Bioengineered Kidney Tubules Efficiently Clear Uremic Toxins in Experimental Dialysis Conditions
Abstract
:1. Introduction
2. Results
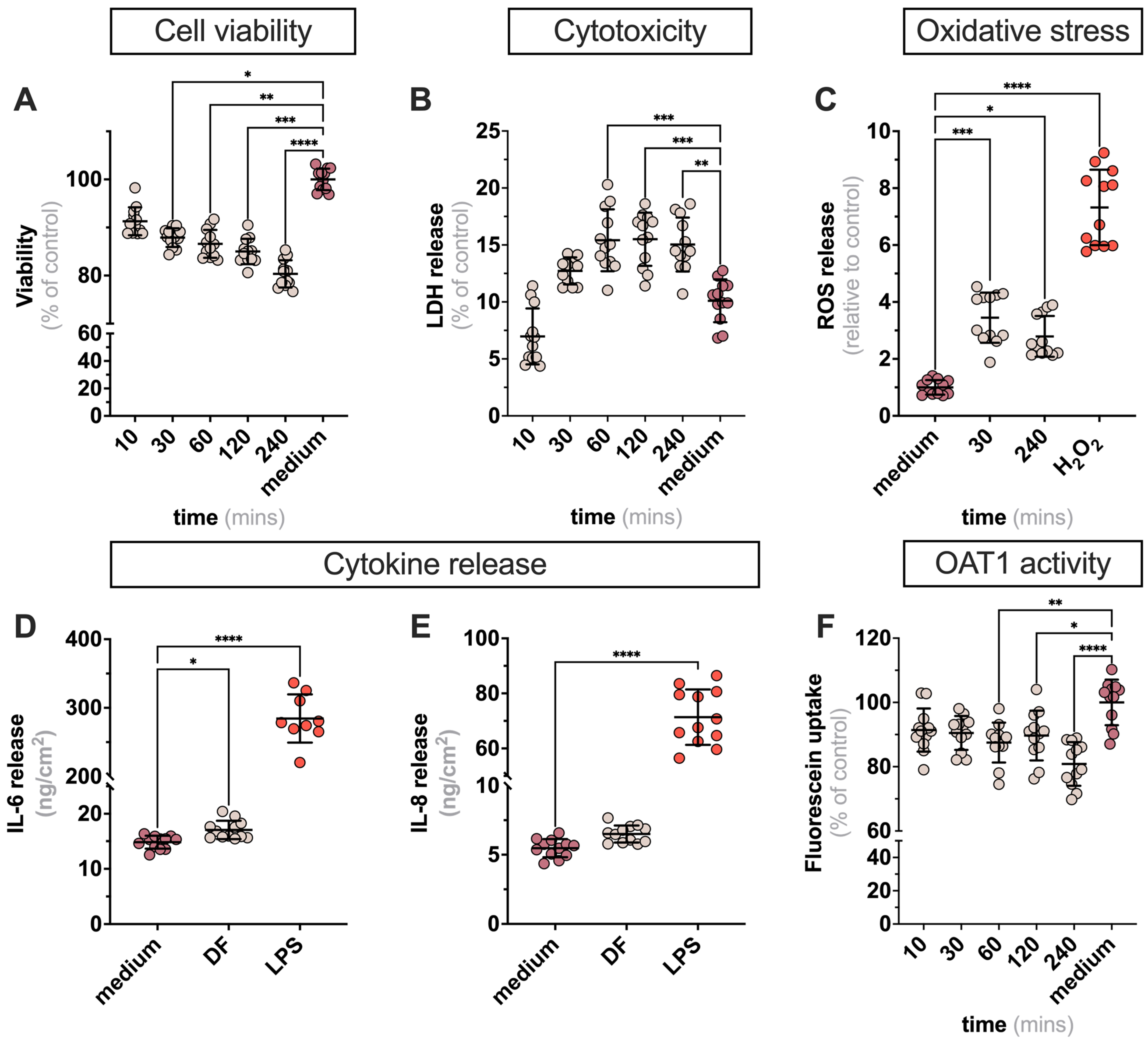
2.1. Exposure of ciPTECs to DF in 2D Leads to Minor Alteration in Cell Viability Markers
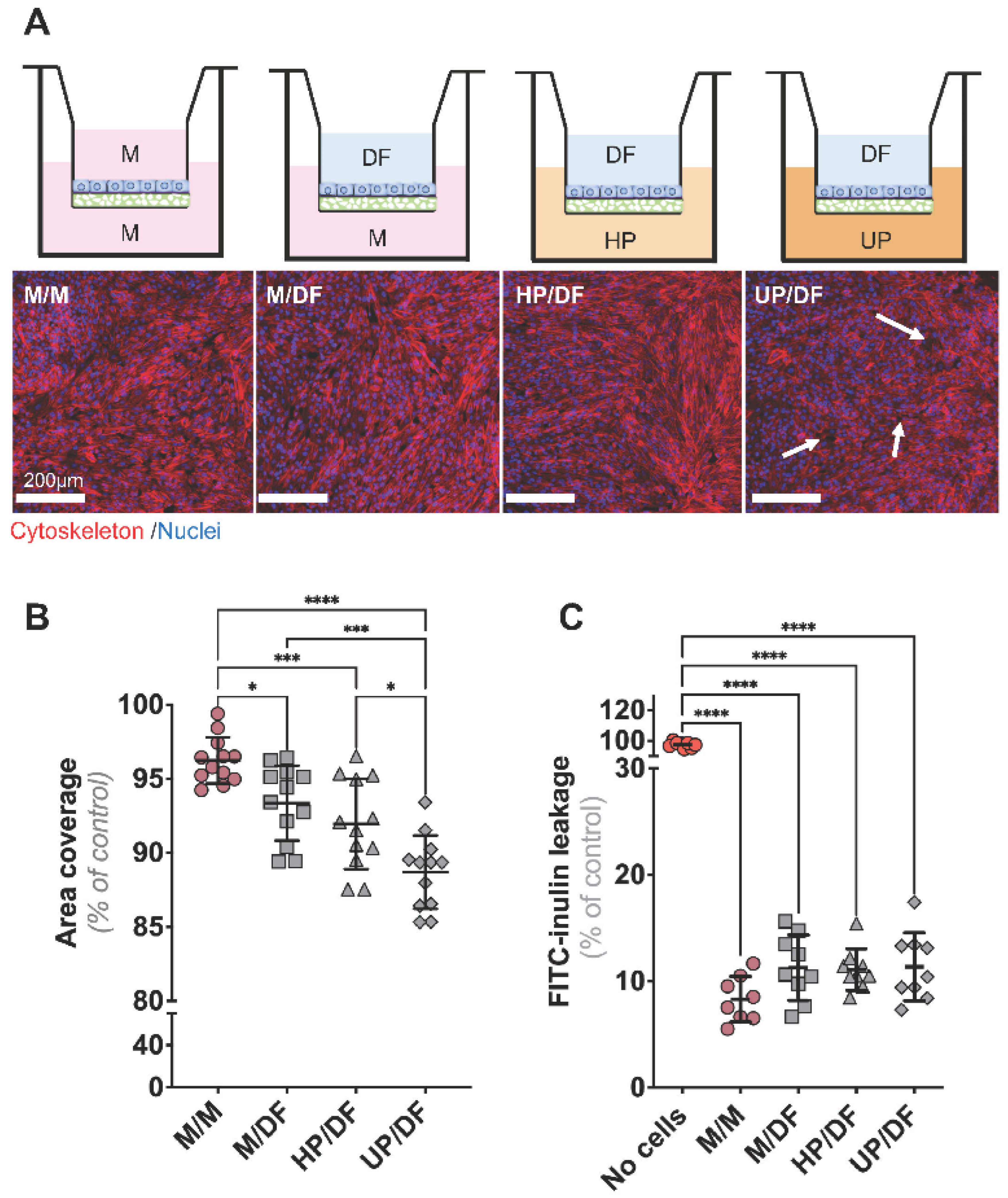
2.2. CiPTECs-OAT1 Cultured on Flat Membranes Show Minor Changes in Barrier Integrity upon Exposure to DF (Apically) and Plasma (Basolaterally)
2.3. CiPTECs-Seeded HFMs Effectively Secreted PBUTs when Exposed to Plasma
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. CiPTECs-OAT1
4.3. Evaluation of the Effects of DF on Flat Cultures of ciPTECs-OAT1
4.3.1. Two-Dimensional Exposure of Cells to DF
4.3.2. Cell Viability Assay
4.3.3. Intracellular Reactive Oxygen Species (ROS) Detection
4.3.4. Lactate Dehydrogenase (LDH) Activity
4.3.5. IL-6 and IL-8 Release
4.3.6. OAT1-Mediated Fluorescein Uptake
4.4. Combinatorial Effect of DF and Plasma on ciPTECs-OAT1 Seeded onto Transwell® Inserts
4.4.1. CiPTECs-OAT1 Culture on Adapted Transwell® Inserts
4.4.2. Exposure to Plasma and Assessment of Monolayer Permeability
4.4.3. Immunocytochemistry
4.5. Assessment of Transepithelial Transport of PBUTs on Bioengineered Kidney Tubules
4.5.1. Culture of ciPTECs-OAT1-Seeded HFM, Perfusion with Plasma, and PBUTs Quantification
4.5.2. Assessment of Monolayer Integrity following Perfusion with Plasma
4.5.3. Evaluation of Albumin Leakage
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nigam, S.K.; Bush, K.T. Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat. Rev. Nephrol. 2019, 15, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masereeuw, R.; Mutsaers, H.A.; Toyohara, T.; Abe, T.; Jhawar, S.; Sweet, D.H.; Lowenstein, J. The kidney and uremic toxin removal: Glomerulus or tubule? Semin. Nephrol. 2014, 34, 191–208. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Bush, K.T.; Nigam, S.K. Key Role for the Organic Anion Transporters, OAT1 and OAT3, in the in vivo Handling of Uremic Toxins and Solutes. Sci. Rep. 2017, 7, 4939. [Google Scholar] [CrossRef] [Green Version]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Nieskens, T.T.; Peters, J.G.; Schreurs, M.J.; Smits, N.; Woestenenk, R.; Jansen, K.; van der Made, T.K.; Röring, M.; Hilgendorf, C.; Wilmer, M.J.; et al. A Human Renal Proximal Tubule Cell Line with Stable Organic Anion Transporter 1 and 3 Expression Predictive for Antiviral-Induced Toxicity. AAPS J. 2016, 18, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Ramada, D.L.; de Vries, J.; Vollenbroek, J.; Noor, N.; Ter Beek, O.; Mihăilă, S.M.; Wieringa, F.; Masereeuw, R.; Gerritsen, K.; Stamatialis, D. Portable, wearable and implantable artificial kidney systems: Needs, opportunities and challenges. Nat. Rev. Nephrol. 2023, 19, 481–490. [Google Scholar] [CrossRef]
- McGill, R.L.; Weiner, D.E. Dialysate Composition for Hemodialysis: Changes and Changing Risk. Semin. Dial. 2017, 30, 112–120. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009; p. 34.
- Nieskens, T.T.G.; Peters, J.G.P.; Dabaghie, D.; Korte, D.; Jansen, K.; Van Asbeck, A.H.; Tavraz, N.N.; Friedrich, T.; Russel, F.G.M.; Masereeuw, R.; et al. Expression of Organic Anion Transporter 1 or 3 in Human Kidney Proximal Tubule Cells Reduces Cisplatin Sensitivity. Drug Metab. Dispos. 2018, 46, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.; Fedecostante, M.; Wilmer, M.J.; Peters, J.G.; Kreuser, U.M.; van den Broek, P.H.; Mensink, R.A.; Boltje, T.J.; Stamatialis, D.; Wetzels, J.F.; et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep. 2016, 6, 26715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaila, S.M.; Faria, J.; Stefens, M.F.J.; Stamatialis, D.; Verhaar, M.C.; Gerritsen, K.G.F.; Masereeuw, R. Drugs Commonly Applied to Kidney Patients May Compromise Renal Tubular Uremic Toxins Excretion. Toxins 2020, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- van der Made, T.K.; Fedecostante, M.; Scotcher, D.; Rostami-Hodjegan, A.; Sastre Toraño, J.; Middel, I.; Koster, A.S.; Gerritsen, K.G.; Jankowski, V.; Jankowski, J.; et al. Quantitative Translation of Microfluidic Transporter in Vitro Data to in Vivo Reveals Impaired Albumin-Facilitated Indoxyl Sulfate Secretion in Chronic Kidney Disease. Mol. Pharm. 2019, 16, 4551–4562. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; Buffington, D.; Westover, A.J.; Roy, S.; Fissell, W.H. The bioartificial kidney: Current status and future promise. Pediatr Nephrol. 2014, 29, 343–351. [Google Scholar] [CrossRef]
- Masereeuw, R.; Stamatialis, D. Creating a bioartificial kidney. Int. J. Artif. Organs 2017, 40, 323–327. [Google Scholar] [CrossRef]
- van Gelder, M.K.; Mihaila, S.M.; Jansen, J.; Wester, M.; Verhaar, M.C.; Joles, J.A.; Stamatialis, D.; Masereeuw, R.; Gerritsen, K. From portable dialysis to a bioengineered kidney. Expert Rev. Med. Devices 2018, 15, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Richards, R.H.; Dowling, J.A.; Vreman, H.J.; Feldman, C.; Weiner, M.W. Acetate levels in human plasma. Proc. Clin. Dial. Transpl. Forum 1976, 6, 73–79. [Google Scholar]
- Coll, E.; Pérez-García, R.; Rodríguez-Benítez, P.; Ortega, M.; Martínez Miguel, P.; Jofré, R.; López-Gómez, J.M. Clinical and analytical changes in hemodialysis without acetate. Nefrologia 2007, 27, 742–748. [Google Scholar]
- Ward, R.A.; Wathen, R.L.; Williams, T.E.; Harding, G.B. Hemodialysate composition and intradialytic metabolic, acid-base and potassium changes. Kidney Int. 1987, 32, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Goshtasbi, N.; Yuan, J.; Jellbauer, S.; Moradi, H.; Raffatellu, M.; Kalantar-Zadeh, K. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am. J. Nephrol. 2012, 36, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Mihajlovic, M.; Janssen, M.J.; Masereeuw, R. The Uremic Toxin Indoxyl Sulfate Accelerates Senescence in Kidney Proximal Tubule Cells. Toxins 2023, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Krebber, M.M.; Yang, Y.; Ahmed, S.; Lozovanu, V.; Andreeva, D.; Verhaar, M.C.; Masereeuw, R. Protein-Bound Uremic Toxins Induce Reactive Oxygen Species-Dependent and Inflammasome-Mediated IL-1beta Production in Kidney Proximal Tubule Cells. Biomedicines 2021, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Besseghir, K.; Mosig, D.; Roch-Ramel, F. Facilitation by serum albumin of renal tubular secretion of organic anions. Am. J. Physiol. 1989, 256 Pt 2, F475–F484. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Kim, S.J.; Lee, W.; Sugiyama, Y. Consideration of albumin-mediated hepatic uptake for highly protein-bound anionic drugs: Bridging the gap of hepatic uptake clearance between in vitro and in vivo. Pharmacol. Ther. 2022, 229, 107938. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Haddad, S. Albumin and Uptake of Drugs in Cells: Additional Validation Exercises of a Recently Published Equation that Quantifies the Albumin-Facilitated Uptake Mechanism(s) in Physiologically Based Pharmacokinetic and Pharmacodynamic Modeling Research. J. Pharm. Sci. 2015, 104, 4448–4458. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, M.K.; Middel, I.R.; Vernooij, R.W.M.; Bots, M.L.; Verhaar, M.C.; Masereeuw, R.; Grooteman, M.P.; Nubé, M.J.; van den Dorpel, M.A.; Blankestijn, P.J.; et al. Protein-Bound Uremic Toxins in Hemodialysis Patients Relate to Residual Kidney Function, Are Not Influenced by Convective Transport, and Do Not Relate to Outcome. Toxins 2020, 1, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, L.L.; O’Brien, F.J.; Sirich, T.L.; Plummer, N.S.; Sheth, R.; Fajardo, C.; Brakeman, P.; Sutherland, S.M.; Meyer, T.W. Association of Plasma Uremic Solute Levels with Residual Kidney Function in Children on Peritoneal Dialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1531–1538. [Google Scholar] [CrossRef]
- Nigam, S.K.; Wu, W.; Bush, K.T.; Hoenig, M.P.; Blantz, R.C.; Bhatnagar, V. Handling of Drugs, Metabolites, and Uremic Toxins by Kidney Proximal Tubule Drug Transporters. Clin. J. Am. Soc. Nephrol. 2015, 10, 2039–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. European Uremic Toxin Work Group Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.; Jansen, K.; Neven, E.; Poesen, R.; Othman, A.; van Mil, A.; Sluijter, J.; Sastre Torano, J.; Zaal, E.A.; Berkers, C.R.; et al. Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion. Proc. Natl. Acad. Sci. USA 2019, 116, 16105–16110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigam, S.K.; Granados, J.C. OAT, OATP, and MRP Drug Transporters and the Remote Sensing and Signaling Theory. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 637–660. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Nigam, S.K.; Burtey, S.; Glorieux, G. What If Not All Metabolites from the Uremic Toxin Generating Pathways Are Toxic? A Hypothesis. Toxins 2022, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ficociello, L.H.; Bazzanella, J.; Mullon, C.; Anger, M.S. Slipping Through the Pores: Hypoalbuminemia and Albumin Loss During Hemodialysis. Int. J. Nephrol. Renov. Dis. 2021, 14, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef]
- Ji, H.; Xu, H.; Jin, L.; Song, X.; He, C.; Liu, X.; Xiong, L.; Zhao, W.; Zhao, C. Surface engineering of low-fouling and hemocompatible polyethersulfone membranes via in-situ ring-opening reaction. J. Membr. Sci. 2019, 581, 373–382. [Google Scholar] [CrossRef]
- Ross, E.J.; Gordon, E.R.; Sothers, H.; Darji, R.; Baron, O.; Haithcock, D.; Prabhakarpandian, B.; Pant, K.; Myers, R.M.; Cooper, S.J.; et al. Three dimensional modeling of biologically relevant fluid shear stress in human renal tubule cells mimics in vivo transcriptional profiles. Sci. Rep. 2021, 11, 14053. [Google Scholar] [CrossRef]
- Vriend, J.; Peters, J.G.P.; Nieskens, T.T.G.; Škovroňová, R.; Blaimschein, N.; Schmidts, M.; Roepman, R.; Schirris, T.J.J.; Russel, F.G.M.; Masereeuw, R.; et al. Flow stimulates drug transport in a human kidney proximal tubule-on-a-chip independent of primary cilia. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129433. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; De Napoli, I.E.; Fedecostante, M.; Schophuizen, C.M.; Chevtchik, N.V.; Wilmer, M.J.; van Asbeck, A.H.; Croes, H.J.; Pertijs, J.C.; Wetzels, J.F.; et al. Human proximal tubule epithelial cells cultured on hollow fibers: Living membranes that actively transport organic cations. Sci. Rep. 2015, 5, 16702. [Google Scholar] [CrossRef] [Green Version]
- Oo, Z.Y.; Kandasamy, K.; Tasnim, F.; Zink, D. A novel design of bioartificial kidneys with improved cell performance and haemocompatibility. J. Cell Mol. Med. 2013, 17, 497–507. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, H.; Wang, Y.; Shen, Y.; Zhu, Q.; Ding, F. Improved Dialysis Removal of Protein-Bound Uraemic Toxins with a Combined Displacement and Adsorption Technique. Blood Purif 2022, 51, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Luo, F.J.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Selectively increasing the clearance of protein-bound uremic solutes. Nephrol. Dial. Transplant. 2012, 27, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Refoyo, R.; Skouras, E.D.; Chectchik, N.V.; Stamatialis, D.; Burganos, V.N. Transport and reaction phenomena in multilayer membranes functioning as bioartificial kidney devices. J. Membr. Sci. 2018, 565, 61–71. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Li Jeon, N.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Silverio, V.; Guha, S.; Keiser, A.; Natu, R.; Reyes, D.R.; van Heeren, H.; Verplanck, N.; Herbertson, L.H. Overcoming technological barriers in microfluidics: Leakage testing. Front. Bioeng. Biotechnol. 2022, 10, 958582. [Google Scholar] [CrossRef]
- Mihajlovic, M.; van den Heuvel, L.P.; Hoenderop, J.G.; Jansen, J.; Wilmer, M.J.; Westheim, A.J.F.; Allebes, W.A.; Stamatialis, D.; Hilbrands, L.B.; Masereeuw, R. Allostimulatory capacity of conditionally immortalized proximal tubule cell lines for bioartificial kidney application. Sci. Rep. 2017, 7, 7103. [Google Scholar] [CrossRef] [Green Version]
- Mutsaers, H.A.; van den Heuvel, L.P.; Ringens, L.H.; Dankers, A.C.; Russel, F.G.; Wetzels, J.F.; Hoenderop, J.G.; Masereeuw, R. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS ONE 2011, 6, e18438. [Google Scholar] [CrossRef] [Green Version]
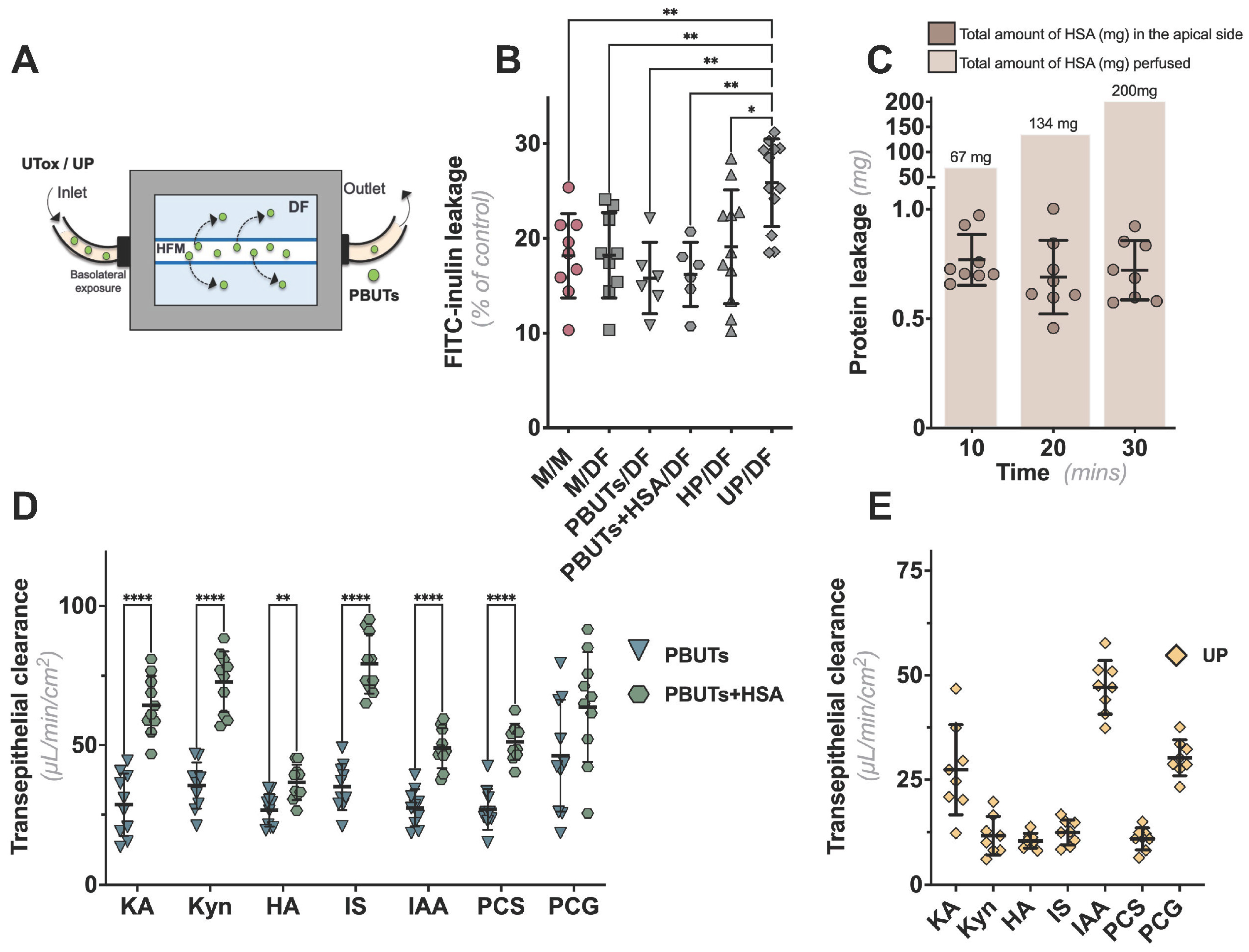
| PBUTs | Clearance Rate (μL/min/cm2) Mean ± SD | ||
|---|---|---|---|
| PBUT | PBUT + HSA | UP | |
| Kynurenic acid | 29 ± 11 | 64 ± 11 (****) | 27 ± 11 |
| Kynurenine | 38 ± 8 | 73 ± 11 (****) | 12 ± 5 |
| Hippuric acid | 27 ± 6 | 37 ± 6 (**) | 10 ± 2 |
| Indoxyl sulfate | 35 ± 8 | 79 ± 11 (****) | 12 ± 3 |
| Indole-3 acetic acid | 27 ± 7 | 49 ± 7 (****) | 47 ± 6 |
| p-cresyl sulfate | 27 ± 7 | 51 ± 7 (****) | 11 ± 3 |
| p-cresyl glucuronide | 46 ± 20 | 64 ± 20 | 30 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.; Ahmed, S.; Stamatialis, D.; Verhaar, M.C.; Masereeuw, R.; Gerritsen, K.G.F.; Mihăilă, S.M. Bioengineered Kidney Tubules Efficiently Clear Uremic Toxins in Experimental Dialysis Conditions. Int. J. Mol. Sci. 2023, 24, 12435. https://doi.org/10.3390/ijms241512435
Faria J, Ahmed S, Stamatialis D, Verhaar MC, Masereeuw R, Gerritsen KGF, Mihăilă SM. Bioengineered Kidney Tubules Efficiently Clear Uremic Toxins in Experimental Dialysis Conditions. International Journal of Molecular Sciences. 2023; 24(15):12435. https://doi.org/10.3390/ijms241512435
Chicago/Turabian StyleFaria, João, Sabbir Ahmed, Dimitrios Stamatialis, Marianne C. Verhaar, Rosalinde Masereeuw, Karin G. F. Gerritsen, and Silvia M. Mihăilă. 2023. "Bioengineered Kidney Tubules Efficiently Clear Uremic Toxins in Experimental Dialysis Conditions" International Journal of Molecular Sciences 24, no. 15: 12435. https://doi.org/10.3390/ijms241512435





