Management and Mitigation of Vibriosis in Aquaculture: Nanoparticles as Promising Alternatives
Abstract
:1. Introduction
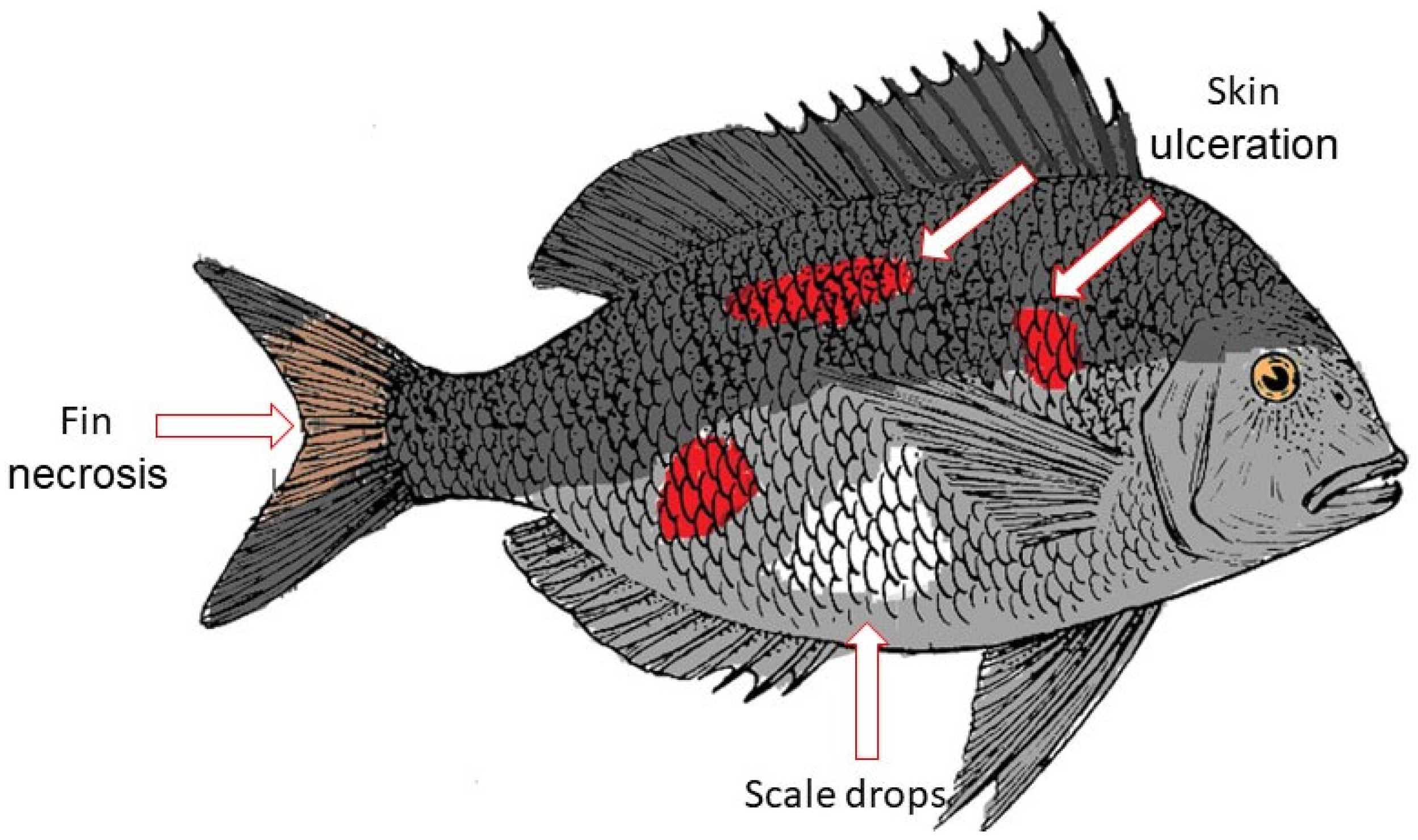
2. Vibriosis
Characteristics of Vibrio Spp.
3. Treatment against Vibriosis and Its Limitation
3.1. Antibiotics
3.2. Vaccination
3.3. Probiotics
3.4. Phytotherapy
4. Nanoparticles
4.1. Nanoparticles in Aquaculture
4.1.1. Silver Nanoparticles
4.1.2. Gold Nanoparticles
4.1.3. Other Types of Nanoparticles
5. Graphene Oxide (GO)
5.1. Application of Graphene Oxide in Aquaculture
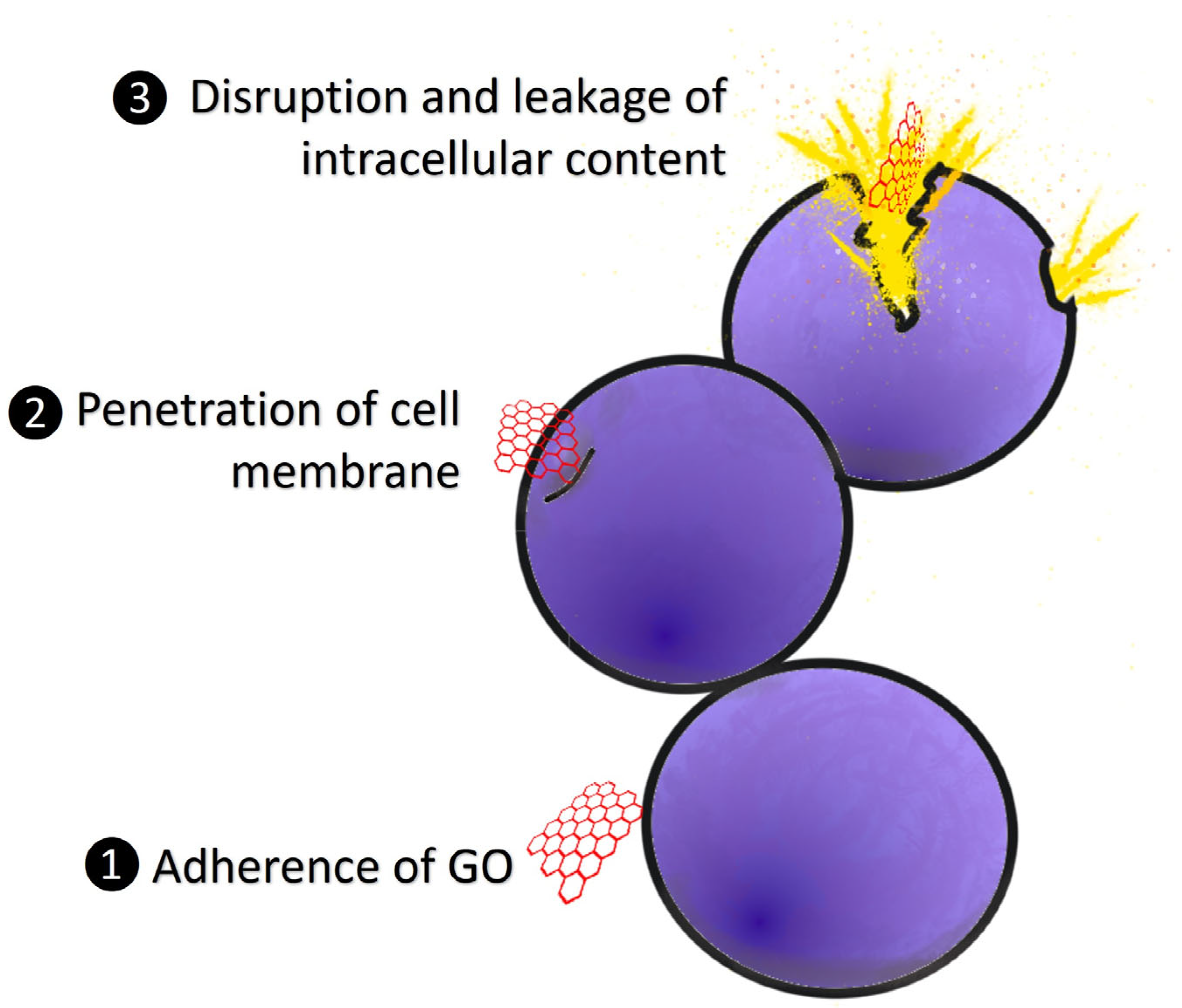
5.2. Antibacterial Effects of Graphene Oxide against Vibrio Spp.
5.3. Challenges and Future Applications of Graphene Oxide in Aquaculture
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanches-Fernandes, G.M.M.; Sá-Correia, I.; Costa, R. Vibriosis Outbreaks in Aquaculture: Addressing Environmental and Public Health Concerns and Preventive Therapies Using Gilthead Seabream Farming as a Model System. Front. Microbiol. 2022, 13, 904815. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yazid, S.H.; Mohd Daud, H.; Azmai, M.N.A.; Mohamad, N.; Mohd Nor, N. Estimating the Economic Loss Due to Vibriosis in Net-Cage Cultured Asian Seabass (Lates calcarifer): Evidence from the East Coast of Peninsular Malaysia. Front. Vet. Sci. 2021, 8, 644009. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, S.; Behera, B.K.; Bossier, P.; Das, B.K. Acute Hepatopancreatic Necrosis Disease (AHPND): Virulence, Pathogenesis and Mitigation Strategies in Shrimp Aquaculture. Toxins 2021, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Chiew, I.K.M.; Salter, A.M.; Lim, Y.S. The significance of major viral and bacterial diseases in Malaysian aquaculture industry. Pertanika J. Trop. Agric. Sci. 2019, 42, 1023–1047. [Google Scholar]
- Mohamad, N.; Amal, M.N.A.; Yasin, I.S.M.; Zamri Saad, M.; Nasruddin, N.S.; Al-saari, N.; Mino, S.; Sawabe, T. Vibriosis in cultured marine fishes: A review. Aquaculture 2019, 512, 734289. [Google Scholar] [CrossRef]
- Istiqomah, I.; Sukardi; Murwantoko; Isnansetyo, A. Review Vibriosis Management in Indonesian Marine Fish Farming. E3S Web Conf. 2020, 147, 01001. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Wang, Y.; Yang, W.; Cai, H.; Zhang, Y.; Huang, L. Strategies for Prevention and Control of Vibriosis in Asian Fish Culture. Vaccines 2023, 11, 98. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Raszl, S.M.; Froelich, B.A.; Vieira, C.R.W.; Blackwood, A.D.; Noble, R.T. Vibrio parahaemolyticus and Vibrio vulnificus in South America: Water, seafood and human infections. J. Appl. Microbiol. 2016, 121, 1201–1222. [Google Scholar] [CrossRef] [PubMed]
- de Souza Valente, C.; Wan, A.H.L. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef]
- Vezzulli, L.; Pezzati, E.; Brettar, I.; Höfle, M.; Pruzzo, C. Effects of Global Warming on Vibrio Ecology. Microbiol. Spectr. 2015, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Koralage, M.S.G.; Alter, T.; Pichpol, D.; Strauch, E.; Zessin, K.-H.; Huehn, S. Prevalence and Molecular Characteristics of Vibrio spp. Isolated from Preharvest Shrimp of the North Western Province of Sri Lanka. J. Food Prot. 2012, 75, 1846–1850. [Google Scholar] [CrossRef]
- Chien, J.; Shih, J.; Hsueh, P.; Yang, P.; Luh, K. Vibrio alginolyticus as the cause of pleural empyema and bacteremia in an immunocompromised patient. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 401–403. [Google Scholar] [CrossRef]
- Ruwandeepika, H.A.D.; Defoirdt, T.; Bhowmick, P.P.; Shekar, M.; Bossier, P.; Karunasagar, I. Presence of typical and atypical virulence genes in vibrio isolates belonging to the Harveyi clade. J. Appl. Microbiol. 2010, 109, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 1, 1–31. [Google Scholar] [CrossRef]
- El-Gohary, M.S.; El Gamal, A.M.; Atia, A.A.; El-Dakroury, M.F. Treatment trial of nile tilapia (Oreochromis niloticus) experimentally infected with vibrio alginolyticus isolated from sea bass (Dicentrarchus labrax). Pakistan J. Biol. Sci. 2020, 23, 1591–1600. [Google Scholar] [CrossRef]
- Kverme, K.O.; Kallekleiv, M.; Larsen, K.; Rønneseth, A.; Wergeland, H.I.; Samuelsen, O.B.; Haugland, G.T. Antibacterial treatment of lumpfish (Cyclopterus lumpus) experimentally challenged with Vibrio anguillarum, atypical Aeromonas salmonicida and Pasteurella atlantica. J. Fish Dis. 2022, 45, 153–163. [Google Scholar] [CrossRef]
- Huang, H.T.; Chang, J.J.; Lin, Y.R.; Chen, Y.Y.; Wu Chang, Y.H.; Chen, B.Y.; Nan, F.H. Synergistic effects of dietary oxolinic acid combined with oxytetracycline on nonspecific immune responses and resistance against Vibrio parahaemolyticus infection of white shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 740–747. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, V.; Yin, W.F.; Lee, L.H.; Chan, K.G. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 2015, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef] [PubMed]
- Amalina, N.Z.; Santha, S.; Zulperi, D.; Amal, M.N.A.; Yusof, M.T.; Zamri-Saad, M.; Ina-Salwany, M.Y. Prevalence, antimicrobial susceptibility and plasmid profiling of Vibrio spp. isolated from cultured groupers in Peninsular Malaysia. BMC Microbiol. 2019, 19, 251. [Google Scholar] [CrossRef] [Green Version]
- Mok, J.S.; Ryu, A.; Kwon, J.Y.; Park, K.; Shim, K.B. Abundance, antimicrobial resistance, and virulence of pathogenic Vibrio strains from molluscan shellfish farms along the Korean coast. Mar. Pollut. Bull. 2019, 149, 110559. [Google Scholar] [CrossRef]
- You, K.G.; Bong, C.W.; Lee, C.W. Antibiotic resistance and plasmid profiling of Vibrio spp. in tropical waters of Peninsular Malaysia. Environ. Monit. Assess. 2016, 188, 171. [Google Scholar] [CrossRef]
- Sudha, S.; Mridula, C.; Silvester, R.; Hatha, A.A.M. Prevalence and antibiotic resistance of pathogenic Vibrios in shellfishes from Cochin market. Indian J. Geo-Mar. Sci. 2014, 43, 815–824. [Google Scholar]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Malandra, R.; Panseri, S.; Arioli, F. Occurrence of antibiotics in mussels and clams from various FAO areas. Food Chem. 2018, 240, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, A.; Foushee, J.; Lantz, E.; Gumina, G. Sulfonamide Allergies. Pharmacy 2019, 7, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Wang, S.; Ma, J.; Liu, Q. A review: Progress in the development of fish Vibrio spp. vaccines. Immunol. Lett. 2020, 226, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.A.; Zamri-Saad, M.; Yasin, I.S.M.; Salleh, A.; Mustaffa-Kamal, F.; Matori, M.F.; Azmai, M.N.A. Efficacy of whole cell inactivated vibrio harveyi vaccine against vibriosis in a marine red hybrid tilapia (Oreochromis niloticus × o. mossambicus) model. Vaccines 2020, 8, 734. [Google Scholar] [CrossRef]
- Pang, H.; Qiu, M.; Zhao, J.; Hoare, R.; Monaghan, S.J.; Song, D.; Chang, Y.; Jian, J. Construction of a Vibrio alginolyticus hopPmaJ (hop) mutant and evaluation of its potential as a live attenuated vaccine in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2018, 76, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Delphino, M.K.V.C.; Barone, R.S.C.; Leal, C.A.G.; Figueiredo, H.C.P.; Gardner, I.A.; Gonçalves, V.S.P. Economic appraisal of vaccination against Streptoccocus agalactiae in Nile tilapia farms in Brazil. Prev. Vet. Med. 2019, 162, 131–135. [Google Scholar] [CrossRef]
- Yamasaki, M.; Araki, K.; Maruyoshi, K.; Matsumoto, M.; Nakayasu, C.; Moritomo, T.; Nakanishi, T.; Yamamoto, A. Comparative analysis of adaptive immune response after vaccine trials using live attenuated and formalin-killed cells of Edwardsiella tarda in ginbuna crucian carp (Carassius auratus langsdorfii). Fish Shellfish Immunol. 2015, 45, 437–442. [Google Scholar] [CrossRef]
- Miccoli, A.; Saraceni, P.R.; Scapigliati, G. Vaccines and immune protection of principal Mediterranean marine fish species. Fish Shellfish Immunol. 2019, 94, 800–809. [Google Scholar] [CrossRef]
- Amatul-Samahah, M.A.; Wan Omar, W.H.H.; Mohd Ikhsan, N.F.; Amal Azmai, M.N.; Zamri-Saad, M.; Ina-Salwany, M.Y. Vaccination trials against vibriosis in shrimp: A review. Aquac. Rep. 2020, 18, 100471. [Google Scholar] [CrossRef]
- Skjermo, J.; Vadstein, O. Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture 1999, 177, 333–343. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. The use of probiotics in aquaculture. Aquaculture 1999, 180, 147–165. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: A review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- El Euony, O.I.; Elblehi, S.S.; Abdel-Latif, H.M.; Abdel-Daim, M.M.; El-Sayed, Y.S. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus). Environ. Sci. Pollut. Res. 2020, 27, 23108–23128. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Khalil, R.H.; Nour, A.M.; Elkhayat, B.K.; Khalifa, E.; Abdel-Latif, H.M.R. Effects of Bacillus subtilis-fermented rice bran on water quality, performance, antioxidants/oxidants, and immunity biomarkers of White leg shrimp (Litopenaeus vannamei) reared at different salinities with zero water exchange. J. Appl. Aquac. 2022, 34, 332–357. [Google Scholar] [CrossRef]
- Bocamdé, T.J.; Marie, K.P.; François, Z.N.; Gondal, M.A.; Kausar, R. Improvement of the Growth Performance, Innate Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Vibrio parahaemolyticus 1T1 following Dietary Application of the Probiotic Strain Lactobacillus plantarum 1KMT. J. Adv. Biol. Biotechnol. 2020, 23, 27–39. [Google Scholar] [CrossRef]
- Geng, X.; Dong, X.H.; Tan, B.P.; Yang, Q.H.; Chi, S.Y.; Liu, H.Y.; Liu, X.Q. Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquac. Nutr. 2012, 18, 46–55. [Google Scholar] [CrossRef]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef]
- Aslam Hosain, M.; Liangyi, X. Impacts of probiotics on feeding technology and its application in aquaculture. SDRP J. Aquac. Fish. Fish Sci. 2020, 3, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Jeyavani, J.; Sibiya, A.; Sivakamavalli, J.; Divya, M.; Preetham, E.; Vaseeharan, B.; Faggio, C. Phytotherapy and combined nanoformulations as a promising disease management in aquaculture: A review. Aquac. Int. 2022, 30, 1071–1086. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Abbasi, Z.; Mohseni, M.; Amiri, E. Vibriosis phytotherapy: A review on the most important world medicinal plants effective on Vibrio spp. J. Appl. Pharm. Sci. 2018, 8, 170–177. [Google Scholar] [CrossRef]
- Karim, R.; Begum, M.M.; Jui, Y.; Islam, T.; Billah, M.; Arafat, Y.; Karim, M.; Khan, A.F.; Rahman, M.S. In-vitro cytotoxic and anti-Vibrio cholerae activities of alcoholic extracts of Desmodium triflorum (L.) whole plant and Terminalia citrina (Roxb.) fruits. Clin. Phytosci. 2021, 7, 36. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Dang, L.T.; Nguyen, H.T.; Hoang, H.H.; Lai, H.T.N.; Nguyen, H.T.T. Screening antibacterial effects of vietnamese plant extracts against pathogens caused acute hepatopancreatic necrosis disease in shrimps. Asian J. Pharm. Clin. Res. 2018, 11, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.M.; Murthy, A.V.S.G.; Choy, C.Y.; Lai, K.S. Phytotherapy in aquaculture: Integration of endogenous application with science. J. Environ. Biol. 2020, 41, 1204–1214. [Google Scholar] [CrossRef]
- Raman, R.P. Applicability, Feasibility and Efficacy of Phytotherapy in Aquatic Animal Health Management. Am. J. Plant Sci. 2017, 8, 257–287. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Loureiro, A.; Azoia, N.G.; Gomes, A.C.; Cavaco-Paulo, A. Albumin-Based Nanodevices as Drug Carriers. Curr. Pharm. Des. 2016, 22, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Khosravi-Katuli, K.; Prato, E.; Lofrano, G.; Guida, M.; Vale, G.; Libralato, G. Effects of nanoparticles in species of aquaculture interest. Environ. Sci. Pollut. Res. 2017, 24, 17326–17346. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Chakraborti, S.; Bera, S.; Sheikh, I.A.; Hoque, K.M.; Chakrabarti, P. The antimicrobial activity of ZnO nanoparticles against Vibrio cholerae: Variation in response depends on biotype. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y. Ben Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Baskaralingam, V.; Sargunar, C.G.; Lin, Y.C.; Chen, J.C. Green synthesis of Silver nanoparticles through Calotropis gigantea leaf extracts and evaluation of antibacterial activity against Vibrio alginolyticus. Nanotechnol. Dev. 2012, 2, 3. [Google Scholar] [CrossRef]
- Nafisi Bahabadi, M.; Hosseinpour Delavar, F.; Mirbakhsh, M.; Niknam, K.; Johari, S.A. Assessment of antibacterial activity of two different sizes of colloidal silver nanoparticle (cAgNPs) against Vibrio harveyi isolated from shrimp Litopenaeus vannamei. Aquac. Int. 2017, 25, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Huq, M.A. Biogenic Silver Nanoparticles Synthesized by Lysinibacillus xylanilyticus MAHUQ-40 to Control Antibiotic-Resistant Human Pathogens Vibrio parahaemolyticus and Salmonella Typhimurium. Front. Bioeng. Biotechnol. 2020, 8, 597502. [Google Scholar] [CrossRef]
- Nalwade, A.R.; Jadhav, A. Biosynthesis of silver nanoparticles using leaf extract of Daturaalba Nees. and evaluation of their antibacterial activity. Arch. Appl. Sci. Res. 2013, 5, 45–49. [Google Scholar]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012, 261, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [Green Version]
- De Silva, C.; Nawawi, N.M.; Karim, M.M.A.; Gani, S.A.; Masarudin, M.J.; Gunasekaran, B.; Ahmad, S.A. The mechanistic action of biosynthesised silver nanoparticles and its application in aquaculture and livestock industries. Animals 2021, 11, 2097. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Shikha, S.; Chaudhuri, S.R.; Bhattacharyya, M.S. Facile One Pot Greener Synthesis of Sophorolipid Capped Gold Nanoparticles and its Antimicrobial Activity having Special Efficacy Against Gram Negative Vibrio cholerae. Sci. Rep. 2020, 10, 1463. [Google Scholar] [CrossRef] [Green Version]
- Tello-Olea, M.; Rosales-Mendoza, S.; Campa-Córdova, A.I.; Palestino, G.; Luna-González, A.; Reyes-Becerril, M.; Velazquez, E.; Hernandez-Adame, L.; Angulo, C. Gold nanoparticles (AuNP) exert immunostimulatory and protective effects in shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2019, 84, 756–767. [Google Scholar] [CrossRef]
- Babu, B.; Palanisamy, S.; Vinosha, M.; Anjali, R.; Kumar, P.; Pandi, B.; Tabarsa, M.; You, S.G.; Prabhu, N.M. Bioengineered gold nanoparticles from marine seaweed Acanthophora spicifera for pharmaceutical uses: Antioxidant, antibacterial, and anticancer activities. Bioprocess Biosyst. Eng. 2020, 43, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Gobi, N.; Ravichandran, S.; Karthi, S.; Ashokkumar, B.; Sivakumar, N. A novel antimicrobial therapy for the control of Aeromonas hydrophila infection in aquaculture using marine polysaccharide coated gold nanoparticle. Microb. Pathog. 2017, 110, 140–151. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Rizvi, S.M.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.S.; Alshammari, F.; Khafagy, E.-S.; Rahamathulla, M.; et al. Cefotaxime Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Reddy, K.R. Green synthesis, morphological and optical studies of CuO nanoparticles. J. Mol. Struct. 2017, 1150, 553–557. [Google Scholar] [CrossRef]
- Ghuglot, R.; Titus, W.; Agnihotri, A.S.; Krishnakumar, V.; Krishnamoorthy, G.; Marimuthu, N. Stable copper nanoparticles as potential antibacterial agent against aquaculture pathogens and human fibroblast cell viability. Biocatal. Agric. Biotechnol. 2021, 32, 101932. [Google Scholar] [CrossRef]
- Chari, N.; Felix, L.O.; Davoodbasha, M.A.; Sulaiman Ali, A.; Nooruddin, T. In vitro and in vivo antibiofilm effect of copper nanoparticles against aquaculture pathogens. Biocatal. Agric. Biotechnol. 2017, 10, 336–341. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Mohd Amin, N.A.A.; Saleh Al-Hammadi, A.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef] [PubMed]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Mater. Sci. Semicond. Process. 2015, 39, 621–628. [Google Scholar] [CrossRef]
- Anand, K.V.; Mahalakshmi, D.; Selvan, S.M.; Ravi, M.; Kannan, M.; Govindaraju, K.; Shalan, A.E. Biomass extract of green macroalga Halimeda opuntia assisted ZnO nanoparticles: Preparation, physico-chemical characterization, and antibacterial activity against Vibrio harveyi. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Singh, R.; Cheng, S.; Singh, S. Oxidative stress-mediated genotoxic effect of zinc oxide nanoparticles on Deinococcus radiodurans. 3 Biotech 2020, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The-antimicrobial-activity-of-nanoparticles—Present-situati. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Tso, C.P.; Zhung, C.M.; Shih, Y.H.; Tseng, Y.M.; Wu, S.C.; Doong, R.A. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 2010, 61, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Bianchini, A.; Playle, R.C.; Wood, C.M.; Walsh, P.J. Short-term silver accumulation in tissues of three marine invertebrates: Shrimp Penaeus duorarum, sea hare Aplysia californica, and sea urchin Diadema antillarum. Aquat. Toxicol. 2007, 84, 182–189. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef] [PubMed]
- Teow, Y.; Asharani, P.V.; Hande, M.P.; Valiyaveettil, S. Health impact and safety of engineered nanomaterials. Chem. Commun. 2011, 47, 7025–7038. [Google Scholar] [CrossRef] [PubMed]
- Larese, F.F.; D’Agostin, F.; Crosera, M.; Adami, G.; Renzi, N.; Bovenzi, M.; Maina, G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009, 255, 33–37. [Google Scholar] [CrossRef]
- Barhoumi, L.; Dewez, D. Toxicity of superparamagnetic iron oxide nanoparticles on green alga Chlorella vulgaris. BioMed Res. Int. 2013, 2013, 647974. [Google Scholar] [CrossRef] [Green Version]
- van der Ploeg, M.J.C.; Handy, R.D.; Waalewijn-Kool, P.L.; van den Berg, J.H.J.; Herrera Rivera, Z.E.; Bovenschen, J.; Molleman, B.; Baveco, J.M.; Tromp, P.; Peters, R.J.B.; et al. Effects of silver nanoparticles (NM-300K) on Lumbricus rubellus earthworms and particle characterization in relevant test matrices including soil. Environ. Toxicol. Chem. 2014, 33, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, J.; Tung, V.C.; Luo, J.; Kim, F.; Huang, J. Graphene oxide as surfactant sheets. Pure Appl. Chem. 2011, 83, 95–110. [Google Scholar] [CrossRef]
- Costinas, C.; Salagean, C.A.; Cotet, L.C.; Baia, M.; Todea, M.; Magyari, K.; Baia, L. Insights into the Stability of Graphene Oxide Aqueous Dispersions. Nanomaterials 2022, 12, 4489. [Google Scholar] [CrossRef]
- Ray, S.C. Application and Uses of Graphene Oxide and Reduced Graphene Oxide. Appl. Graphene Graphene-Oxide Based Nanomater. 2015, 6, 39–55. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef]
- de Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Han, H. A new function of graphene oxide emerges: Inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J. Nanoparticle Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Wu, X.; Tan, S.; Xing, Y.; Pu, Q.; Wu, M.; Zhao, J.X. Graphene oxide as an efficient antimicrobial nanomaterial for eradicating multi-drug resistant bacteria in vitro and in vivo. Colloids Surf. B Biointerfaces 2017, 157, 1–9. [Google Scholar] [CrossRef]
- Shamsi, S.; Elias, N.; Narti Edayu Sarchio, S.; Md Yasin, F. Gallic Acid Loaded Graphene Oxide Based Nanoformulation (GAGO) as Potential Anti-bacterial Agent against Staphylococcus aureus. Mater. Today Proc. 2018, 5, S160–S165. [Google Scholar] [CrossRef]
- Shamsi, S.; Ghafor, A.A.H.A.; Norjoshukrudin, N.H.; Ng, I.M.J.; Abdullah, S.N.S.; Sarchio, S.N.E.; Yasin, F.M.; Gani, S.A.; Desa, M.N.M. Stability, Toxicity, and Antibacterial Potential of Gallic Acid-Loaded Graphene Oxide (GAGO) Against Methicillin-Resistant Staphylococcus aureus (MRSA) Strains. Int. J. Nanomed. 2022, 17, 5781–5807. [Google Scholar] [CrossRef]
- Ng, I.M.J.; Shamsi, S. Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2022, 23, 9096. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene Induces Formation of Pores That Kill Spherical and Rod-Shaped Bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef]
- Sarkar, B.; Mahanty, A.; Gupta, S.K.; Choudhury, A.R.; Daware, A.; Bhattacharjee, S. Nanotechnology: A next-generation tool for sustainable aquaculture. Aquaculture 2022, 546, 737330. [Google Scholar] [CrossRef]
- Natarajan, A.; Devi, K.S.S.; Raja, S.; Senthil Kumar, A. An Elegant Analysis of White Spot Syndrome Virus Using a Graphene Oxide/Methylene Blue based Electrochemical Immunosensor Platform. Sci. Rep. 2017, 7, 46169. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, X.; Li, W.; Wu, W.; Wang, S.; Guo, Z.; Zhou, J.; Su, X. A label-free multi-functionalized graphene oxide based electrochemiluminscence immunosensor for ultrasensitive and rapid detection of Vibrio parahaemolyticus in seawater and seafood. Talanta 2016, 147, 220–225. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, Y.; Hu, Y.; Yu, Z.; Tao, Y.; Wu, Y.; Zeng, M.; Wang, S.; Li, X.; Zhou, J.; et al. Faraday cage-type electrochemiluminescence immunosensor for ultrasensitive detection of Vibrio vulnificus based on multi-functionalized graphene oxide. Anal. Bioanal. Chem. 2016, 408, 7203–7211. [Google Scholar] [CrossRef]
- Wei, L.S.; Mustakim, M.T.; An’amt, M.N.; Wee, W.; Huang, N.M. The potential of graphene oxide as antimicrobial agent against pathogenic bacteria isolated from aquaculture sites. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 220–224. [Google Scholar]
- Lee, J.H.; Yoo, H.; Ahn, Y.J.; Kim, H.J.; Kwon, S.R. Evaluation of the Antimicrobial Effect of Graphene Oxide Fiber on Fish Bacteria for Application in Aquaculture Systems. Materials 2022, 15, 966. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Ghafor, A.A.H.A.; Elias, N.; Shamsi, S.; Yasin, F.M.; Sarchio, S.N.E. Toxicity assessment of gallic acid loaded graphene oxide (GAGO) nano-formulation in zebrafish (danio rerio) embryos. Pertanika J. Sci. Technol. 2020, 28, 311–326. [Google Scholar]
- Souza, J.P.; Baretta, J.F.; Santos, F.; Paino, I.M.M.; Zucolotto, V. Toxicological effects of graphene oxide on adult zebrafish (Danio rerio). Aquat. Toxicol. 2017, 186, 11–18. [Google Scholar] [CrossRef]
- Chen, M.; Yin, J.; Liang, Y.; Yuan, S.; Wang, F.; Song, M.; Wang, H. Oxidative stress and immunotoxicity induced by graphene oxide in zebrafish. Aquat. Toxicol. 2016, 174, 54–60. [Google Scholar] [CrossRef]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Cai, Z.; Li, B. A mechanism study on toxicity of graphene oxide to Daphnia magna: Direct link between bioaccumulation and oxidative stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef]
- Cavion, F.; Fusco, L.; Sosa, S.; Manfrin, C.; Alonso, B.; Zurutuza, A.; Della Loggia, R.; Tubaro, A.; Prato, M.; Pelin, M. Ecotoxicological impact of graphene oxide: Toxic effects on the model organism: Artemia franciscana. Environ. Sci. Nano 2020, 7, 3605–3615. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Alagan, A.A.; Sarchio, S.N.E.; Md Yasin, F. Synthesis, characterization, and toxicity assessment of Pluronic F127-functionalized graphene oxide on the embryonic development of Zebrafish (Danio Rerio). Int. J. Nanomed. 2020, 15, 8311–8329. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticles | Concentration | Bacteria | Findings | Reference |
|---|---|---|---|---|
| AgNPs from Calotropis gigantea leaf extract | g/mL | V. alginolyticus | V. alginolyticus was completely inhibited at 20 μg/mL and infected brine shrimp treated with AgNPs had a higher survival rate than the non-treated group | [63] |
| AgNPs | - | V. harveyi | Smaller AgNPs (16.62 nm) had greater antibacterial activity compared to larger particles (22.22 nm) | [64] |
| AgNPs produced using Lysinibacillus xylanilyticus strain MAHUQ-40 | g/mL | V. parahaemolyticus and Salmonella Typhimurium | AgNPs exhibited a MIC of 3.12 and 6.25 μg/mL for V. parahaemolyticus and S. Typhimurium | [65] |
| AuNPs | g/g feed | V. parahaemolyticus | AuNPs enhanced survival rate of challenged shrimps and did not cause any histological damage or toxic effects | [73] |
| AuNPs from marine red alga Acanthophora spicifera | g/mL | V. harveyi and S. aureus | AuNPs showed greater antibacterial effect against V. harveyi than against S. aureus | [74] |
| fucoidan coated AuNPs | g/mL | Aeromonas hydrophila | AuNPs effectively inhibited A. hydrophila and reduced the mortality rate of infected tilapia | [75] |
| CuNPs from Trigonella foenum-graecum leaf extract | 0.5–2.5 mM | V. harveyi, V. parahaemolyticus, and V. vulnificus | CuNPs showed bactericidal effect against the bacteria | [79] |
| CuNPs | 100 ng/mL | V. alginolyticus, V. parahaemolyticus, and A. hydrophila | 60% and increased the survival rate of brine shrimp | [80] |
| ZnONPs from Halimeda opuntia extract | g/mL | V. harveyi | Growth inhibition by ZnO increased with exposure duration and dose | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kah Sem, N.A.D.; Abd Gani, S.; Chong, C.M.; Natrah, I.; Shamsi, S. Management and Mitigation of Vibriosis in Aquaculture: Nanoparticles as Promising Alternatives. Int. J. Mol. Sci. 2023, 24, 12542. https://doi.org/10.3390/ijms241612542
Kah Sem NAD, Abd Gani S, Chong CM, Natrah I, Shamsi S. Management and Mitigation of Vibriosis in Aquaculture: Nanoparticles as Promising Alternatives. International Journal of Molecular Sciences. 2023; 24(16):12542. https://doi.org/10.3390/ijms241612542
Chicago/Turabian StyleKah Sem, Nuan Anong Densaad, Shafinaz Abd Gani, Chou Min Chong, Ikhsan Natrah, and Suhaili Shamsi. 2023. "Management and Mitigation of Vibriosis in Aquaculture: Nanoparticles as Promising Alternatives" International Journal of Molecular Sciences 24, no. 16: 12542. https://doi.org/10.3390/ijms241612542
APA StyleKah Sem, N. A. D., Abd Gani, S., Chong, C. M., Natrah, I., & Shamsi, S. (2023). Management and Mitigation of Vibriosis in Aquaculture: Nanoparticles as Promising Alternatives. International Journal of Molecular Sciences, 24(16), 12542. https://doi.org/10.3390/ijms241612542





