Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress
Abstract
1. Introduction
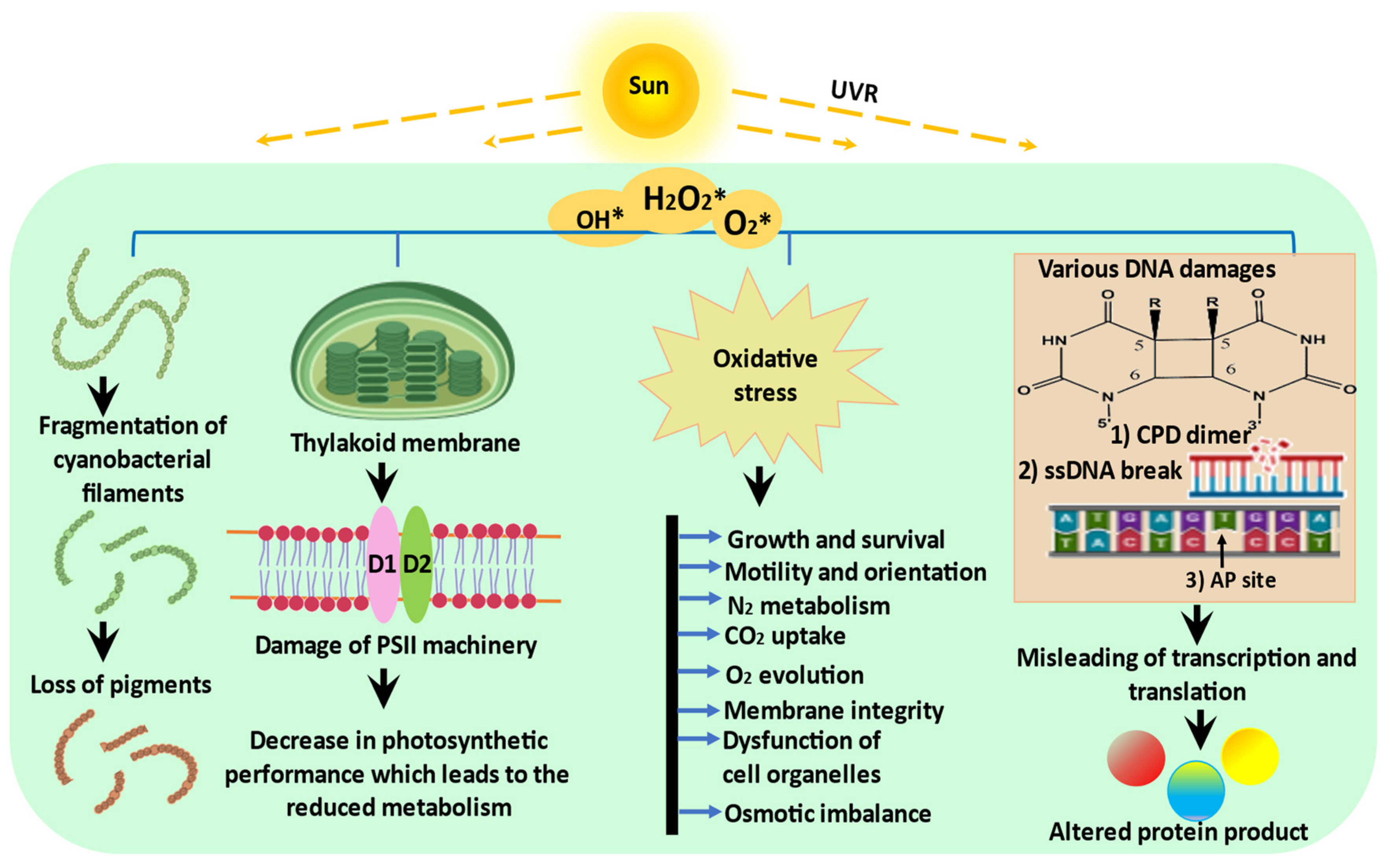
2. Impact of UVR on Cyanobacteria
2.1. Photosynthesis
2.2. Growth, Cell Differentiation, and Motility
2.3. Nitrogen Metabolism
2.4. Biomolecules
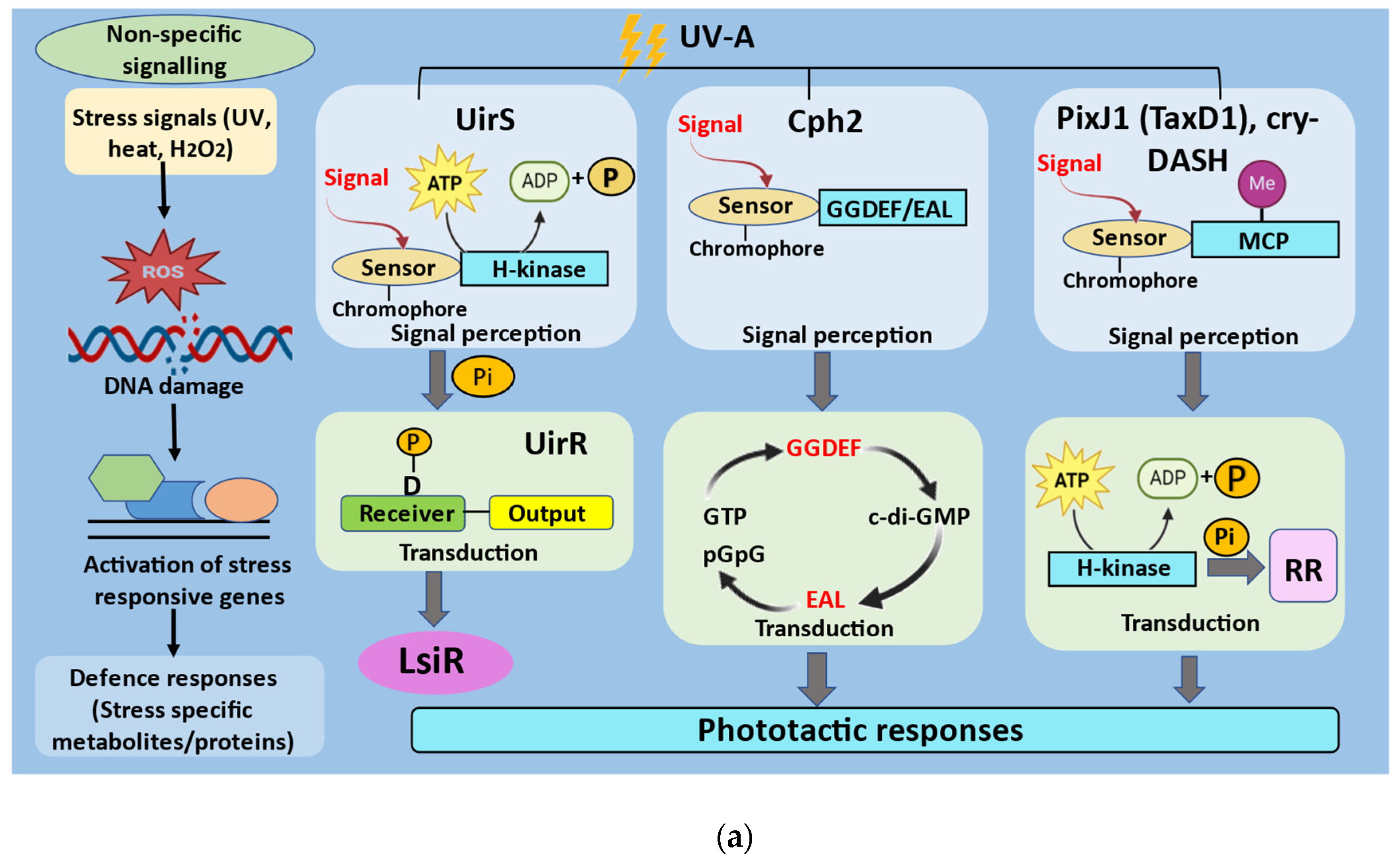
3. UV-Mediated Signal Transduction in Cyanobacteria
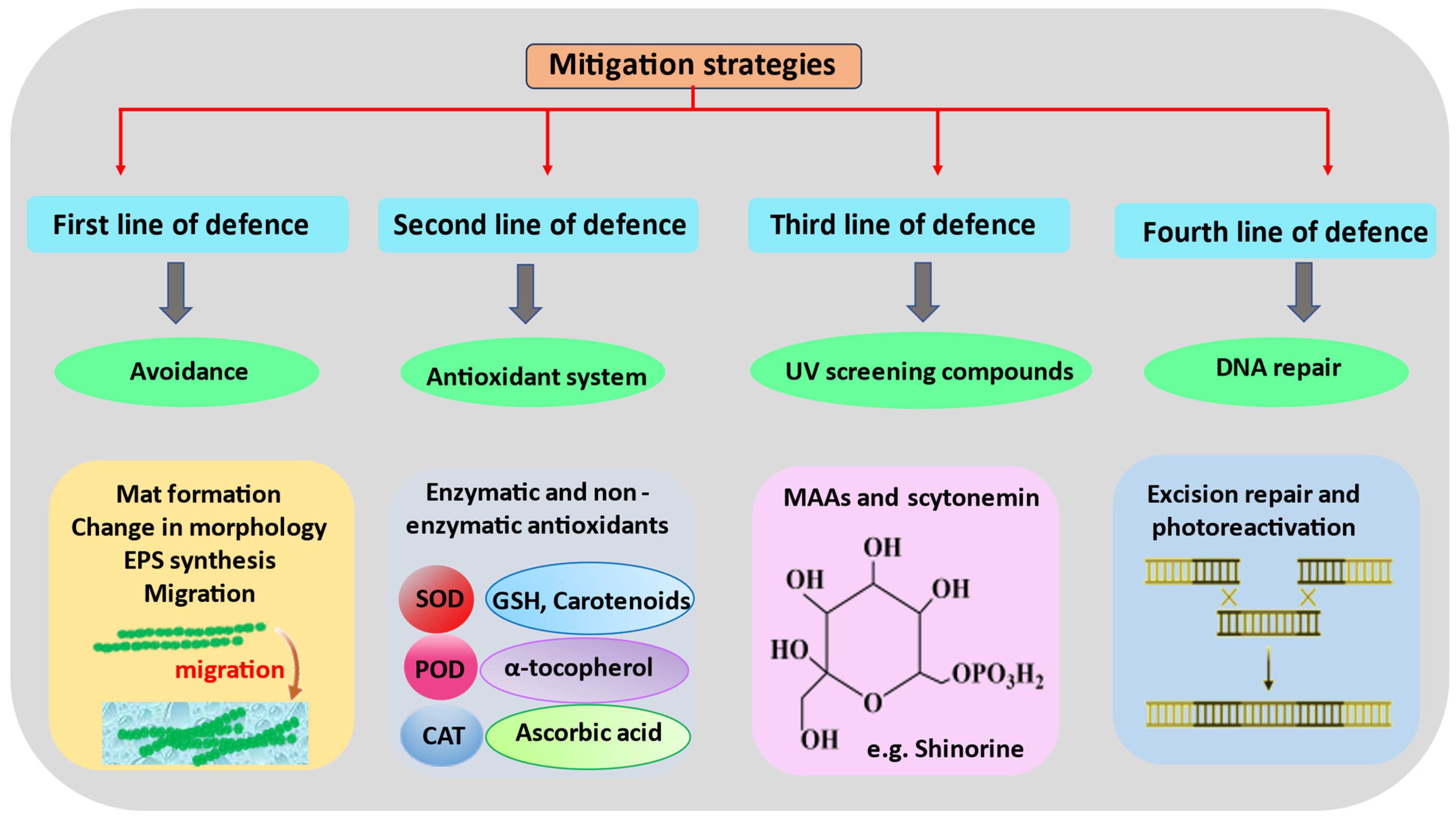
4. UV Stress Tolerance and Mitigation Strategies in Cyanobacteria
4.1. Avoidance and Migration
4.2. Mat Formation
4.3. Antioxidant Systems
4.4. UV-Screening Compounds and Their Molecular Mechanisms
4.5. Repair and Resynthesis
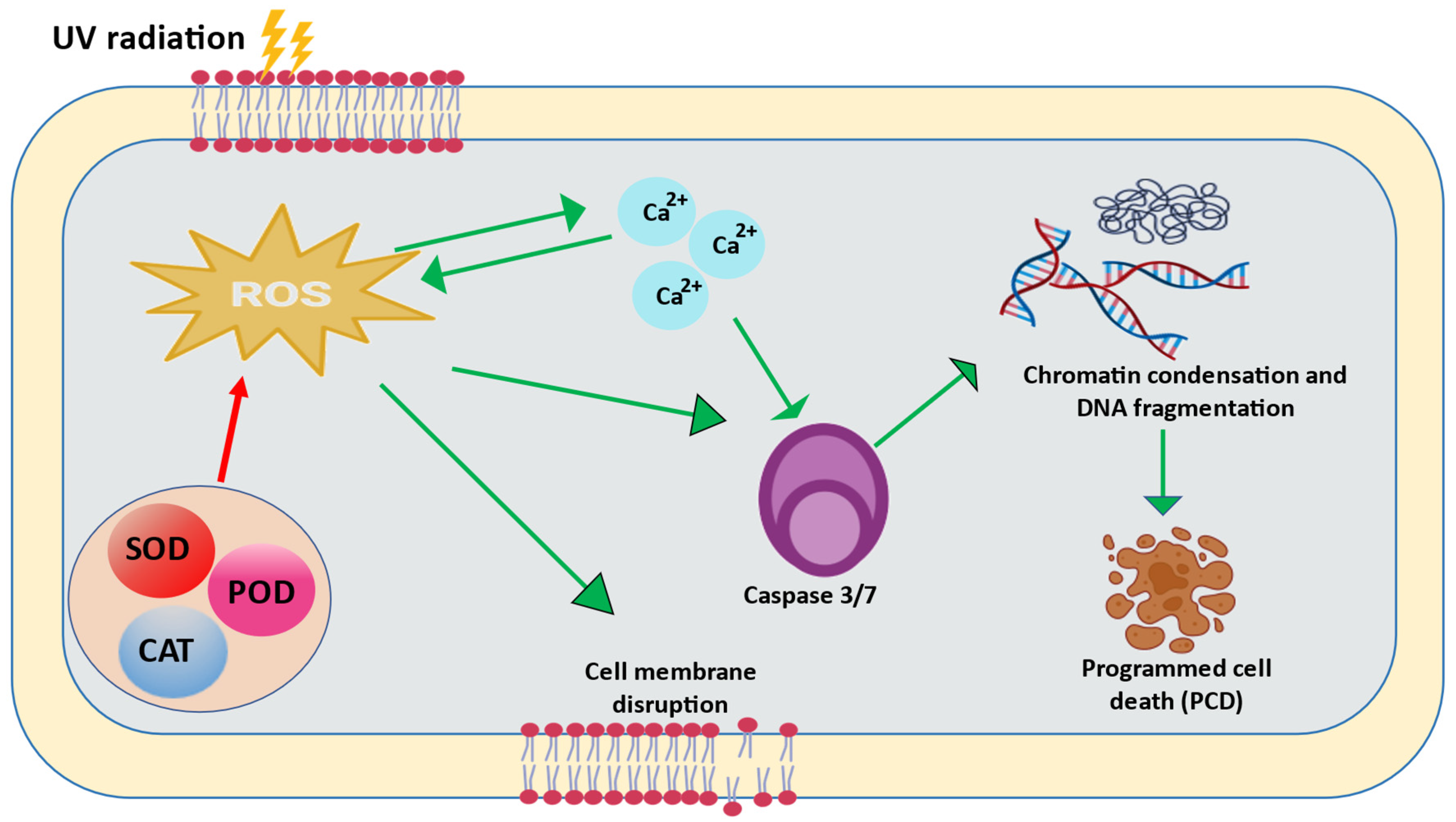
5. UV-Mediated Cell Death/Apoptosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.P.; Häder, D.-P.; Sinha, R.P. Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res. Rev. 2010, 9, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.-P.; et al. UVR and cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169. [Google Scholar] [CrossRef]
- Häder, D.-P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2007, 6, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.M.; Björn, L.O.; Bornman, J.F.; Flint, S.D.; Kulandaivelu, G.; Teramura, A.H.; Tevini, M. Effects of increased solar UVR on terrestrial ecosystems. J. Photochem. Photobiol. B Biol. 1998, 46, 40–52. [Google Scholar] [CrossRef]
- Araújo, R.G.; Alcantar-Rivera, B.; Meléndez-Sánchez, E.R.; Martínez-Prado, M.A.; Sosa-Hernández, J.E.; Iqbal, H.M.; Parra-Saldivar, R.; Martínez-Ruiz, M. Effects of UV and UV-vis irradiation on the production of microalgae and macroalgae: New alternatives to produce photobioprotectors and biomedical compounds. Molecules 2022, 27, 5334. [Google Scholar] [CrossRef] [PubMed]
- Karentz, D.; Cleaver, J.E.; Mitchell, D.L. DNA damage in the Antarctic. Nature 1991, 350, 28. [Google Scholar] [CrossRef]
- Vincent, W.F.; Neale, P.J. Mechanisms of UV damage to aquatic organisms. In The Effects of UV Radiation on Marine Ecosystems; de Mora, S.J., Demers, S., Vernet, M., Eds.; Cambridge Univ. Press: Cambridge, UK, 2000; pp. 149–176. [Google Scholar]
- Hargreaves, A.; Taiwo, F.A.; Duggan, O.; Kirk, S.H.; Ahmad, S.I. Near-ultraviolet photolysis of b-phenylpyruvic acid generates free radicals and results in DNA damage. J. Photochem. Photobiol. B Biol. 2007, 89, 110–116. [Google Scholar] [CrossRef]
- Gao, K.; Yu, H.; Brown, M.T. Solar PAR and UV radiation affects the physiology and morphology of the cyanobacterium Anabaena sp. PCC 7120. J. Photochem. Photobiol. B Biol. 2007, 89, 117–124. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.-P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289. [Google Scholar] [CrossRef]
- Kavitha, G.; Kurinjimalar, C.; Thevanathan, R.; Rengasamy, R. Evaluation of Ultraviolet-B radiation induced changes in biochemical response of Arthrospira platensis (Gomont). Int. Res. J. Biol. Sci. 2015, 4, 52–59. [Google Scholar]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and red macroalgae as potential sources of antioxidants and UVR-absorbing compounds for cosmeceutical applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.R.; He, J.; Chow, W.S.; Anderson, J.M. Changes in mRNA levels and polypeptide subunits of ribulose 1, 5-bisphosphate carboxylase in response to supplementary ultraviolet-B radiation. Plant Cell Environ. 1992, 15, 91–98. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.-P. Effects of ultraviolet-B radiation in three rice field cyanobacteria. J. Plant Physiol. 1998, 153, 763–769. [Google Scholar] [CrossRef]
- Campbell, D.; Eriksson, M.J.; Öquist, G.; Gustafsson, P.; Clarke, A.K. The cyanobacterium Synechococcus resists UV-B by exchanging photosystem II reaction-center D1 proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 364–369. [Google Scholar] [CrossRef]
- Bhandari, R.; Sharma, P.K. High-light-induced changes on photosynthesis, pigments, sugars, lipids and antioxidant enzymes in freshwater (Nostoc spongiaeforme) and marine (Phormidium corium) cyanobacteria. Photochem. Photobiol. 2006, 82, 702–710. [Google Scholar] [CrossRef]
- Singh, S.P.; Rastogi, R.P.; Sinha, R.P.; Häder, D.-P. Photosynthetic performance of Anabaena variabilis PCC 7937 under simulated solar radiation. Photosynthetica 2013, 51, 259–266. [Google Scholar] [CrossRef]
- Gupta, R.; Bhadauriya, P.; Chauhan, V.S.; Bisen, P.S. Impact of UV-B radiation on thylakoid membrane and fatty acid profile of Spirulina platensis. Curr. Microbiol. 2008, 56, 156–161. [Google Scholar] [CrossRef]
- Häder, D.-P. Effects of enhanced solar UVR on aquatic ecosystems. In Biophysics of Photoreceptors and Photomovements in Microorganisms; Lenci, F., Ghetti, F., Colombetti, G., Häder, D.-P., Song, P.-S., Eds.; Springer: New York, NY, USA, 1991; pp. 157–172. [Google Scholar]
- He, Y.Y.; Häder, D.-P. Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochem. Photobiol. Sci. 2002, 1, 729–736. [Google Scholar] [CrossRef]
- Pathak, J.; Singh, P.R.; Häder, D.P.; Sinha, R.P. UV-induced DNA damage and repair: A cyanobacterial perspective. Plant Gene 2019, 19, 100194. [Google Scholar] [CrossRef]
- Döhler, G.; Biermann, I.; Zink, J. Impact of UV-B radiation on photosynthetic assimilation of IT-bicarbonate and inorganic ‘SN-compounds by cyanobacteria. Z. Naturforsch. 1986, 41, 426–432. [Google Scholar] [CrossRef]
- Häder, D.-P.; Watanabe, M.; Furuya, M. Inhibition of motility in the cyanobacterium Phormidium uncinatum by solar and monochromatic UV irradiation. Plant Cell Physiol. 1986, 27, 887–894. [Google Scholar] [CrossRef]
- Newton, J.W.; Tyler, D.D.; Slodki, M.E. Effect of ultraviolet-B (280–320 nm) radiation on blue-green algae (Cyanobacteria), possible biological indicators of stratospheric ozone depletion. Appl. Environ. Microbiol. 1979, 37, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Richa, S.R.; Sinha, P. UV-mediated stress and its mitigation in cyanobacteria. Int. J. Plant Anim. Environ. Sci. 2011, 1, 155–166. [Google Scholar]
- Sinha, R.P.; Singh, N.; Kumar, A.; Kumar, H.D.; Häder, D.-P. Effects of UV irradiation on certain physiological and biochemical processes in cyanobacteria. J. Photochem. Photobiol. B Biol. 1996, 32, 107–113. [Google Scholar] [CrossRef]
- Kumar, A.; Tyagi, M.B.; Jha, P.N.; Srinivas, G.; Singh, A. Inactivation of cyanobacterial nitrogenase after exposure to ultraviolet-B radiation. Curr. Microbiol. 2003, 46, 0380–0384. [Google Scholar] [CrossRef]
- Tyagi, R.; Srinivas, G.; Vyas, D.; Kumar, A.; Kumar, H.D. Differential effect of ultraviolet-B radiation on certain metabolic processes in a chromatically adapting Nostoc. Photochem. Photobiol. 1992, 55, 401–407. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit. Rev. Microbiol. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Effects of PAR and UV radiation on the structural and functional integrity of phycocyanin, phycoerythrin and allophycocyanin isolated from the marine cyanobacterium Lyngbya sp. A09DM. Photochem. Photobiol. 2015, 91, 837–844. [Google Scholar] [CrossRef]
- Wu, H.; Gao, K.; Villafañe, V.E.; Watanabe, T.; Helbling, E.W. Effects of solar UVR on morphology and photosynthesis of filamentous cyanobacterium Arthrospira platensis. Appl. Environ. Microbiol. 2005, 71, 5004–5013. [Google Scholar] [CrossRef]
- Bhandari, R.; Sharma, P.K. Effect of UV-B and high visual radiation on photosynthesis in freshwater (Nostoc spongiaeforme) and marine (Phormidium corium) cyanobacteria. Indian J. Biochem. Biophys. 2007, 44, 231–239. [Google Scholar]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y.; Liu, Y.; Liu, Y. Ultraviolet-B exposure induces photo-oxidative damage and subsequent repair strategies in a desert cyanobacterium Microcoleus vaginatus Gom. Eur. J. Soil Biol. 2009, 45, 377–382. [Google Scholar] [CrossRef]
- Sinha, R.P.; Dautz, M.; Häder, D.-P. A simple and efficient method for the quantitative analysis of thymine dimers in cyanobacteria, phytoplankton and macroalgae. Acta Protozool. 2001, 40, 187–195. [Google Scholar]
- Mosca, C.; Rothschild, L.J.; Napoli, A.; Ferré, F.; Pietrosanto, M.; Fagliarone, C.; Billi, D. Over-expression of UV-damage DNA repair genes and ribonucleic acid persistence contribute to the resilience of dried biofilms of the desert cyanobacetrium Chroococcidiopsis exposed to Mars-like UV flux and long-term desiccation. Front. Microbiol. 2019, 10, 2312. [Google Scholar] [CrossRef] [PubMed]
- Montogomery, B.L. Sensing the light: Photoreceptive systems and signal transduction in cyanobacteria. Mol. Microbiol. 2007, 64, 16–27. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Ishizuka, T. Cyanobacteriochromes: A new superfamily of tetrapyrrole-binding photoreceptors in cyanobacteria. Photochem. Photobiol. Sci. 2008, 7, 1159–1167. [Google Scholar] [CrossRef]
- Hirose, Y.; Narikawa, R.; Katayama, M.; Ikeuchi, M. Cyanobacteriochrome CcaS regulates phycoerythrin accumulation in Nostoc punctiforme, a group II chromatic adapter. Proc. Natl. Acad. Sci. USA 2010, 107, 8854–8859. [Google Scholar] [CrossRef]
- Terauchi, K.; Ohmori, M. Blue light stimulates cyanobacterial motility via a cAMP signal transduction system. Mol. Microbiol. 2004, 52, 303–309. [Google Scholar] [CrossRef]
- Ng, W.O.; Grossman, A.R.; Bhaya, D. Multiple light inputs control phototaxis in Synechocystis sp. strain PCC6803. J. Bacteriol. 2003, 185, 1599–1607. [Google Scholar] [CrossRef]
- Moon, Y.J.; Lee, E.M.; Park, Y.M.; Park, Y.S.; Chung, W.I.; Chung, Y.H. The role of cyanopterin in UV/blue light signal transduction of cyanobacterium Synechocystis sp. PCC 6803 phototaxis. Plant Cell Physiol. 2010, 51, 969–980. [Google Scholar] [CrossRef]
- Moon, Y.J.; Kim, S.J.; Park, Y.M.; Chung, Y.H. Sensing UV/blue: Pterin as a UV-A absorbing chromophore of cryptochrome. Plant Signal. Behav. 2010, 5, 1127–1130. [Google Scholar] [CrossRef]
- Bhaya, D.; Takahashi, A.; Grossman, A.R. Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC 6803. Proc. Natl. Acad. Sci. USA 2001, 98, 7540–7545. [Google Scholar] [CrossRef]
- Moon, Y.J.; Kim, S.I.; Chung, Y.H. Sensing and responding to UV-A in cyanobacteria. Int. J. Mol. Sci. 2012, 13, 16303–16332. [Google Scholar] [CrossRef]
- Song, J.Y.; Cho, H.S.; Cho, J.I.; Jeon, J.S.; Lagarias, J.C.; Park, Y.I. Near-UV cyanobacteriochrome signaling system elicits negative phototaxis in the cyanobacterium Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2011, 108, 10780–10785. [Google Scholar] [CrossRef] [PubMed]
- Ulijasz, A.T.; Cornilescu, G.; von Stetten, D.; Cornilescu, C.; Velazquez Escobar, F.; Zhang, J.; Stankey, R.J.; Rivera, M.; Hildebrandt, P.; Vierstra, R.D. Cyanochromes are blue/green light photoreversible photoreceptors defined by a stable double cysteine linkage to a phycoviolobilin-type chromophore. J. Biol. Chem. 2009, 284, 29757–29772. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Postier, B.L.; Burnap, R.L. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 2004, 279, 5739–5751. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Singh, A.K.; McIntyre, L.M.; Sherman, L.A. Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC 6803. J. Bacteriol. 2004, 186, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.; Desikan, R.; Harrison, J.; Bright, J.; Hooley, R.; Neill, S. Doing the unexpected: Proteins involved in hydrogen peroxide perception. J. Exp. Bot. 2006, 57, 1711–1718. [Google Scholar] [CrossRef]
- Richter, P.; Ntefidou, M.; Streb, C.; Lebert, M.; Häder, D.-P. The role of reactive oxygen species (ROS) in signaling of light stress. Recent Res. Dev. Biochem. 2003, 4, 957–970. [Google Scholar]
- Richter, P.; Krywult, M.; Sinha, R.P.; Häder, D.-P. Calcium signals from heterocysts of Anabaena sp. after UV irradiation. J. Plant Physiol. 1999, 154, 137–139. [Google Scholar] [CrossRef]
- Quesada, A.; Vincent, W.F. Strategies of adaptation by Antarctic cyanobacteria to ultraviolet radiation. Eur. J. Phycol. 1997, 32, 335–342. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Scherer, S. UV protection in cyanobacteria. Eur. J. Phycol. 1999, 34, 329–338. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Mechling, M.; Castenholz, R.W. Diel migrations of microorganisms within a benthic, hypersaline mat community. Appl. Environ. Microbiol. 1994, 60, 1500–1511. [Google Scholar] [CrossRef]
- Bebout, B.M.; Garcia-Pichel, F. UV-B induced vertical migrations of cyanobacteria in a microbial mat. Appl. Environ. Microbiol. 1995, 61, 4215–4222. [Google Scholar] [CrossRef] [PubMed]
- Castenholz, R.W. Multiple strategies for UV tolerance in cyanobacteria. Spectrum 1997, 10, 10–16. [Google Scholar]
- Büdel, B. Ecology and diversity of rock-inhabiting cyanobacteria in tropical regions. Eur. J. Phycol. 1999, 34, 361–370. [Google Scholar] [CrossRef]
- Kovacik, L. Cyanobacteria and algae as agents of biodeterioration of stone substrata of historical buildings and other cultural monuments. In Proceedings of the New Millenium International Forum on Conservation of Cultural Property, Daejeon, Republic of Korea, 5–8 December 2000; Choi, S., Suh, M., Eds.; Kongju National University: Kongju, Republic of Korea, 2000; pp. 44–56. [Google Scholar]
- Javor, B.J.; Castenholz, R.W. Productivity studies of microbial mats, Laguna Guerrero Negro, Mexico. In Microbial Mats: Stomatolites; Cohen, Y., Hulvorson, H., Castenholz, R.W., Eds.; Alan, R. Liss. Inc.: New York, NY, USA, 1984; pp. 149–170. [Google Scholar]
- Karsten, U.; Maier, J.; Garcia-Pichel, F. Seasonality in UV-absorbing compounds of cyanobacterial mat communities from an intertidal mangrove flat. Aquat. Microb. Ecol. 1998, 16, 37–44. [Google Scholar] [CrossRef]
- Canfield, D.E.; Sorensen, K.B.; Oren, A. Biogeochemistry of a gypsum-encrusted microbial ecosystem. Geobiology 2004, 2, 133–150. [Google Scholar] [CrossRef]
- Büdel, B.; Karsten, U.; Garcia-Pichel, F. Ultraviolet-absorbing scytonemin and mycosporine-like amino acid derivatives in exposed rock inhabiting cyanobacterial lichens. Oecologia 1997, 112, 165–172. [Google Scholar] [CrossRef]
- Tripathy, P.; Roy, A.; Anand, N.; Adhikary, S.P. Blue-green algal flora on the rock surface of temples and monuments of India. Feddes Repert. 1999, 110, 133–144. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef]
- Lakatos, M.; Bilger, W.; Büdel, B. Carotenoid composition of terrestrial cyanobacteria: Response to natural light conditions in open rock habitats in Venezuela. Eur. J. Phycol. 2001, 36, 367–375. [Google Scholar] [CrossRef]
- Jiang, H.; Qiu, B. Photosynthetic adaptation of a bloom-forming cyanobacterium Microcystis aeruginosa (cyanophyceae) to prolonged UV-B exposure. J. Phycol. 2005, 41, 983–992. [Google Scholar] [CrossRef]
- Goetz, T.; Windhoevel, U.; Boeger, P.; Sandmann, G. Protection of photosynthesis against ultraviolet-B radiation by carotenoids in transformants of the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1999, 120, 599–604. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetics and molecular approaches. Annu. Rev. Plant Physiol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- He, Y.-Y.; Häder, D.-P. UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: Protective effects of ascorbic acid and N-acetyl-L-cysteine. J. Photochem. Photobiol. B Biol. 2002, 66, 115–124. [Google Scholar] [CrossRef]
- Shirkey, B.; Kovarcik, D.-P.; Wright, D.J.; Wilmoth, G.; Prickett, T.F.; Helm, R.F.; Gregory, E.M.; Potts, M. Active Fe-containing superoxide dismutase and abundant sodF mRNA in Nostoc commune (Cyanobacteria) after years of desiccation. J. Bacteriol. 2000, 182, 189–197. [Google Scholar] [CrossRef]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Kumari, N.; Pathak, J.; Dwivedy, A.K.; Sinha, R.P. Bioprospection of UV-screening compounds from lichens inhabiting the Indian state of Sikkim. Plant Arch. 2021, 21, 1168–1177. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural sun-screening compounds and DNA-repair enzymes: Photoprotection and photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Shick, J.M. The continuity and intensity of ultraviolet irradiation affect the kinetics of biosynthesis, accumulation, and conversion of mycosporine-like amino acids (MAAs) in the coral Stylophora pistillata. Limnol. Oceanogr. 2004, 49, 442–458. [Google Scholar] [CrossRef]
- Singh, V.K.; Jha, S.; Rana, P.; Gupta, A.; Singh, A.P.; Kumari, N.; Mishra, S.; Singh, P.R.; Jaiswal, J.; Sinha, R.P. Application of synthetic biology approaches to high-yield production of mycosporine-like amino acids. Fermentation 2023, 9, 669. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Ind. J. Exp. Biol. 2008, 46, 7–17. [Google Scholar]
- Geraldes, V.; Pinto, E. Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef]
- Hartmann, A.; Glaser, K.; Holzinger, A.; Ganzera, M.; Karsten, U. Klebsormidin A and B, two new UV-sunscreen compounds in green microalgal Interfilum and Klebsormidium species (Streptophyta) from terrestrial habitats. Front. Microbiol. 2020, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563. [Google Scholar] [CrossRef]
- Sen, S.; Mallick, N. Scytonemin: Unravelling major progress and prospects. Algal Res. 2022, 64, 102678. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Castenholz, R.W. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J. Phycol. 1991, 27, 395–409. [Google Scholar] [CrossRef]
- Fleming, E.D.; Castenholz, R.W. Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ. Microbiol. 2007, 9, 1448–1455. [Google Scholar] [CrossRef]
- Gao, X.; Jing, X.; Liu, X.; Lindblad, P. Biotechnological production of the sunscreen pigment scytonemin in cyanobacteria: Progress and strategy. Mar. Drugs 2021, 19, 129. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Levine, E.; Thiel, T. UV-inducible DNA repair in the cyanobacteria Anabaena spp. J. Bacteriol. 1987, 169, 3988–3993. [Google Scholar] [CrossRef]
- Ng, W.-O.; Pakrasi, H.B. DNA photolyase homologs are the major UV resistance factors in the cyanobacterium Synechocystis sp. PCC 6803. Mol. Genet. Genom. 2001, 264, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Owttrim, G.W.; Coleman, J.R. Regulation of expression and nucleotide sequence of the Anabaena variabilis recA gene. J. Bacteriol. 1989, 171, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, U. The FAPY-DNA glycosylase (Fpg) is required for survival of the cyanobacterium Synechococcus elongatus under high light irradiance. FEMS Microbiol. Lett. 2000, 187, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Schulz, S.; Wait, R.; Görg, A.; Scherer, S. The UV-B stimulon of the terrestrial cyanobacterium Nostoc commune comprises early shock proteins and late acclimation proteins. Mol. Microbiol. 2002, 46, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Berman-Frank, I.; Bidle, K.D.; Haramaty, L.; Falkowski, P.G. The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol. Oceanogr. 2004, 49, 997–1005. [Google Scholar] [CrossRef]
- Frangeul, L.; Quillardet, P.; Castets, A.-M.; Humbert, J.-F.; Matthijs, H.C.; Cortez, D.; Tolonen, A.; Zhang, C.-C.; Gribaldo, S.; Kehr, J.-C.; et al. Highly plastic genome of Microcystis aeruginosa PCC 7806, a ubiquitous toxic freshwater cyanobacterium. BMC Genom. 2008, 9, 274. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Swapnil, P.; Yadav, A.K.; Srivastav, S.; Sharma, N.K.; Srikrishna, S.; Rai, A.K. Biphasic ROS accumulation and programmed cell death in a cyanobacterium exposed to salinity (NaCl and Na2SO4). Algal Res. 2017, 23, 88–95. [Google Scholar] [CrossRef]
- Lu, Z.; Sha, J.; Tian, Y.; Zhang, X.; Liu, B.; Wu, Z. Polyphenolic allelochemical pyrogallic acid induces caspase-3(like)-dependent programmed cell death in the cyanobacterium Microcystis aeruginosa. Algal Res. 2017, 21, 148–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, X.; Qu, F.; Lv, M.; Liu, Q.; Li, J.; Li, L.; Zhang, B.; Zhao, Y. ROS mediated programmed cell death (PCD) of Thalassiosira pseudonana under the stress of BDE-47. Environ. Pollut. 2020, 262, 114342. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Jia, Y.; Li, J.; Wu, Z.; Li, L.; Song, L. Paraquat induces different programmed cell death patterns in Microcystis aeruginosa and Chlorella luteoviridis. Ecotoxicol. Environ. Saf. 2023, 249, 114429. [Google Scholar] [CrossRef] [PubMed]



| Order | Organism | MAAs (λmax nm) |
|---|---|---|
| Synechococcales | Synechocystis sp. PCC 6803 | Mycosporine-taurine (309); Dehydroxyl-usujirene (357) |
| Chroococcales | Gloeocapsa sp. | Shinorine (334); Mycosporine-glycine (310) |
| Aphanothece halophytica | Mycosporine-2-glycine (334) | |
| Euhalothece sp. | Euhalothece (362); Mycosporine-2-glycine (334) | |
| Microcystis aeruginosa | Shinorine (334); Porphyra (334) | |
| Oscillatoriales | Lyngbya sp. CU2555 | Palythine (320); Asterina (330) |
| Microcoleus chthonoplastes | Shinorine (334) | |
| Oscillatoria spongelidae | Mycosporine-glycine (310); Usujirene (357); Palythene (360) | |
| Trichodesmium sp. | Asterina (330); Shinorine (334); Porphyra (334); Palythene (360) | |
| Nostocales | Anabaena sp. | Shinorine (334) |
| Anabaena doliolum | Mycosporine-glycine (310); Porphyra (334); Shinorine (334) | |
| Anabaena variabilis PCC 7937 | Shinorine (334); Palythine-Serine (320); MAA-glycine (310) | |
| Nostoc commune | Shinorine (334) | |
| Scytonema sp. | Shinorine (334); MAA-315 (315); Asterina (330) | |
| Nostoc punctiforme ATCC 29133 | Shinorine (334) | |
| Nostoc sp. HKAR-2 and HKAR-6 | Shinorine (334); Porphyra (334) | |
| Nodularia sp. | Shinorine (334); Porphyra (334) | |
| Aphanizomenon flos-aquae | Porphyra (334) | |
| Chlorogloeopsis PCC 6912 | Mycosporine-glycine (310); Shinorine (334) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.K.; Jha, S.; Rana, P.; Mishra, S.; Kumari, N.; Singh, S.C.; Anand, S.; Upadhye, V.; Sinha, R.P. Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress. Int. J. Mol. Sci. 2023, 24, 12381. https://doi.org/10.3390/ijms241512381
Singh VK, Jha S, Rana P, Mishra S, Kumari N, Singh SC, Anand S, Upadhye V, Sinha RP. Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress. International Journal of Molecular Sciences. 2023; 24(15):12381. https://doi.org/10.3390/ijms241512381
Chicago/Turabian StyleSingh, Varsha K., Sapana Jha, Palak Rana, Sonal Mishra, Neha Kumari, Suresh C. Singh, Shekhar Anand, Vijay Upadhye, and Rajeshwar P. Sinha. 2023. "Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress" International Journal of Molecular Sciences 24, no. 15: 12381. https://doi.org/10.3390/ijms241512381
APA StyleSingh, V. K., Jha, S., Rana, P., Mishra, S., Kumari, N., Singh, S. C., Anand, S., Upadhye, V., & Sinha, R. P. (2023). Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress. International Journal of Molecular Sciences, 24(15), 12381. https://doi.org/10.3390/ijms241512381






