Asbestos and Iron
Abstract
1. Introduction
2. The Crystal Lattice of Asbestos and Structural Iron
3. The Surface of Asbestos and Complexed Iron
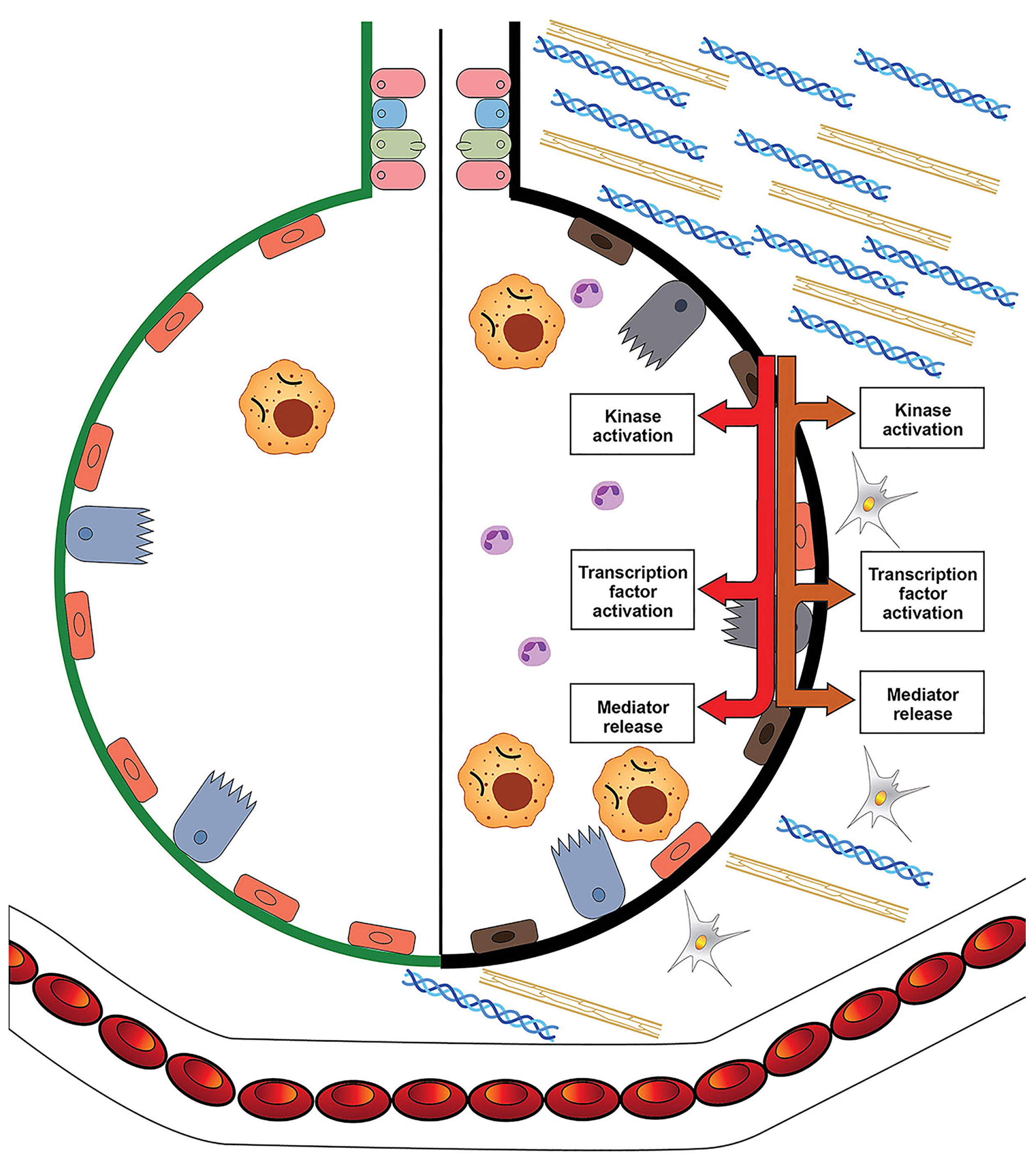
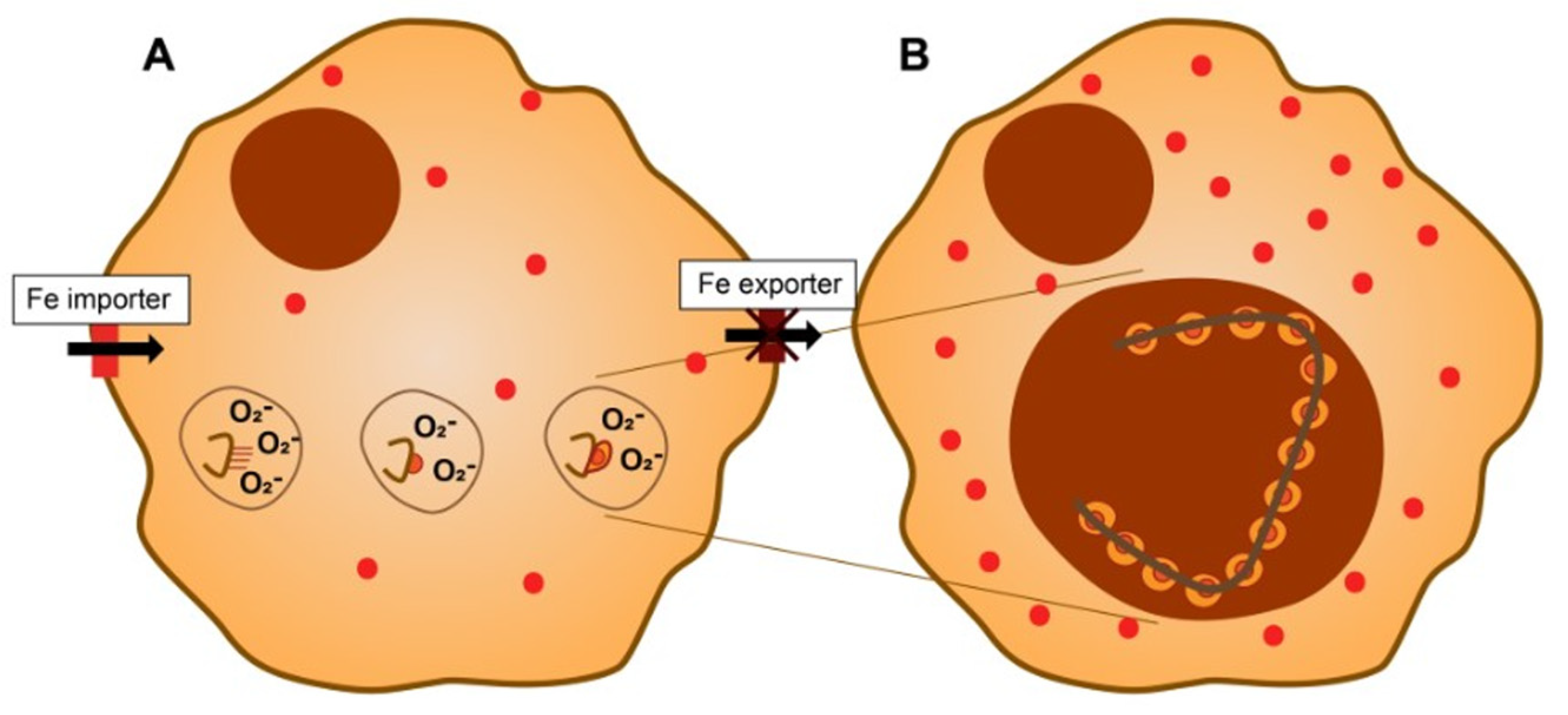
4. Asbestos, Cell Iron Homeostasis, and Their Biological Effects
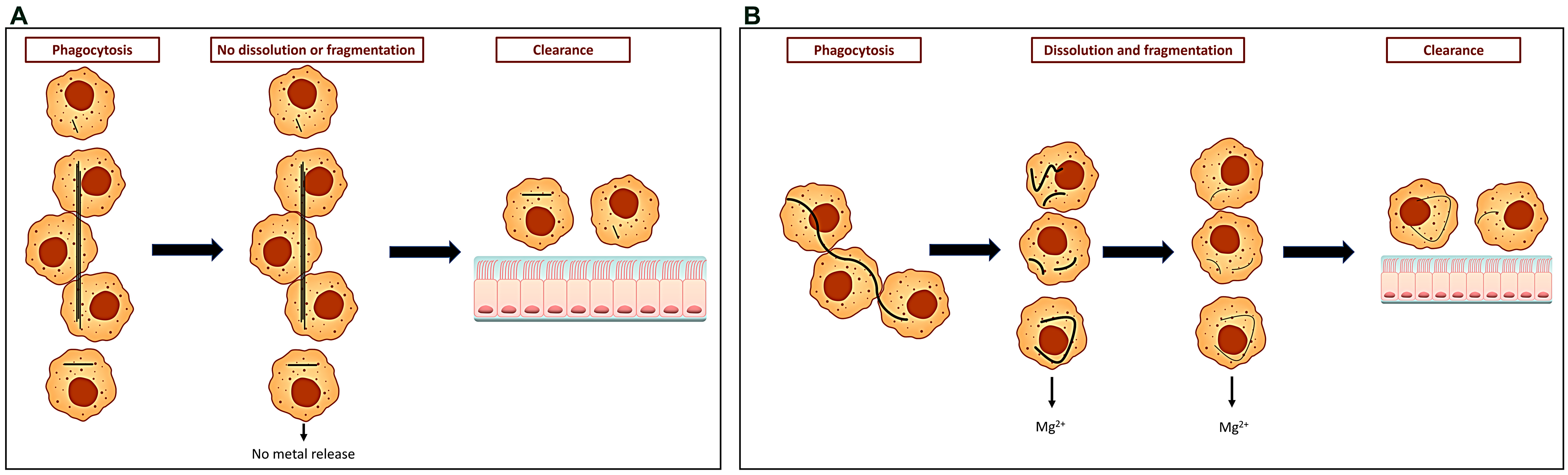
5. The Formation of Ferruginous Bodies and Iron
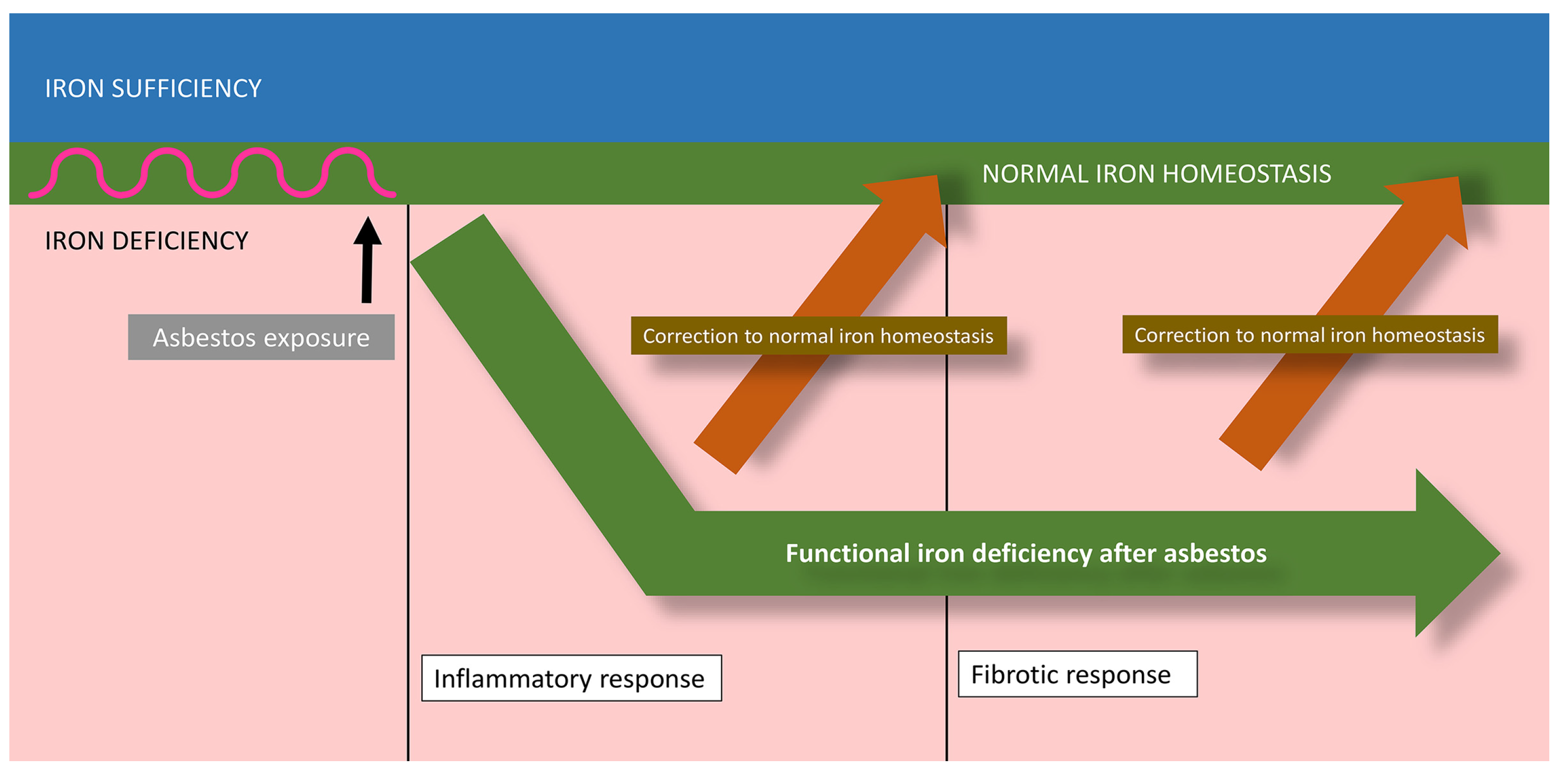
6. Disparities between the Biological Impacts of Asbestos and Iron
Author Contributions
Funding
Conflicts of Interest
References
- Cooke, W.E. Fibrosis of the lungs due to the inhalation of asbestos dust. Br. Med. J. 1924, 2, 147. [Google Scholar] [CrossRef]
- Breslow, L. Industrial aspects of bronchiogenic neoplasms. Dis. Chest 1955, 28, 421–430. [Google Scholar] [CrossRef]
- Doll, R. Mortality from lung cancer in asbestos workers. Br. J. Ind. Med. 1955, 12, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Sleggs, C.A.; Marchand, P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br. J. Ind. Med. 1960, 17, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yasui, H.; Toyokuni, S. Deferasirox induces mesenchymal-epithelial transition in crocidolite-induced mesothelial carcinogenesis in rats. Cancer Prev. Res. 2013, 6, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Chew, S.H.; Shibata, T.; Okazaki, Y.; Yamashita, K.; Toyokuni, S. Phlebotomy as a preventive measure for crocidolite-induced mesothelioma in male rats. Cancer Sci. 2018, 109, 330–339. [Google Scholar] [CrossRef]
- Ito, F.; Kato, K.; Yanatori, I.; Murohara, T.; Toyokuni, S. Ferroptosis-dependent extracellular vesicles from macrophage contribute to asbestos-induced mesothelial carcinogenesis through loading ferritin. Redox Biol. 2021, 47, 102174. [Google Scholar] [CrossRef]
- Ghio, A.J.; Kennedy, T.P.; Schapira, R.M.; Crumbliss, A.L.; Hoidal, J.R. Hypothesis: Is lung disease after silicate inhalation caused by oxidant generation? Lancet 1990, 336, 967–969. [Google Scholar]
- Ghio, A.J.; Stonehuerner, J.; Richards, J.; Devlin, R.B. Iron homeostasis in the lung following asbestos exposure. Antioxid. Redox Signal. 2008, 10, 371–377. [Google Scholar] [CrossRef]
- Case, B.W.; Abraham, J.L.; Meeker, G.; Pooley, F.D.; Pinkerton, K.E. Applying definitions of “asbestos” to environmental and “low-dose” exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Foresti, E.; Fornero, E.; Lesci, I.G.; Rinaudo, C.; Zuccheri, T.; Roveri, N. Asbestos health hazard: A spectroscopic study of synthetic geoinspired Fe-doped chrysotile. J. Hazard. Mater. 2009, 167, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Morgan, A.; Sandalls, J. Determination of iron, chromium, cobalt, nickel, and scandium in asbestos by neutron activation analysis. Am. Ind. Hyg. Assoc. J. 1971, 32, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Borghi, E.; Occhiuzzi, M.; Foresti, E.; Lesci, I.G.; Roveri, N. Spectroscopic characterization of Fe-doped synthetic chrysotile by EPR, DRS and magnetic susceptibility measurements. Phys. Chem. Chem. Phys. 2010, 12, 227–238. [Google Scholar] [CrossRef] [PubMed]
- David, S.R.; Ihiawakrim, D.; Regis, R.; Geoffroy, V.A. Efficiency of pyoverdines in iron removal from flocking asbestos waste: An innovative bacterial bioremediation strategy. J. Hazard. Mater. 2020, 394, 122532. [Google Scholar] [CrossRef]
- Parker, C.W.; Senko, J.M.; Auler, A.S.; Sasowsky, I.D.; Schulz, F.; Woyke, T.; Barton, H.A. Enhanced terrestrial Fe(II) mobilization identified through a novel mechanism of microbially driven cave formation in Fe(III)-rich rocks. Sci. Rep. 2022, 12, 17062. [Google Scholar] [CrossRef]
- Pollastri, S.; D’Acapito, F.; Trapananti, A.; Colantoni, I.; Andreozzi, G.B.; Gualtieri, A.F. The chemical environment of iron in mineral fibres. A combined X-ray absorption and Mossbauer spectroscopic study. J. Hazard. Mater. 2015, 298, 282–293. [Google Scholar] [CrossRef]
- Fels, A.O.; Cohn, Z.A. The alveolar macrophage. J. Appl. Physiol. 1986, 60, 353–369. [Google Scholar] [CrossRef]
- Speil, S.; Leineweber, J.P. Asbestos minerals in modern technology. Environ. Res. 1969, 2, 166–208. [Google Scholar] [CrossRef]
- Gandolfi, N.B.; Gualtieri, A.F.; Pollastri, S.; Tibaldi, E.; Belpoggi, F. Assessment of asbestos body formation by high resolution FEG-SEM after exposure of Sprague-Dawley rats to chrysotile, crocidolite, or erionite. J. Hazard. Mater. 2016, 306, 95–104. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Lusvardi, G.; Zoboli, A.; Di Giuseppe, D.; Lassinantti Gualtieri, M. Biodurability and release of metals during the dissolution of chrysotile, crocidolite and fibrous erionite. Environ. Res. 2019, 171, 550–557. [Google Scholar] [CrossRef]
- Tanji, T.; Yada, K.; Akatsuka, Y. Alternation of Clinochrysotile and Orthochrysotile in a Single Fiber as Revealed by High-Resolution Electron-Microscopy. Clays Clay Miner. 1984, 32, 429–432. [Google Scholar] [CrossRef]
- Titulaer, M.K.; Vanmiltenburg, J.C.; Jansen, J.B.H.; Geus, J.W. Characterization of Tubular Chrysotile by Thermoporometry, Nitrogen Sorption, Drifts, and Tem. Clays Clay Miner. 1993, 41, 496–513. [Google Scholar] [CrossRef]
- Bernstein, D.M. The health risk of chrysotile asbestos. Curr. Opin. Pulm. Med. 2014, 20, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Morgan, A. Leaching of constituents of chrysotile asbestos in vivo. Nature 1967, 215, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Tanaka, A.; Mita, K.; Furuichi, R. Surface hydroxyl site densities on metal oxides as a measure for the ion-exchange capacity. J. Colloid Interface Sci. 1999, 209, 225–231. [Google Scholar] [CrossRef]
- Crumbliss, A.L.; Garrison, J.M. A comparison of some aspects of the coordination chemistry of aluminum (III) and iron (III). Comm. Inorg. Chem. 1988, 8, 1–26. [Google Scholar] [CrossRef]
- Fordham, A.W. Sorption and precipitation of iron on kaolinite. Factors involved in sorption equilibria. Aust. J. Soil Res. 1969, 7, 185–197. [Google Scholar] [CrossRef]
- Herrera, R.; Peech, M. Reaction of montmorillonite with iron (III). Soil Sci. Soc. Am. Proc. 1970, 34, 740–745. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rauf, N. Removal of Fe(II) from the wastewater of a galvanized pipe manufacturing industry by adsorption onto bentonite clay. J. Environ. Manag. 2004, 73, 285–292. [Google Scholar] [CrossRef]
- Bakalar, T.; Kanuchova, M.; Girova, A.; Pavolova, H.; Hromada, R.; Hajduova, Z. Characterization of Fe(III) Adsorption onto Zeolite and Bentonite. Int. J. Environ. Res. Public Health 2020, 17, 5718. [Google Scholar] [CrossRef]
- Duenas-Ramirez, P.; Bertagnolli, C.; Müller, R.; Sartori, K.; Boos, A.; Elhabiri, M.; Bégin-Colin, S.; Mertz, D. Highly chelating stellate mesoporous silica nanoparticles for specific iron removal from biological media. J. Colloid Interface Sci. 2020, 579, 140–151. [Google Scholar] [CrossRef]
- Flieger, J.; Kawka, J.; Plazinski, W.; Panek, R.; Madej, J. Sorption of Heavy Metal Ions of Chromium, Manganese, Selenium, Nickel, Cobalt, Iron from Aqueous Acidic Solutions in Batch and Dynamic Conditions on Natural and Synthetic Aluminosilicate Sorbents. Materials 2020, 13, 5271. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; de Baar, H.J.W.; Follows, M.; et al. Mesoscale iron enrichment experiments 1993–2005, synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef]
- Carver, P.L. The Battle for Iron between Humans and Microbes. Curr. Med. Chem. 2018, 25, 85–96. [Google Scholar] [CrossRef]
- Sanchez, M.; Sabio, L.; Galvez, N.; Capdevila, M.; Dominguez-Vera, J.M. Iron chemistry at the service of life. IUBMB Life 2017, 69, 382–388. [Google Scholar] [CrossRef]
- Fukuchi, K.; Tomoyasu, S.; Watanabe, K.; Watanabe, H.; Takagi, Y.; Tsuruoka, N.; Gomi, K. Enhanced c-fos expression after intracellular iron deprivation. Biochem. Mol. Biol. Int. 1993, 30, 403–409. [Google Scholar]
- Ido, Y.; Muto, N.; Inada, A.; Kohroki, J.; Mano, M.; Odani, T.; Itoh, N.; Yamamoto, K.; Tanaka, K. Induction of apoptosis by hinokitiol, a potent iron chelator, in teratocarcinoma F9 cells is mediated through the activation of caspase-3. Cell Prolif. 1999, 32, 63–73. [Google Scholar] [CrossRef]
- Georgiou, N.A.; van der Bruggen, T.; Oudshoorn, M.; Hider, R.C.; Marx, J.J.; van Asbeck, B.S. Human immunodeficiency virus type 1 replication inhibition by the bidentate iron chelators CP502 and CP511 is caused by proliferation inhibition and the onset of apoptosis. Eur. J. Clin. Investig. 2002, 32 (Suppl. 1), 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kakhlon, O.; Cabantchik, Z.I. The labile iron pool: Characterization, measurement, and participation in cellular processes. Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef]
- Ghio, A.J.; Soukup, J.M.; Dailey, L.A.; Richards, J.H.; Tong, H. The biological effect of asbestos exposure is dependent on changes in iron homeostasis. Inhal. Toxicol. 2016, 28, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kennedy, T.P.; Whorton, A.R.; Crumbliss, A.L.; Hatch, G.E.; Hoidal, J.R. Role of surface complexed iron in oxidant generation and lung inflammation induced by silicates. Am. J. Physiol. 1992, 263 Pt 1, L511–L518. [Google Scholar] [CrossRef]
- Ghio, A.J.; Kennedy, T.P.; Stonehuerner, J.G.; Crumbliss, A.L.; Hoidal, J.R. DNA strand breaks following in vitro exposure to asbestos increase with surface-complexed [Fe3+]. Arch. Biochem. Biophys. 1994, 311, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Allerson, C.R.; Polycarpou-Schwarz, M.; Rofts, A.; Rogers, J.T.; Kishi, F.; Hentze, M.W.; Rouault, T.A.; Andrews, N.C.; Hediger, M.A. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001, 509, 309–316. [Google Scholar] [CrossRef]
- Ghio, A.J.; Pavlisko, E.N.; Roggli, V.L. Iron and Iron-Related Proteins in Asbestosis. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 277–285. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef] [PubMed]
- Grivennikova, V.G.; Vinogradov, A.D. Generation of superoxide by the mitochondrial Complex, I. Biochim. Biophys. Acta 2006, 1757, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Dendorfer, A.; Heidbreder, M.; Hellwig-Burgel, T.; Johren, O.; Qadri, F.; Dominiak, P. Deferoxamine induces prolonged cardiac preconditioning via accumulation of oxygen radicals. Free Radic. Biol. Med. 2005, 38, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Callens, C.; Coulon, S.; Naudin, J.; Radford-Weiss, I.; Boissel, N.; Raffoux, E.; Wang, P.H.M.; Agarwal, S.; Tamouza, H.; Paubelle, E.; et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J. Exp. Med. 2010, 207, 731–750. [Google Scholar] [CrossRef]
- Cakmak, I.; van de Wetering, D.A.; Marschner, H.; Bienfait, H.F. Involvement of superoxide radical in extracellular ferric reduction by iron-deficient bean roots. Plant Physiol. 1987, 85, 310–314. [Google Scholar] [CrossRef]
- Turi, J.L.; Jaspers, I.; Dailey, L.A.; Madden, M.C.; Brighton, L.E.; Carter, J.D.; Nozik-Grayck, E.; Piantadosi, C.A.; Ghio, A.J. Oxidative stress activates anion exchange protein 2 and AP-1 in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L791–L798. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shukla, A.; Ramos-Nino, M.; Mossman, B. Cell signaling and transcription factor activation by asbestos in lung injury and disease. Int. J. Biochem. Cell Biol. 2003, 35, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Tong, H.; Soukup, J.M.; Dailey, L.A.; Cheng, W.Y.; Samet, J.M.; Kesic, M.J.; Bromberg, P.A.; Turi, J.L.; Upadhyay, D.; et al. Sequestration of mitochondrial iron by silica particle initiates a biological effect. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L712–L724. [Google Scholar] [CrossRef][Green Version]
- Ghio, A.J.; Soukup, J.M.; Dailey, L.A.; Tong, H.; Kesic, M.J.; Budinger, G.S.; Mutlu, G.M. Wood Smoke Particle Sequesters Cell Iron to Impact a Biological Effect. Chem. Res. Toxicol. 2015, 28, 2104–2111. [Google Scholar] [CrossRef]
- Knorr-Wittmann, C.; Hengstermann, A.; Gebel, S.; Alam, J.; Muller, T. Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radic. Biol. Med. 2005, 39, 1438–1448. [Google Scholar] [CrossRef]
- Deng, X.; Rui, W.; Zhang, F.; Ding, W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol. Toxicol. 2013, 29, 143–157. [Google Scholar] [CrossRef]
- Cheng, S.E.; Lee, I.T.; Lin, C.C.; Kou, Y.R.; Yang, C.M. Cigarette smoke particle-phase extract induces HO-1 expression in human tracheal smooth muscle cells: Role of the c-Src/NADPH oxidase/MAPK/Nrf2 signaling pathway. Free Radic. Biol. Med. 2010, 48, 1410–1422. [Google Scholar] [CrossRef]
- Chan, J.K.; Charrier, J.G.; Kodani, S.D.; Vogel, C.F.; Kado, S.Y.; Anderson, D.S.; Anastasio, C.; Van Winkle, L.S. Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates Nrf2 antioxidants differently in neonatal and adult rat lungs. Part. Fibre Toxicol. 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sun, S.; Kuhn, D.C.; Gaydos, L.J.; Shi, X.; Lu, Y.; Demers, L.M. Involvement of NF-kappaB in silica-induced cyclooxygenase II gene expression in rat alveolar macrophages. Am. J. Physiol. 1997, 272 Pt 1, L779–L786. [Google Scholar] [CrossRef]
- Li, N.; Kim, S.; Wang, M.; Froines, J.; Sioutas, C.; Nel, A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal. Toxicol. 2002, 14, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Karin, M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 1996, 8, 402–411. [Google Scholar] [CrossRef]
- Xiao, G.G.; Wang, M.; Li, N.; Loo, J.A.; Nel, A.E. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J. Biol. Chem. 2003, 278, 50781–50790. [Google Scholar] [CrossRef]
- Ghio, A.J.; Stonehuerner, J.; Soukup, J.M.; Dailey, L.A.; Kesic, M.J.; Cohen, M.D. Iron diminishes the in vitro biological effect of vanadium. J. Inorg. Biochem. 2015, 147, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chua, F.; Gauldie, J.; Laurent, G.J. Pulmonary fibrosis: Searching for model answers. Am. J. Respir. Cell Mol. Biol. 2005, 33, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rosmus, J.; Vancikova, O.; Marc, J.; Deyl, Z. Studies on the structure of collagen V. The site of binding of trivalent iron on collagen. Experientia 1967, 23, 898. [Google Scholar] [CrossRef] [PubMed]
- Sannes, P.L. Cytochemical visualization of anions in collagenous and elastic fiber-associated connective tissue matrix in neonatal and adult rat lungs using iron-containing stains. Histochemistry 1986, 84, 49–56. [Google Scholar] [CrossRef]
- Tang, R.; Liao, X.P.; Liu, X.; Shi, B. Collagen fiber immobilized Fe(III): A novel catalyst for photo-assisted degradation of dyes. Chem. Commun. 2005, 47, 5882–5884. [Google Scholar] [CrossRef]
- Nakatani, S.; Naito, I.; Momota, R.; Hinenoya, N.; Horiuchi, K.; Nishida, K.; Ohtsuka, A. In situ preparation of colloidal iron by microwave irradiation for transmission electron microscopy. Acta Med. Okayama 2006, 60, 59–64. [Google Scholar]
- Kinberger, G.A.; Taulane, J.P.; Goodman, M. Fe(III)-binding collagen mimetics. Inorg. Chem. 2006, 45, 961–963. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wu, C.H.; Yang, J.I.; Li, Y.H.; Kuo, J.M. Evaluation of iron-binding activity of collagen peptides prepared from the scales of four cultivated fishes in Taiwan. J. Food Drug Anal. 2015, 23, 671–678. [Google Scholar] [CrossRef]
- Kanagy, J.R.; Kronstadt, R.A. Iron as a tanning agent. J. Res. Natl. Bur. Stand. 1943, 31, 279–292. [Google Scholar] [CrossRef]
- Fathima, N.N.; Rao, J.R.; Nair, B.U. Effect of UV irradiation on the physico-chemical properties of iron crosslinked collagen. J. Photochem. Photobiol. B 2011, 105, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Pieraggi, M.; Nejjar, I.; Julian, M.; Bouissou, H. Staining of elastic tissue by Verhoeff’s iron hematoxylin. Ann. Pathol. 1986, 6, 74–77. [Google Scholar]
- Tilson, M.D. Histochemistry of aortic elastin in patients with nonspecific abdominal aortic aneurysmal disease. Arch. Surg. 1988, 123, 503–505. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Mukhopadhyay, S.; Zanardi, C.; Dexter, E. Clinically occult interstitial fibrosis in smokers: Classification and significance of a surprisingly common finding in lobectomy specimens. Hum. Pathol. 2010, 41, 316–325. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Tun, H.C. The degradation of hyaluronic acid by ferrous ions. Carbohydr. Res. 1972, 22, 43–51. [Google Scholar] [CrossRef]
- Merce, A.L.; Marques Carrera, L.C.; Santos Romanholi, L.K.; Lobo Recio, M.A. Aqueous and solid complexes of iron(III) with hyaluronic acid. Potentiometric titrations and infrared spectroscopy studies. J. Inorg. Biochem. 2002, 89, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Bracke, K.R.; Dentener, M.A.; Papakonstantinou, E.; Vernooy, J.H.J.; Demoor, T.; Pauwels, N.S.; Cleutjens, J.; van Suylen, R.J.; Joos, G.F.; Brusselle, G.G.; et al. Enhanced deposition of low-molecular-weight hyaluronan in lungs of cigarette smoke-exposed mice. Am. J. Respir. Cell Mol. Biol. 2010, 42, 753–761. [Google Scholar] [CrossRef]
- Ikeda, H.; Wu, G.Y.; Wu, C.H. Evidence that an iron chelator regulates collagen synthesis by decreasing the stability of procollagen mRNA. Hepatology 1992, 15, 282–287. [Google Scholar] [CrossRef]
- Gardi, C.; Arezzini, B.; Fortino, V.; Comporti, M. Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. Biochem. Pharmacol. 2002, 64, 1139–1145. [Google Scholar] [CrossRef]
- Bunda, S.; Kaviani, N.; Hinek, A. Fluctuations of intracellular iron modulate elastin production. J. Biol. Chem. 2005, 280, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, F.; Fan, D.; Wang, Y.; Yu, Y. Higher iron bioavailability of a human-like collagen iron complex. J. Biomater. Appl. 2017, 32, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, G.; Pigman, W. Catalytic role of copper and iron ions in the depolymerization of hyaluronic acid by ascorbic acid. Arch. Biochem. Biophys. 1965, 110, 526–533. [Google Scholar] [CrossRef]
- Harris, M.J.; Herp, A.; Pigman, W. Metal catalysis in the depolymerization of hyaluronic acid by autoxidants. J. Am. Chem. Soc. 1972, 94, 7570–7572. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Yeates, D.B.; Albert, R.E. Deposition, retention, and clearance of inhaled particles. Br. J. Ind. Med. 1980, 37, 337–362. [Google Scholar] [CrossRef]
- Hiller, F.C. Deposition of sidestream cigarette smoke in the human respiratory tract. Prev. Med. 1984, 13, 602–607. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, C.S. Mathematical analysis of particle deposition in human lungs: An improved single path transport model. Inhal. Toxicol. 2007, 19, 925–939. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sim, S.; Choi, H.G. Active and passive smoking impacts on asthma with quantitative and temporal relations: A Korean Community Health Survey. Sci. Rep. 2018, 8, 8614. [Google Scholar] [CrossRef]
- Lambert, A.R.; O’Shaughnessy, P.; Tawhai, M.H.; Hoffman, E.A.; Lin, C.L. Regional deposition of particles in an image-based airway model: Large-eddy simulation and left-right lung ventilation asymmetry. Aerosol. Sci. Technol. 2011, 45, 11–25. [Google Scholar] [CrossRef]
- Brown, J.S.; Zeman, K.L.; Bennett, W.D. Regional deposition of coarse particles and ventilation distribution in healthy subjects and patients with cystic fibrosis. J. Aerosol. Med. 2001, 14, 443–454. [Google Scholar] [CrossRef]
- Heyder, J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Gawda, A.; Majka, G.; Nowak, B.; Srottek, M.; Walczewska, M.; Marcinkiewicz, J. Air particulate matter SRM 1648a primes macrophages to hyperinflammatory response after LPS stimulation. Inflamm. Res. 2018, 67, 765–776. [Google Scholar] [CrossRef]
- Boulanger, G.; Andujar, P.; Pairon, J.-C.; Billon-Galland, M.-A.; Dion, C.; Dumortier, P.; Brochard, P.; Sobaszek, A.; Bartsch, P.; Paris, C.; et al. Quantification of short and long asbestos fibers to assess asbestos exposure: A review of fiber size toxicity. Environ. Health 2014, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M.; Rogers, R.A.; Sepulveda, R.; Donaldson, K.; Schuler, D.; Gaering, S.; Kunzendorf, P.; Chevalier, J.; Holm, S.E.; et al. Quantification of the pathological response and fate in the lung and pleura of chrysotile in combination with fine particles compared to amosite-asbestos following short-term inhalation exposure. Inhal. Toxicol. 2011, 23, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M. Synthetic vitreous fibers: A review toxicology, epidemiology and regulations. Crit. Rev. Toxicol. 2007, 37, 839–886. [Google Scholar] [CrossRef]
- Bernstein, D.; Dunnigan, J.; Hesterberg, T.; Brown, R.; Velasco, J.A.L.; Barrera, R.; Hoskins, J.; Gibbs, A. Health risk of chrysotile revisited. Crit. Rev. Toxicol. 2013, 43, 154–183. [Google Scholar] [CrossRef]
- Pascolo, L.; Gianoncelli, A.; Kaulich, B.; Rizzardi, C.; Schneider, M.; Bottin, C.; Polentarutti, M.; Kiskinova, M.; Longoni, A. Synchrotron soft X-ray imaging and fluorescence microscopy reveal novel features of asbestos body morphology and composition in human lung tissues. Part. Fibre Toxicol. 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, F.; Veronesi, G.; Capella, S.; Bellis, D.; Charlet, L.; Cedola, A.; Belluso, E. New insights on the biomineralisation process developing in human lungs around inhaled asbestos fibres. Sci. Rep. 2017, 7, 44862. [Google Scholar] [CrossRef]
- Churg, A.M.; Warnock, M.L. Asbestos and other ferruginous bodies: Their formation and clinical significance. Am. J. Pathol. 1981, 102, 447–456. [Google Scholar]
- Bardelli, F.; Brun, F.; De Panfilis, S.; Cloetens, P.; Capella, S.; Belluso, E.; Bellis, D.; Di Napoli, A.; Cedola, A. Chemo-physical properties of asbestos bodies in human lung tissues studied at the nano-scale by non-invasive, label free X-ray imaging and spectroscopic techniques. Toxicol. Lett. 2021, 348, 18–27. [Google Scholar] [CrossRef]
- Roggli, V.L. Asbestos bodies and non-asbestos ferruginous bodies. In Pathology of Asbestos-Associated Disease, 3rd ed.; Oury, T.D., Sporn, T.A., Roggli, V.L., Eds.; Springer: Berlin, Germany, 2014; Chapter 3; pp. 25–51. [Google Scholar]
- Koerten, H.K.; de Bruijn, J.D.; Daems, W.T. The formation of asbestos bodies by mouse peritoneal macrophages. An in vitro study. Am. J. Pathol. 1990, 137, 121–134. [Google Scholar] [PubMed]
- Botham, S.K.; Holt, P.F. Development of asbestos bodies on amosite, chrysotile and crocidolite fibres in guinea-pig lungs. J. Pathol. 1971, 105, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Roggli, V.L. Perls’ Prussian Blue Stains of Lung Tissue, Bronchoalveolar Lavage, and Sputum. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pooley, F.D. Asbestos Bodies, Their Formation, Composition and Character. Environ. Res. 1972, 5, 363–379. [Google Scholar] [CrossRef]
- Treffry, A.; Harrison, P.M.; Cleton, M.I.; de Bruijn, W.C.; Mann, S. A note on the composition and properties of ferritin iron cores. J. Inorg. Biochem. 1987, 31, 1–6. [Google Scholar] [CrossRef]
- Vigliaturo, R.; Jamnik, M.; Dražić, G.; Podobnik, M.; Žnidarič, M.T.; Della Ventura, G.; Redhammer, G.J.; Žnidaršič, N.; Caserman, S.; Gieré, R. Nanoscale transformations of amphiboles within human alveolar epithelial cells. Sci. Rep. 2022, 12, 1782. [Google Scholar] [CrossRef]
- Warnock, M.L.; Churg, A.M. Asbestos bodies. Chest 1980, 77, 129–130. [Google Scholar] [CrossRef]
- Shen, Z.; Bosbach, D.; Hochella, M.F.; Bish, D.L., Jr.; Williams, M.G.; Dodson, R.F., Jr.; Aust, A.E. Using in vitro iron deposition on asbestos to model asbestos bodies formed in human lung. Chem. Res. Toxicol. 2000, 13, 913–921. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Obst, M.; Kappler, A.; Hegler, F.; Schädler, S.; Bouchez, C.; Guyot, F.; Morin, G. Extracellular iron biomineralization by photoautotrophic iron-oxidizing bacteria. Appl. Environ. Microbiol. 2009, 75, 5586–5591. [Google Scholar] [CrossRef]
- Boland, D.D.; Collins, R.N.; Miller, C.J.; Glover, C.J.; Waite, T.D. Effect of solution and solid-phase conditions on the Fe(II)-accelerated transformation of ferrihydrite to lepidocrocite and goethite. Environ. Sci. Technol. 2014, 48, 5477–5485. [Google Scholar] [CrossRef]
- Xiao, W.; Jones, A.M.; Li, X.; Collins, R.N.; Waite, T.D. Effect of Shewanella oneidensis on the Kinetics of Fe(II)-Catalyzed Transformation of Ferrihydrite to Crystalline Iron Oxides. Environ. Sci. Technol. 2018, 52, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Benner, S.G.; Neiss, J.; Dohnalkova, A.; Kukkadapu, R.K.; Fendorf, S. Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochim. Cosmochim. Acta 2003, 67, 2977–2992. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Pascolo, L.; Gianoncelli, A.; Schneider, G.; Salomé, M.; Schneider, M.; Calligaro, C.; Kiskinova, M.; Melato, M.; Rizzardi, C. The interaction of asbestos and iron in lung tissue revealed by synchrotron-based scanning X-ray microscopy. Sci. Rep. 2013, 3, srep01123. [Google Scholar] [CrossRef]
- Hansel, C.M.; Benner, S.G.; Fendorf, S. Competing Fe (II)-induced mineralization pathways of ferrihydrite. Environ. Sci. Technol. 2005, 39, 7147–7153. [Google Scholar] [CrossRef]
- Dlamini, C.L.; De Kock, L.A.; Kefeni, K.K.; Mamba, B.B.; Msagati, T.A.M. Polymeric ion exchanger supported ferric oxide nanoparticles as adsorbents for toxic metal ions from aqueous solutions and acid mine drainage. J. Environ. Health Sci. 2019, 17, 719–730. [Google Scholar]
- Ajith, N.; Satpati, A.K.; Debnath, A.K.; Swain, K.K. Evidences on As(III) and As(V) interaction with iron(III) oxides: Hematite and goethite. J. Environ. Sci. Health Part A 2021, 56, 1007–1018. [Google Scholar] [CrossRef]
- Shi, M.Q.; Min, X.B.; Ke, Y.; Lin, Z.; Yang, Z.H.; Wang, S.; Peng, N.; Yan, X.; Luo, S.; Wu, J.; et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr)oxides. Sci. Total Environ. 2021, 752, 141930. [Google Scholar] [CrossRef]
- Boily, J.F.; Song, X.W. Direct identification of reaction sites on ferrihydrite. Commun. Chem. 2020, 3, 79. [Google Scholar] [CrossRef]
- Ghio, A.J.; Roggli, V.L.; Richards, J.H.; Crissman, K.M.; Stonehuerner, J.D.; Piantadosi, C.A. Oxalate deposition on asbestos bodies. Hum. Pathol. 2003, 34, 737–742. [Google Scholar] [CrossRef]
- Pascolo, L.; Zabucchi, G.; Gianoncelli, A.; Kourousias, G.; Trevisan, E.; Pascotto, E.; Casarsa, C.; Ryan, C.; Lucattelli, M.; Lungarella, G.; et al. Synchrotron X-ray microscopy reveals early calcium and iron interaction with crocidolite fibers in the lung of exposed mice. Toxicol. Lett. 2016, 241, 111–120. [Google Scholar] [CrossRef]
- Nakamura, E.; Makishima, A.; Hagino, K.; Okabe, K. Accumulation of radium in ferruginous protein bodies formed in lung tissue: Association of resulting radiation hotspots with malignant mesothelioma and other malignancies. Proc. Jpn. Acad. Ser. B 2009, 85, 229–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ryan, A.J.; Larson-Casey, J.L.; He, C.; Murthy, S.; Carter, A.B. Asbestos-induced disruption of calcium homeostasis induces endoplasmic reticulum stress in macrophages. J. Biol. Chem. 2014, 289, 33391–33403. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Aust, A.E. Induction of ferritin synthesis in human lung epithelial cells treated with crocidolite asbestos. Arch. Biochem. Biophys. 1997, 340, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Borelli, V.; Brochetta, C.; Melato, M.; Rizzardi, C.; Polentarutti, M.; Busatto, C.; Vita, F.; Abbate, R.; Gotter, R.; Zabucchi, G. A procedure for the isolation of asbestos bodies from lung tissue by exploiting their magnetic properties: A new approach to asbestos body study. J. Toxicol. Environ. Health A 2007, 70, 1232–1240. [Google Scholar] [CrossRef]
- Ghio, A.; Tan, R.J.; Ghio, K.; Fattman, C.L.; Oury, T.D. Iron accumulation and expression of iron-related proteins following murine exposure to crocidolite. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 153–162. [Google Scholar] [CrossRef]
- Pascolo, L.; Borelli, V.; Canzonieri, V.; Gianoncelli, A.; Birarda, G.; Bedolla, D.E.; Salomé, M.; Vaccari, L.; Calligaro, C.; Cotte, M.; et al. Differential protein folding and chemical changes in lung tissues exposed to asbestos or particulates. Sci. Rep. 2015, 5, 12129. [Google Scholar] [CrossRef]
- La, A.; Nguyen, T.; Tran, K.; Sauble, E.; Tu, D.; Gonzalez, A.; Kidane, T.Z.; Soriano, C.; Morgan, J.; Doan, M.; et al. Mobilization of iron from ferritin: New steps and details. Metallomics 2018, 10, 154–168. [Google Scholar] [CrossRef]
- Dumortier, P.; Çoplü, L.; Broucke, I.; Emri, S.; Selcuk, T.; de Maertelaer, V.; De Vuyst, P.; Baris, I. Erionite bodies and fibres in bronchoalveolar lavage fluid (BALF) of residents from Tuzkoy, Cappadocia, Turkey. Occup. Environ. Med. 2001, 58, 261–266. [Google Scholar] [CrossRef]
- Ghio, A.J.; Soukup, J.M.; Dailey, L.A.; Madden, M.C. Air pollutants disrupt iron homeostasis to impact oxidant generation, biological effects, and tissue injury. Free Radic. Biol. Med. 2020, 151, 38–55. [Google Scholar] [CrossRef]
- Ghio, A.J.; LeFurgey, A.; Roggli, V.L. In vivo accumulation of iron on crocidolite is associated with decrements in oxidant generation by the fiber. J. Toxicol. Environ. Health 1997, 50, 125–142. [Google Scholar] [PubMed]
- Lewinski, N.; Graczyk, H.; Riediker, M. Human inhalation exposure to iron oxide particles. BiioNanoMat 2013, 14, 5–23. [Google Scholar] [CrossRef][Green Version]
- Askri, D.; Ouni, S.; Galai, S.; Chovelon, B.; Arnaud, J.; Lehmann, S.G.; Sakly, M.; Sève, M.; Amara, S. Sub-acute intravenous exposure to Fe(2)O(3) nanoparticles does not alter cognitive performances and catecholamine levels, but slightly disrupts plasma iron level and brain iron content in rats. J. Trace Elem. Med. Biol. 2018, 50, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Koksal, D.; Karcioglu, O.; Babaoglu, E.; Sarinc Ulasli, S.; Onder, S. The contribution of bronchoalveolar lavage in the diagnosis of welder’s lung in a patient with pulmonary fibrosis. Arch. Environ. Occup. Health 2020, 75, 56–59. [Google Scholar] [CrossRef]
- Guo, C.; Weber, R.J.M.; Buckley, A.; Mazzolini, J.; Robertson, S.; Delgado-Saborit, J.M.; Rappoport, J.Z.; Warren, J.; Hodgson, A.; Sanderson, P.; et al. Environmentally Relevant Iron Oxide Nanoparticles Produce Limited Acute Pulmonary Effects in Rats at Realistic Exposure Levels. Int. J. Mol. Sci. 2021, 22, 556. [Google Scholar] [CrossRef]
- Vargas, G.; Cypriano, J.; Correa, T.; Leao, P.; Bazylinski, D.A.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef]
- Amor, M.; Mathon, F.P.; Monteil, C.L.; Busigny, V.; Lefevre, C.T. Iron-biomineralizing organelle in magnetotactic bacteria: Function, synthesis and preservation in ancient rock samples. Environ. Microbiol. 2020, 22, 3611–3632. [Google Scholar] [CrossRef]
- Werckmann, J.; Cypriano, J.; Lefèvre, C.T.; Dembelé, K.; Ersen, O.; Bazylinski, D.A.; Lins, U.; Farina, M. Localized iron accumulation precedes nucleation and growth of magnetite crystals in magnetotactic bacteria. Sci. Rep. 2017, 7, 8291. [Google Scholar] [CrossRef]
- Khan, N.; Seshadri, B.; Bolan, N.; Saint, C.; Kirkham, M.; Chowdhury, S.; Yamaguchi, N.; Lee, D.; Li, G.; Kunhikrishnan, A.; et al. Root Iron Plaque on Wetland Plants as a Dynamic Pool of Nutrients and Contaminants. Adv. Agron. 2016, 138, 1–96. [Google Scholar]
- Singha, K.T.; Sebastian, A.; Prasad, M.N.V. Iron plaque formation in the roots of Pistia stratiotes L.: Importance in phytoremediation of cadmium. Int. J. Phytoremediation 2019, 21, 120–128. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Tripathi, P.; Dwivedi, S.; Kumar, A.; Mishra, A.; Chauhan, P.S.; Norton, G.J.; Nautiyal, C.S. Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics 2014, 6, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, X.; Tao, L.; Yang, Y.; Gao, L.; Xiong, J. Aeration Increases Cadmium (Cd) Retention by Enhancing Iron Plaque Formation and Regulating Pectin Synthesis in the Roots of Rice (Oryza sativa) Seedlings. Rice 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Hume, L.A.; Rimstidt, J.D. The Biodurability of Chrysotile Asbestos. Am. Miner. 1992, 77, 1125–1128. [Google Scholar]
- Bernstein, D.M.; Rogers, R.A.; Sepulveda, R.; Kunzendorf, P.; Bellmann, B.; Ernst, H.; Creutzenberg, O.; Phillips, J.I. Evaluation of the fate and pathological response in the lung and pleura of brake dust alone and in combination with added chrysotile compared to crocidolite asbestos following short-term inhalation exposure. Toxicol. Appl. Pharmacol. 2015, 283, 20–34. [Google Scholar] [CrossRef]
- Bernstein, D.M.; Rogers, R.; Smith, P. The biopersistence of brazilian chrysotile asbestos following inhalation. Inhal. Toxicol. 2004, 16, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M.; Rogers, R.; Smith, P. The biopersistence of Canadian chrysotile asbestos following inhalation. Inhal. Toxicol. 2003, 15, 1247–1274. [Google Scholar] [CrossRef]
- Bernstein, D.M.; Chevalier, J.; Smith, P. Comparison of Calidria chrysotile asbestos to pure tremolite: Final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal. Toxicol. 2005, 17, 427–449. [Google Scholar] [CrossRef]
- Bernstein, D.; Rogers, R.; Smith, P. The biopersistence of Canadian chrysotile asbestos following inhalation: Final results through 1 year after cessation of exposure. Inhal. Toxicol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Koerten, H.K.; Brederoo, P.; Ginsel, L.A.; Daems, W.T. The endocytosis of asbestos by mouse peritoneal macrophages and its long-term effect on iron accumulation and labyrinth formation. Eur. J. Cell Biol. 1986, 40, 25–36. [Google Scholar]
- Breuer, W.; Epsztejn, S.; Cabantchik, Z.I. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 1995, 270, 24209–24215. [Google Scholar] [CrossRef]

| Actinolite | Ca2(Mg,Fe2+)5Si8O22(OH)2 |
| Amosite | (Mg,Fe2+)7Si8O22(OH)2 |
| Anthophyllite | Mg7Si8O22(OH)2 |
| Crocidolite | Na2Fe32+Fe23+Si8O22(OH)2 |
| Tremolite | Ca2Mg5Si8O22(OH)2 |
| Chrysotile | Mg3Si2O5(OH)4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghio, A.J.; Stewart, M.; Sangani, R.G.; Pavlisko, E.N.; Roggli, V.L. Asbestos and Iron. Int. J. Mol. Sci. 2023, 24, 12390. https://doi.org/10.3390/ijms241512390
Ghio AJ, Stewart M, Sangani RG, Pavlisko EN, Roggli VL. Asbestos and Iron. International Journal of Molecular Sciences. 2023; 24(15):12390. https://doi.org/10.3390/ijms241512390
Chicago/Turabian StyleGhio, Andrew J., Matthew Stewart, Rahul G. Sangani, Elizabeth N. Pavlisko, and Victor L. Roggli. 2023. "Asbestos and Iron" International Journal of Molecular Sciences 24, no. 15: 12390. https://doi.org/10.3390/ijms241512390
APA StyleGhio, A. J., Stewart, M., Sangani, R. G., Pavlisko, E. N., & Roggli, V. L. (2023). Asbestos and Iron. International Journal of Molecular Sciences, 24(15), 12390. https://doi.org/10.3390/ijms241512390







