.6 | 37.8 ± 10.5 | 0.9188 | 0.8285 | 0.6870 |
| IFN-γ | 1115 ± 595.1 | 276.9 ± 85.5 | 54.6 ± 16 | 0.0143 * | 0.0031 * | 0.3946 |
| TNF-α | 358.9 ± 181.2 | 109.9 ± 25.2 | 3.9 ± 1 | 0.0120 * | 0.0006 * | 0.1488 |
| IL-2 | 1.3 ± 1.1 | 0.6 ± 0.3 | 1.9 ± 0.5 | 0.6623 | 0.4732 | 0.1561 |
| IL-6 | 6839 ± 2265 | 15537± 5973 | 5337 ± 1543 | 0.8550 | 0.5829 | 0.6337 |
| IL-4 | ND | 0.2 ± 0.9 | 0.3 ± 0.7 | 0.4299 | 0.1948 | 0.5084 |
| IL-10 | 1494 ± 340.3 | 1021 ± 289.1 | 328.9 ± 77.5 | 0.2392 | 0.0064 * | 0.0298 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-García, S.; Martínez-Peinado, P.; López-Jaén, A.B.; Navarro-Blasco, F.J.; Montoyo-Pujol, Y.G.; Roche, E.; Peiró, G.; Sempere-Ortells, J.M. Analysis of Novel Immunological Biomarkers Related to Rheumatoid Arthritis Disease Severity. Int. J. Mol. Sci. 2023, 24, 12351. https://doi.org/10.3390/ijms241512351
Pascual-García S, Martínez-Peinado P, López-Jaén AB, Navarro-Blasco FJ, Montoyo-Pujol YG, Roche E, Peiró G, Sempere-Ortells JM. Analysis of Novel Immunological Biomarkers Related to Rheumatoid Arthritis Disease Severity. International Journal of Molecular Sciences. 2023; 24(15):12351. https://doi.org/10.3390/ijms241512351
Chicago/Turabian StylePascual-García, Sandra, Pascual Martínez-Peinado, Ana B. López-Jaén, Francisco J. Navarro-Blasco, Yoel G. Montoyo-Pujol, Enrique Roche, Gloria Peiró, and José M. Sempere-Ortells. 2023. "Analysis of Novel Immunological Biomarkers Related to Rheumatoid Arthritis Disease Severity" International Journal of Molecular Sciences 24, no. 15: 12351. https://doi.org/10.3390/ijms241512351






