Lipid Polyunsaturated Fatty Acid Chains in Mouse Kidneys Were Increased within 5 min of a Single High Dose Whole Body Irradiation
Abstract
1. Introduction
2. Results
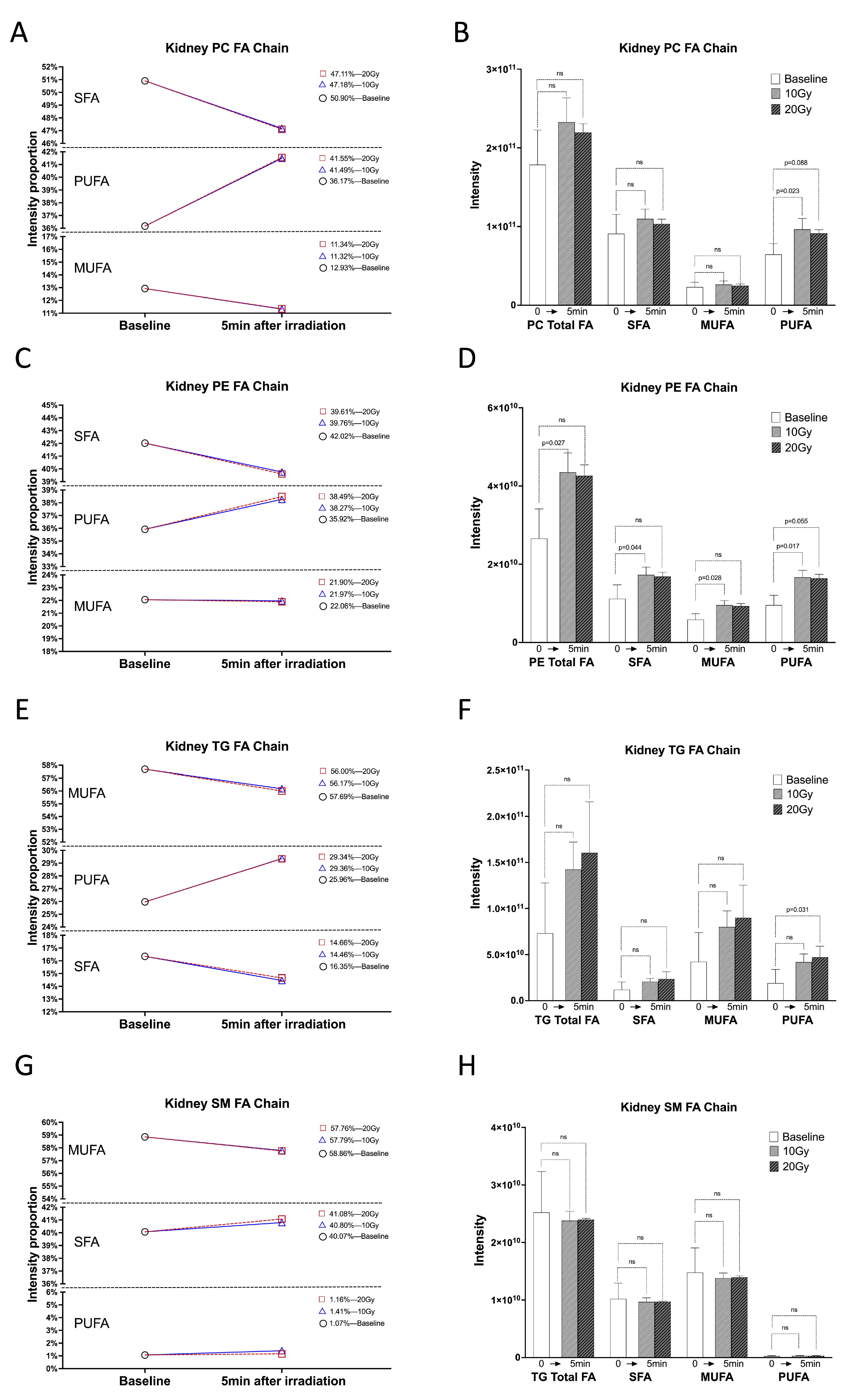
2.1. Within 5 min of Irradiation, the PUFA Chain of Kidney Lipids Significantly Increased
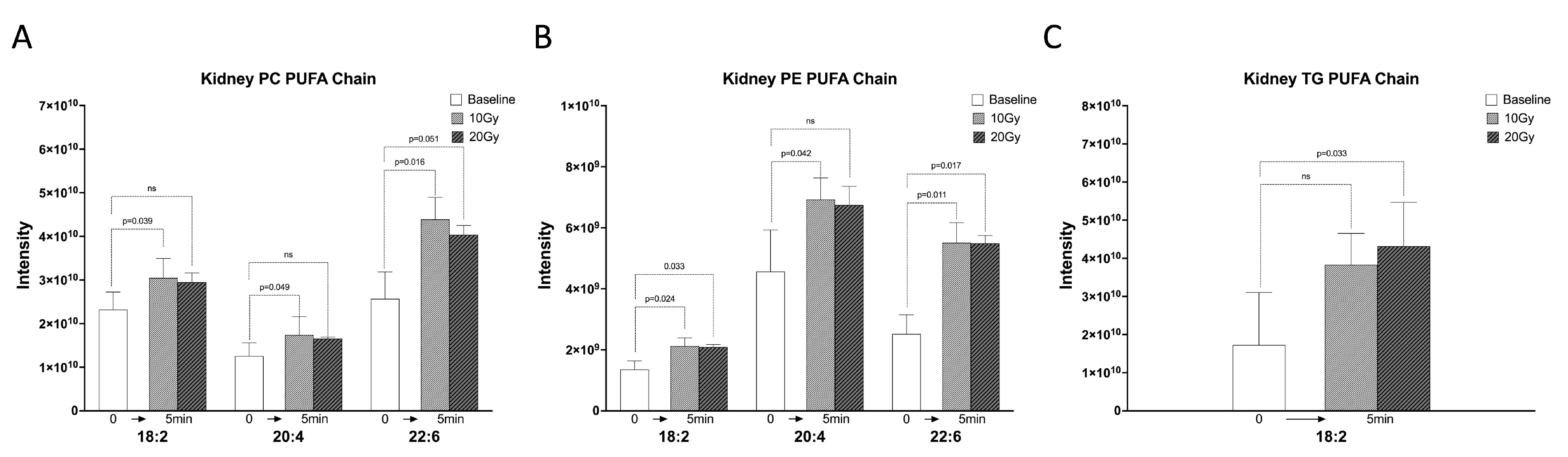
2.2. The C18:2, C20:4, and C22:6 FA Chains of Kidney Lipids Were Significantly Increased within 5 min of Irradiation
2.3. Significant Increase in Kidney Lipidomes within 5 min of Irradiation
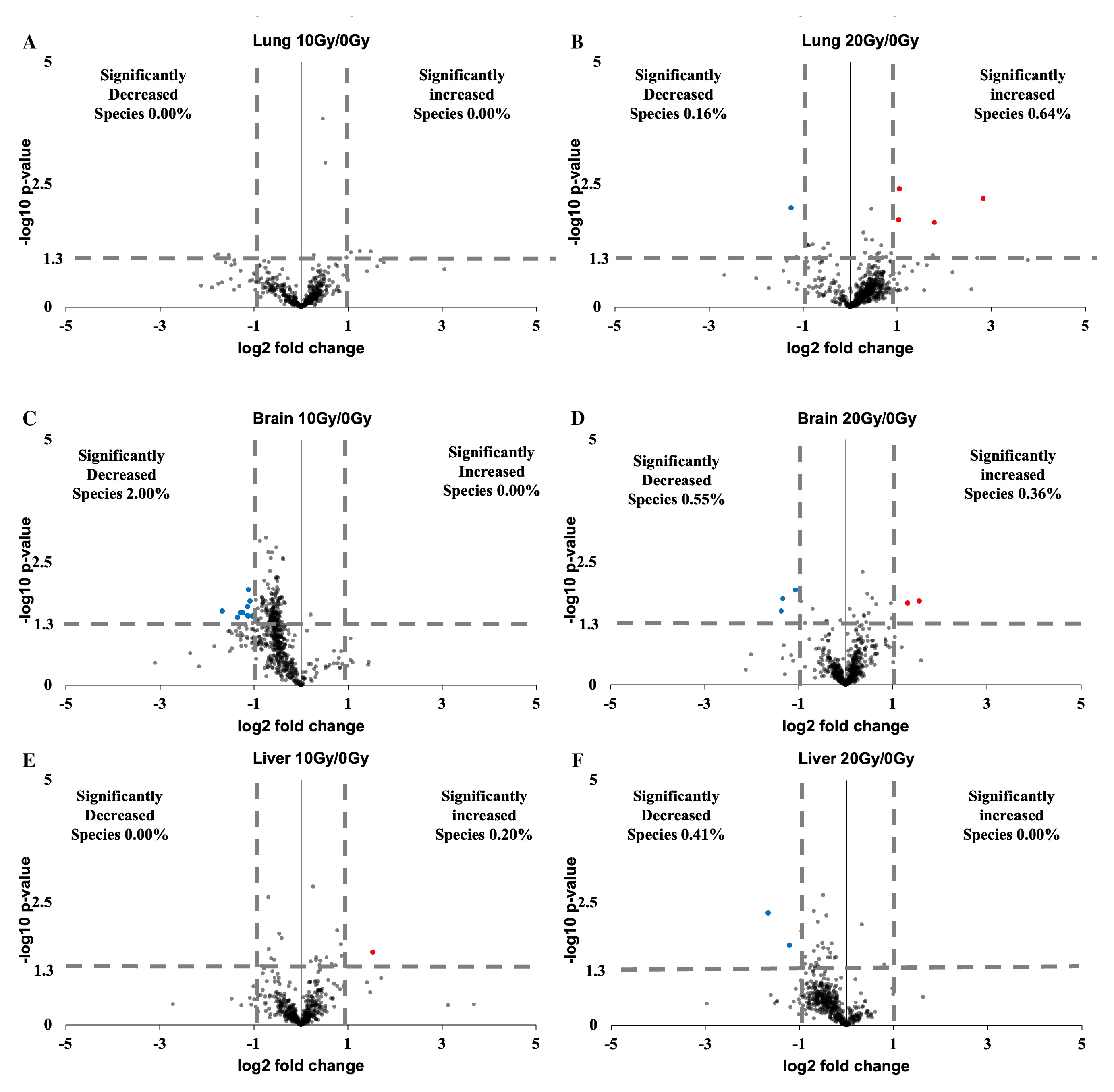
2.4. Lung, Brain, and Liver Showed Slight Lipidomic Alteration without Significant PUFA Change
3. Discussion
4. Materials and Methods
4.1. Animals Furthermore, X-ray Irradiation
4.2. Lipid Extraction
4.3. LC-MS and LC-MS/MS of Kidney, Lung, Brain, and Liver Lipids
4.4. Lipid Identification Furthermore, Quantification
4.5. Data Processing
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yap, M.L.; Zubizarreta, E.; Bray, F.; Ferlay, J.; Barton, M. Global Access to Radiotherapy Services: Have We Made Progress During the Past Decade? J. Glob. Oncol. 2016, 2, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Aili, A.; Xue, L.; Jiang, P.; Wang, J. Advances in Radiobiology of Stereotactic Ablative Radiotherapy. Front. Oncol. 2020, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Folkert, M.R.; Timmerman, R.D. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv. Drug Deliv. Rev. 2017, 109, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Nahum, A.E. The radiobiology of hypofractionation. Clin. Oncol. (R. Coll. Radiol. (Great Br.)) 2015, 27, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Wenk, M.R. Lipidomics: New tools and applications. Cell 2010, 143, 888–895. [Google Scholar] [CrossRef]
- Köfeler, H.C.; Fauland, A.; Rechberger, G.N.; Trötzmüller, M. Mass Spectrometry Based Lipidomics: An Overview of Technological 391 Platforms. Metabolites 2012, 2, 19–38. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, Q.; Halim, A.; Song, G. Targeting lipid metabolism of cancer cells: A promising therapeutic strategy for cancer. Cancer Lett. 2017, 401, 39–45. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef]
- Nishizawa, H.; Matsumoto, M.; Chen, G.; Ishii, Y.; Tada, K.; Onodera, M.; Kato, H.; Muto, A.; Tanaka, K.; Igarashi, K. Lipid peroxidation and the subsequent cell death transmitting from ferroptotic cells to neighboring cells. Cell Death Dis. 2021, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Wiernicki, B.; Dubois, H.; Tyurina, Y.Y.; Hassannia, B.; Bayir, H.; Kagan, V.E.; Vandenabeele, P.; Wullaert, A.; Berghe, T.V. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020, 11, 922. [Google Scholar] [CrossRef]
- Benderitter, M.; Vincent-Genod, L.; Pouget, J.P.; Voisin, P. The cell membrane as a biosensor of oxidative stress induced by radiation exposure: A multiparameter investigation. Radiat. Res. 2003, 159, 471–483. [Google Scholar] [CrossRef]
- Li, J.; Ren, S.; Piao, H.L.; Wang, F.; Yin, P.; Xu, C.; Lu, X.; Ye, G.; Shao, Y.; Yan, M.; et al. Integration of lipidomics and transcriptomics unravels aberrant lipid metabolism and defines cholesteryl oleate as potential biomarker of prostate cancer. Sci. Rep. 2016, 6, 20984. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Liu, T.; Wang, H.; Xue, L.X.; Wang, J.J. Fatty Acids Metabolism: The Bridge Between Ferroptosis and Ionizing Radiation. Front. Cell Dev. Biol. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Cheng, J.X. Direct Visualization of De novo Lipogenesis in Single Living Cells. Sci. Rep. 2014, 4, 6807. [Google Scholar] [CrossRef]
- O’Donnell, V.B. New appreciation for an old pathway: The Lands Cycle moves into new arenas in health and disease. Biochem. Soc. Trans. 2022, 50, 1–11. [Google Scholar] [CrossRef]
- Higgins, A.J.; Lees, P. The acute inflammatory process, arachidonic acid metabolism and the mode of action of anti-inflammatory drugs. Equine Vet. J. 1984, 16, 163–175. [Google Scholar] [CrossRef]
- Takeda, R.; Islam, A.; Sato, T.; Kurita, H.; Kahyo, T.; Urano, T.; Setou, M. The stability of the metabolic turnover of arachidonic acid in human unruptured intracranial aneurysmal walls is sustained. Clin. Neurol. Neurosurg. 2021, 208, 106881. [Google Scholar] [CrossRef]
- Kehwar, T. Analytical approach to estimate normal tissue complication probability using best fit of normal tissue tolerance doses into the NTCP equation of the linear quadratic model. J. Cancer Res. Ther. 2005, 1, 168. [Google Scholar] [CrossRef]
- Scheenstra, A.E.; Rossi, M.M.; Belderbos, J.S.; Damen, E.M.; Lebesque, J.V.; Sonke, J.J. Alpha/Beta Ratio for Normal Lung Tissue as Estimated From Lung Cancer Patients Treated With Stereotactic Body and Conventionally Fractionated Radiation Therapy. Int. J. Radiat. Oncol. 2014, 88, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, R.; Ishikawa, M. Precise and global identification of phospholipid molecular species by an Orbitrap mass spectrometer and automated search engine Lipid Search. J. Chromatogr. A 2010, 1217, 4229–4239. [Google Scholar] [CrossRef]
- Peake, D.; Kiyonami, R.; Yokoi, Y.; Fukamachi, Y.; Huang, Y. Processing of a Complex Lipid Dataset for the NIST Inter-laboratory Comparison Exercise for Lipidomics Measurements in Human Serum and Plasma. In Proceedings of the LIPID MAPS Annual Meeting, Saint Louis, MO, USA, 31 May–4 June 2015; pp. 5–7. [Google Scholar]
- Taguchi, R.; Nishijima, M.; Shimizu, T. Basic Analytical Systems for Lipidomics by Mass Spectrometry in Japan. Methods Enzymol. 2007, 432, 185–211. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, C.; Aramaki, S.; Xu, L.; Tsuge, S.; Sakamoto, T.; Mamun, M.A.; Islam, A.; Hayakawa, T.; Takanashi, Y.; et al. Lipid Polyunsaturated Fatty Acid Chains in Mouse Kidneys Were Increased within 5 min of a Single High Dose Whole Body Irradiation. Int. J. Mol. Sci. 2023, 24, 12439. https://doi.org/10.3390/ijms241512439
Li W, Zhang C, Aramaki S, Xu L, Tsuge S, Sakamoto T, Mamun MA, Islam A, Hayakawa T, Takanashi Y, et al. Lipid Polyunsaturated Fatty Acid Chains in Mouse Kidneys Were Increased within 5 min of a Single High Dose Whole Body Irradiation. International Journal of Molecular Sciences. 2023; 24(15):12439. https://doi.org/10.3390/ijms241512439
Chicago/Turabian StyleLi, Wenxin, Chi Zhang, Shuhei Aramaki, Lili Xu, Shogo Tsuge, Takumi Sakamoto, Md. Al Mamun, Ariful Islam, Takamitsu Hayakawa, Yusuke Takanashi, and et al. 2023. "Lipid Polyunsaturated Fatty Acid Chains in Mouse Kidneys Were Increased within 5 min of a Single High Dose Whole Body Irradiation" International Journal of Molecular Sciences 24, no. 15: 12439. https://doi.org/10.3390/ijms241512439
APA StyleLi, W., Zhang, C., Aramaki, S., Xu, L., Tsuge, S., Sakamoto, T., Mamun, M. A., Islam, A., Hayakawa, T., Takanashi, Y., Dubail, M., Konishi, K., Sato, T., Kahyo, T., Fouillade, C., Nakamura, K., & Setou, M. (2023). Lipid Polyunsaturated Fatty Acid Chains in Mouse Kidneys Were Increased within 5 min of a Single High Dose Whole Body Irradiation. International Journal of Molecular Sciences, 24(15), 12439. https://doi.org/10.3390/ijms241512439







